It was with great interest that we read the article by Velayos-Galán et al.1 We would like to report a similar case of Guillain-Barré syndrome (GBS) that manifested after bilateral pneumonia due to SARS-CoV-2 infection, and analyse the data published on this entity.
On 4 May 2020, a 70-year-old man presented subacute weakness in all 4 limbs, which worsened over the following 5 days. Three weeks earlier, he had presented bilateral pneumonia due to SARS-CoV-2 infection: a chest CT scan showed ground-glass opacities in both lungs, and PCR results were positive for SARS-CoV-2 in nasopharyngeal and oropharyngeal swabs. The patient was treated with oxygen therapy, hydroxychloroquine, azithromycin, ceftriaxone, and dexamethasone. The patient had no relevant medical history. His body temperature was 36.5°C and baseline oxygen saturation was 99%. Pulmonary auscultation revealed no alterations. A neurological examination revealed asymmetrical weakness (Medical Research Council grade 4/5 in the right hand; 4+/5 in the left hand; 4/5 in the left leg, and 3+/5 in the right leg) and areflexia in the legs and feet. Two days after admission, symptoms worsened. Muscle strength was 4/5 in the arms and hands and 3/5 in the legs and feet. Light touch and pin prick distal sensitivity was decreased in distal regions.
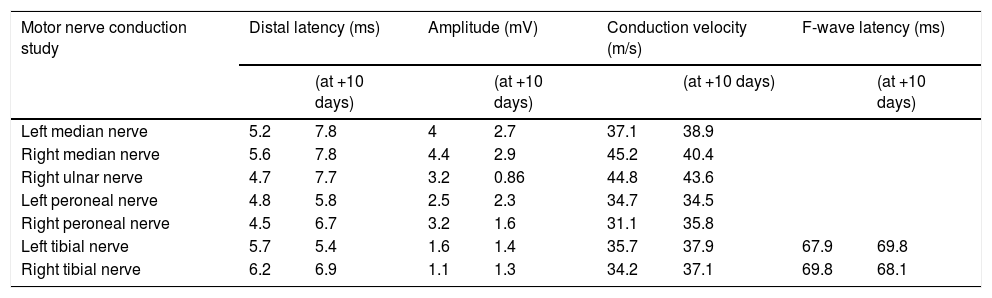
Laboratory analysis at admission revealed lymphocytopaenia (0.52 × 103 cells/L; normal range: 1.1-3.2 × 103 cells/L) and thrombocytopaenia (113 × 103 cells/L; normal range: 125-300 × 103 cells/L). CSF analysis showed normal cell count (0 × 103 cells/L; normal range: 0-8 × 103 cells/L) and a slightly increased protein level (49 mg/dL; normal range: 8-43 mg/dL). Nerve conduction studies performed on day 6 revealed delayed distal latencies and absence of F waves in the early phase, in the context of mixed-type (axonal and demyelinating) acute motor polyneuropathy of moderate and symmetrical intensity in all 4 limbs, with associated axonal sensory involvement, loss of motor units, and signs of neurogenic involvement of the muscles analysed without acute denervation (Table 1). The patient was diagnosed with GBS and started treatment on high-dose intravenous immunoglobulins (0.4 g/kg/day for 5 days) 8 hours after admission; symptoms improved on day 3 of treatment.
Motor and sensory nerve conduction findings.
