Adrenoleukodystrophy is an X-linked genetic disease caused by a mutation that dysregulates metabolism of very long-chain fatty acids. This leads to a defect in axonal myelination and white matter involvement, with neurological symptoms varying according to the form of disease presentation1; in some cases, it manifests with symptoms of spastic paraparesis.2 Furthermore, it is associated with damage to the adrenal cortex, with subsequent hormonal involvement.
Due to its X-linked inheritance pattern, it predominantly affects boys, although girls may also present a certain degree of involvement, which is generally milder but in some cases it may be as disabling as in boys.2,3
The clinical spectrum includes:
- -
A classic form, with onset during childhood, which clinically presents as learning and behavioural problems with psychomotor developmental regression. It also manifests with visual alterations due to optic atrophy, auditory deficits, and progressive motor impairment, causing spastic tetraparesis that generally leads to dependence within a short period (2-3 years).
- -
A form with onset during young adulthood (approximately in the third decade of life), known as adrenomyeloneuropathy. It generally manifests as a progressive gait disorder with spastic tetraparesis and sensory ataxia. It may be associated with neurogenic bladder, sexual dysfunction, and occasionally adrenal insufficiency, which may also precede the other symptoms. Psychiatric symptoms, such as depression and psychosis, have also been described. Although it inevitably presents a progressive course, progression is slower than in the childhood-onset form.
- -
Lastly, in cases of predominantly hormonal involvement, symptoms coincide with those of classic Addison syndrome, with adrenal insufficiency being diagnosed in the first years of life, although the majority of patients subsequently present some neurological symptom.
No curative treatment is currently available, although several strategies that may change disease outcomes are being studied.4–6
Given the current lack of disease-modifying treatments, symptom progression is inevitable and these patients present high rates of physical dependence.
Considering current evidence on gait improvement in patients with multiple sclerosis and treated with fampridine,7 we administered the drug as compassionate use in 2 patients with adrenoleukodystrophy and significant gait impairment; both patients gave written informed consent.
Both patients presented adult-onset adrenoleukodystrophy: patient 1 was a 26-year-old man and patient 2 was a 41-year-old woman with weakness in the lower limbs and gait alteration with a spastic paretic pattern. Neither patient presented any relevant history.
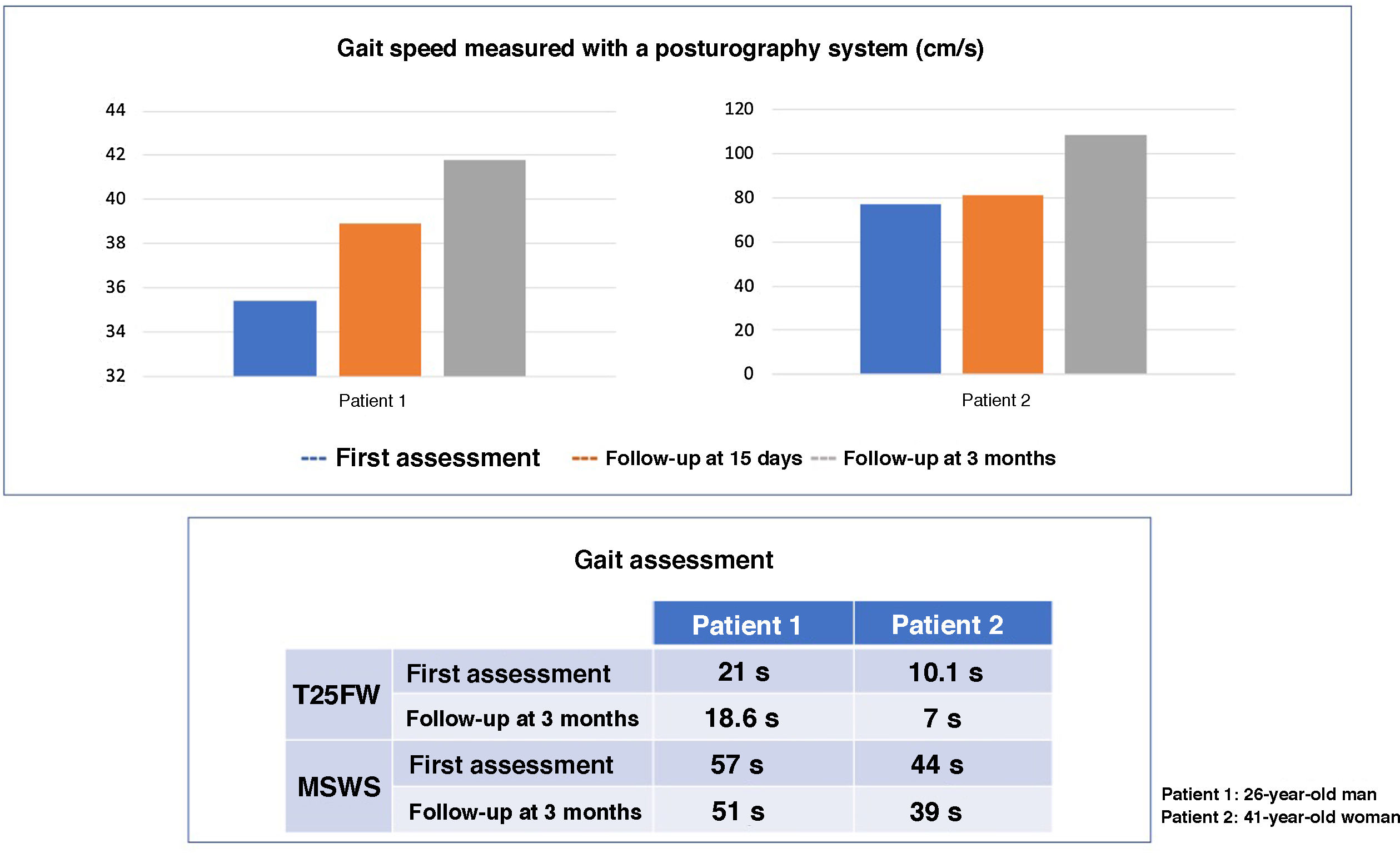
Both patients underwent neurological examination, the Timed-25 Foot Walk Test, the Multiple Sclerosis Walking Scale assessment, and a posturographic study (Neurocom Balance Manager System). As reflected by the scales, gait impairment was more pronounced in the male patient, but both patients showed an improvement at day 15 of treatment with fampridine at 10mg/12h, which persisted in a follow-up consultation at 3 months (Fig. 1). A noteworthy improvement was also observed in the pyramidal signs, with improvement or resolution of the ankle clonus in the examination. Neither patient presented any adverse reaction.
Subjectively, both patients also reported improved stability when walking and standing. In a follow-up visit at 6 months, scores on the tests performed remained stable.
In the light of these results, and always taking into account the limitations mentioned in the current summary of product characteristics and the administration of the drug as compassionate use, we may consider treatment with fampridine on a case-by-case basis in patients with symptoms of gait alteration with spastic paraparesis, provided that there is no other alternative with proven efficacy. There is a need for further studies assessing fampridine treatment in larger numbers of patients with gait alterations due to spastic paraparesis secondary to diseases other than multiple sclerosis.
Conflicts of interestThe authors have no conflicts of interest to declare. No external funding was received for this study.
Please cite this article as: Morales-Casado MI, López-Ariztegui N, Muñoz-Escudero F, Navarro-Bejarano A, Pérez-Matos JA. Mejoría de la marcha en pacientes con adrenoleucodistrofia tratados con fampridina como uso compasivo. Neurología. 2021;36:393–395.