| Motor nerve conduction study | Distal latency (ms) | Amplitude (mV) | Conduction velocity (m/s) | F-wave latency (ms) | ||||
|---|---|---|---|---|---|---|---|---|
| (at +10 days) | (at +10 days) | (at +10 days) | (at +10 days) | |||||
| Left median nerve | 5.2 | 7.8 | 4 | 2.7 | 37.1 | 38.9 | ||
| Right median nerve | 5.6 | 7.8 | 4.4 | 2.9 | 45.2 | 40.4 | ||
| Right ulnar nerve | 4.7 | 7.7 | 3.2 | 0.86 | 44.8 | 43.6 | ||
| Left peroneal nerve | 4.8 | 5.8 | 2.5 | 2.3 | 34.7 | 34.5 | ||
| Right peroneal nerve | 4.5 | 6.7 | 3.2 | 1.6 | 31.1 | 35.8 | ||
| Left tibial nerve | 5.7 | 5.4 | 1.6 | 1.4 | 35.7 | 37.9 | 67.9 | 69.8 |
| Right tibial nerve | 6.2 | 6.9 | 1.1 | 1.3 | 34.2 | 37.1 | 69.8 | 68.1 |
| Sensory nerve conduction study | Amplitude (μV) | Conduction velocity (m/s) | ||
|---|---|---|---|---|
| (at +10 days) | (at +10 days) | |||
| Left median nerve | 2.4 | 46.9 | ||
| Right median nerve | 4.4 | 6.2 | 47.3 | 49.7 |
| Right ulnar nerve | 8.3 | Absent | 50 | Absent |
| Left sural nerve | 4.2 | 2.9 | 46.5 | 45.1 |
| Right sural nerve | 4.6 | 3.9 | 48.5 | 48.5 |
Upon discharge, 14 days after admission, he only presented mild weakness in the interosseous muscles of the hands (4+/5) and dorsiflexor muscles of both feet (4+/5), as well as generalised areflexia. The PCR results for SARS-CoV-2 at discharge were negative.
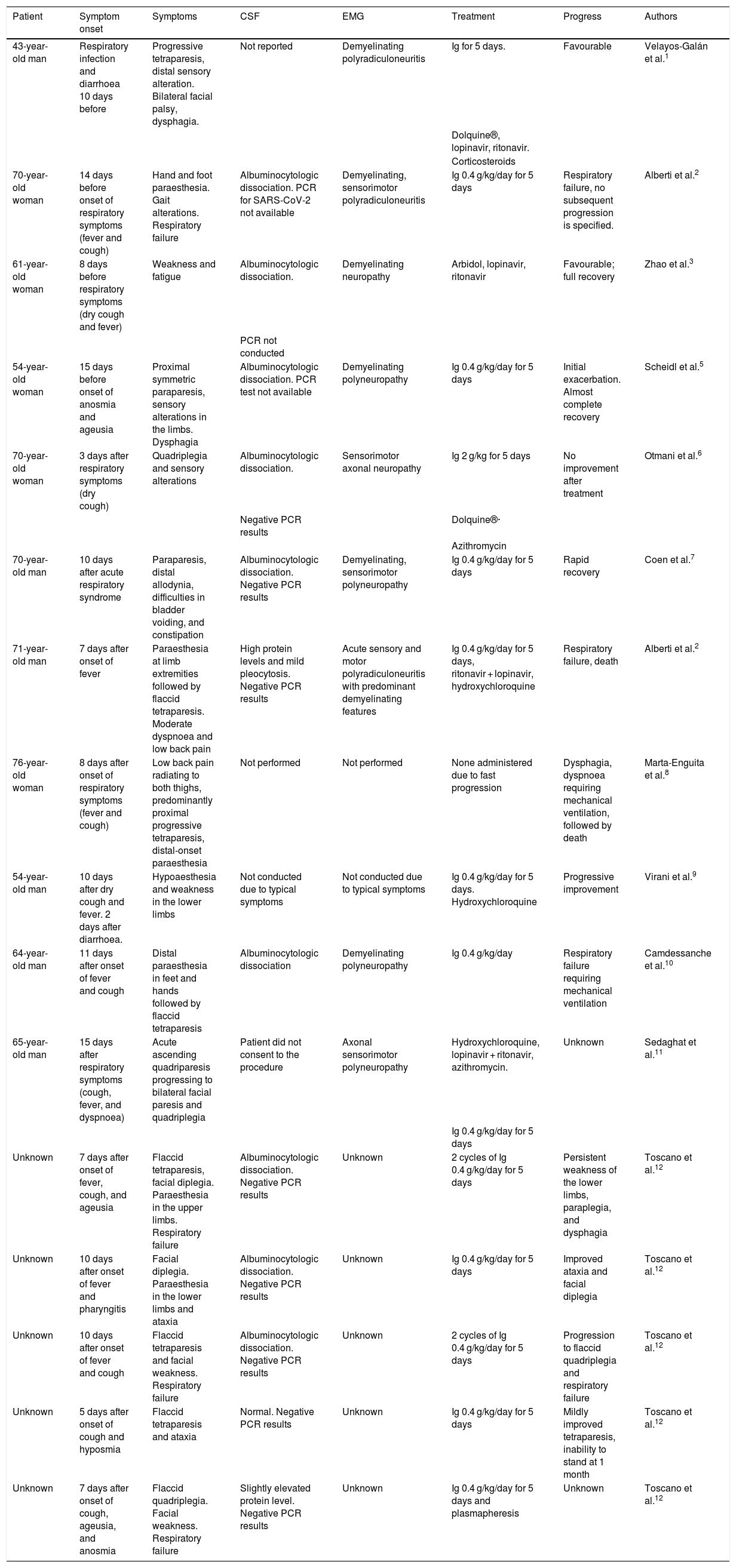
To our knowledge, 3 cases of concomitant GBS and SARS-CoV-2 infection with parainfectious profiles1–3 and 13 cases of GBS subsequent to SARS-CoV-2 infection4–12 have been reported to date (Table 2). The favourable progression of most of the patients who presented GBS after the infection is noteworthy.
Patient profiles.
| Patient | Symptom onset | Symptoms | CSF | EMG | Treatment | Progress | Authors |
|---|---|---|---|---|---|---|---|
| 43-year-old man | Respiratory infection and diarrhoea 10 days before | Progressive tetraparesis, distal sensory alteration. Bilateral facial palsy, dysphagia. | Not reported | Demyelinating polyradiculoneuritis | Ig for 5 days. | Favourable | Velayos-Galán et al.1 |
| Dolquine®, lopinavir, ritonavir. | |||||||
| Corticosteroids | |||||||
| 70-year-old woman | 14 days before onset of respiratory symptoms (fever and cough) | Hand and foot paraesthesia. Gait alterations. Respiratory failure | Albuminocytologic dissociation. PCR for SARS-CoV-2 not available | Demyelinating, sensorimotor polyradiculoneuritis | Ig 0.4 g/kg/day for 5 days | Respiratory failure, no subsequent progression is specified. | Alberti et al.2 |
| 61-year-old woman | 8 days before respiratory symptoms (dry cough and fever) | Weakness and fatigue | Albuminocytologic dissociation. | Demyelinating neuropathy | Arbidol, lopinavir, ritonavir | Favourable; full recovery | Zhao et al.3 |
| PCR not conducted | |||||||
| 54-year-old woman | 15 days before onset of anosmia and ageusia | Proximal symmetric paraparesis, sensory alterations in the limbs. Dysphagia | Albuminocytologic dissociation. PCR test not available | Demyelinating polyneuropathy | Ig 0.4 g/kg/day for 5 days | Initial exacerbation. Almost complete recovery | Scheidl et al.5 |
| 70-year-old woman | 3 days after respiratory symptoms (dry cough) | Quadriplegia and sensory alterations | Albuminocytologic dissociation. | Sensorimotor axonal neuropathy | Ig 2 g/kg for 5 days | No improvement after treatment | Otmani et al.6 |
| Negative PCR results | Dolquine®· | ||||||
| Azithromycin | |||||||
| 70-year-old man | 10 days after acute respiratory syndrome | Paraparesis, distal allodynia, difficulties in bladder voiding, and constipation | Albuminocytologic dissociation. Negative PCR results | Demyelinating, sensorimotor polyneuropathy | Ig 0.4 g/kg/day for 5 days | Rapid recovery | Coen et al.7 |
| 71-year-old man | 7 days after onset of fever | Paraesthesia at limb extremities followed by flaccid tetraparesis. Moderate dyspnoea and low back pain | High protein levels and mild pleocytosis. Negative PCR results | Acute sensory and motor polyradiculoneuritis with predominant demyelinating features | Ig 0.4 g/kg/day for 5 days, ritonavir + lopinavir, hydroxychloroquine | Respiratory failure, death | Alberti et al.2 |
| 76-year-old woman | 8 days after onset of respiratory symptoms (fever and cough) | Low back pain radiating to both thighs, predominantly proximal progressive tetraparesis, distal-onset paraesthesia | Not performed | Not performed | None administered due to fast progression | Dysphagia, dyspnoea requiring mechanical ventilation, followed by death | Marta-Enguita et al.8 |
| 54-year-old man | 10 days after dry cough and fever. 2 days after diarrhoea. | Hypoaesthesia and weakness in the lower limbs | Not conducted due to typical symptoms | Not conducted due to typical symptoms | Ig 0.4 g/kg/day for 5 days. Hydroxychloroquine | Progressive improvement | Virani et al.9 |
| 64-year-old man | 11 days after onset of fever and cough | Distal paraesthesia in feet and hands followed by flaccid tetraparesis | Albuminocytologic dissociation | Demyelinating polyneuropathy | Ig 0.4 g/kg/day | Respiratory failure requiring mechanical ventilation | Camdessanche et al.10 |
| 65-year-old man | 15 days after respiratory symptoms (cough, fever, and dyspnoea) | Acute ascending quadriparesis progressing to bilateral facial paresis and quadriplegia | Patient did not consent to the procedure | Axonal sensorimotor polyneuropathy | Hydroxychloroquine, lopinavir + ritonavir, azithromycin. | Unknown | Sedaghat et al.11 |
| Ig 0.4 g/kg/day for 5 days | |||||||
| Unknown | 7 days after onset of fever, cough, and ageusia | Flaccid tetraparesis, facial diplegia. Paraesthesia in the upper limbs. Respiratory failure | Albuminocytologic dissociation. Negative PCR results | Unknown | 2 cycles of Ig 0.4 g/kg/day for 5 days | Persistent weakness of the lower limbs, paraplegia, and dysphagia | Toscano et al.12 |
| Unknown | 10 days after onset of fever and pharyngitis | Facial diplegia. Paraesthesia in the lower limbs and ataxia | Albuminocytologic dissociation. Negative PCR results | Unknown | Ig 0.4 g/kg/day for 5 days | Improved ataxia and facial diplegia | Toscano et al.12 |
| Unknown | 10 days after onset of fever and cough | Flaccid tetraparesis and facial weakness. Respiratory failure | Albuminocytologic dissociation. Negative PCR results | Unknown | 2 cycles of Ig 0.4 g/kg/day for 5 days | Progression to flaccid quadriplegia and respiratory failure | Toscano et al.12 |
| Unknown | 5 days after onset of cough and hyposmia | Flaccid tetraparesis and ataxia | Normal. Negative PCR results | Unknown | Ig 0.4 g/kg/day for 5 days | Mildly improved tetraparesis, inability to stand at 1 month | Toscano et al.12 |
| Unknown | 7 days after onset of cough, ageusia, and anosmia | Flaccid quadriplegia. Facial weakness. Respiratory failure | Slightly elevated protein level. Negative PCR results | Unknown | Ig 0.4 g/kg/day for 5 days and plasmapheresis | Unknown | Toscano et al.12 |
CSF: cerebrospinal fluid; EMG: electromyography; Ig: immunoglobulins; PCR: polymerase chain reaction.
In our patient, the gradual progression of neurological symptoms resembles that observed with postinfectious aetiologies. Therefore, we suspect an association between acute polyneuropathy and SARS-CoV-2 infection. This association is supported by the fact that the patient observed home isolation for 21 days before neurological symptom onset, as well as the negative results for antiganglioside antibodies. However, the postinfectious onset, acute clinical course, and typical neurophysiological findings of GBS (mixed-type polyneuropathy of motor and sensory fibres), together with the absence of history of autoimmune, neoplastic, or neurological disease, suggest postinfectious aetiology. A significant limitation is the lack of availability of SARS-CoV-2 serology tests and CSF PCR tests at our centre.
While our case is suggestive of a possible association between GBS and SARS-CoV-2 infection, further case reports with epidemiological data are needed to demonstrate a causal relationship. This case also underscores the need to consider possible neurological symptoms of SARS-CoV-2 infection.
The authors agree that there is a need for careful observation of neurological complications of SARS-CoV-2 infection.
The Spanish Society of Neurology is currently conducting a national observational study on neurological presentations and manifestations of COVID-19.
Please cite this article as: Guijarro-Castro C, Rosón-González M, Abreu A, García-Arratibel A, Ochoa-Mulas M. Síndrome de Guillain-Barré tras infección por SARS-CoV-2. Comentarios tras la publicación de 16 nuevos casos. Neurología. 2020;35:412–415.