Inserting, maintaining, or removing a central venous catheter (CVC) is an invasive procedure and, as such, is not free of risk; it may even lead to severe complications that compromise the patient's life. One example is air embolism,1–3 the entry of gas into the arteries or veins.4
We describe the case of a patient who presented ischaemic stroke due to an air embolism after her CVC was manipulated during the replacement of a parenteral nutrition bag.
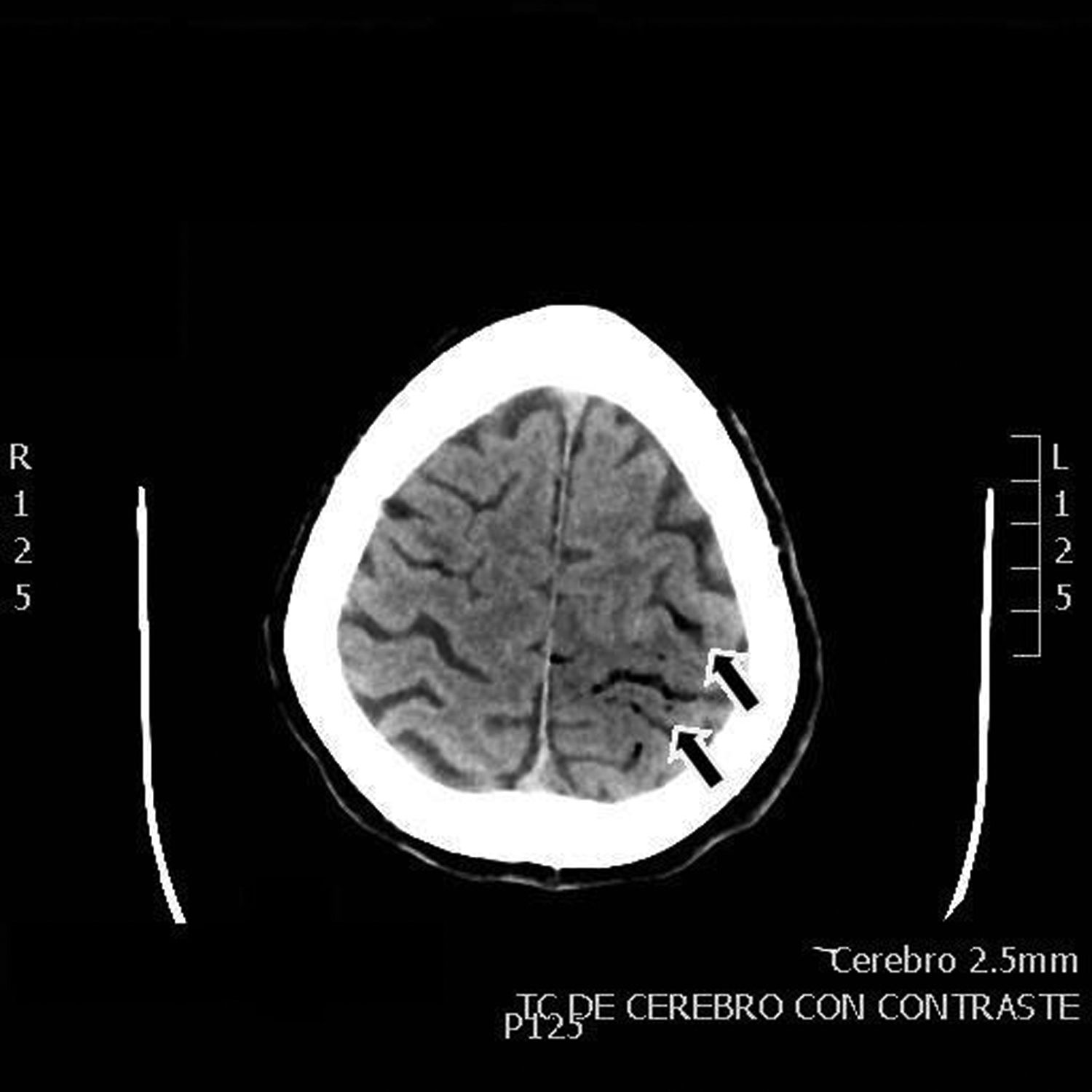
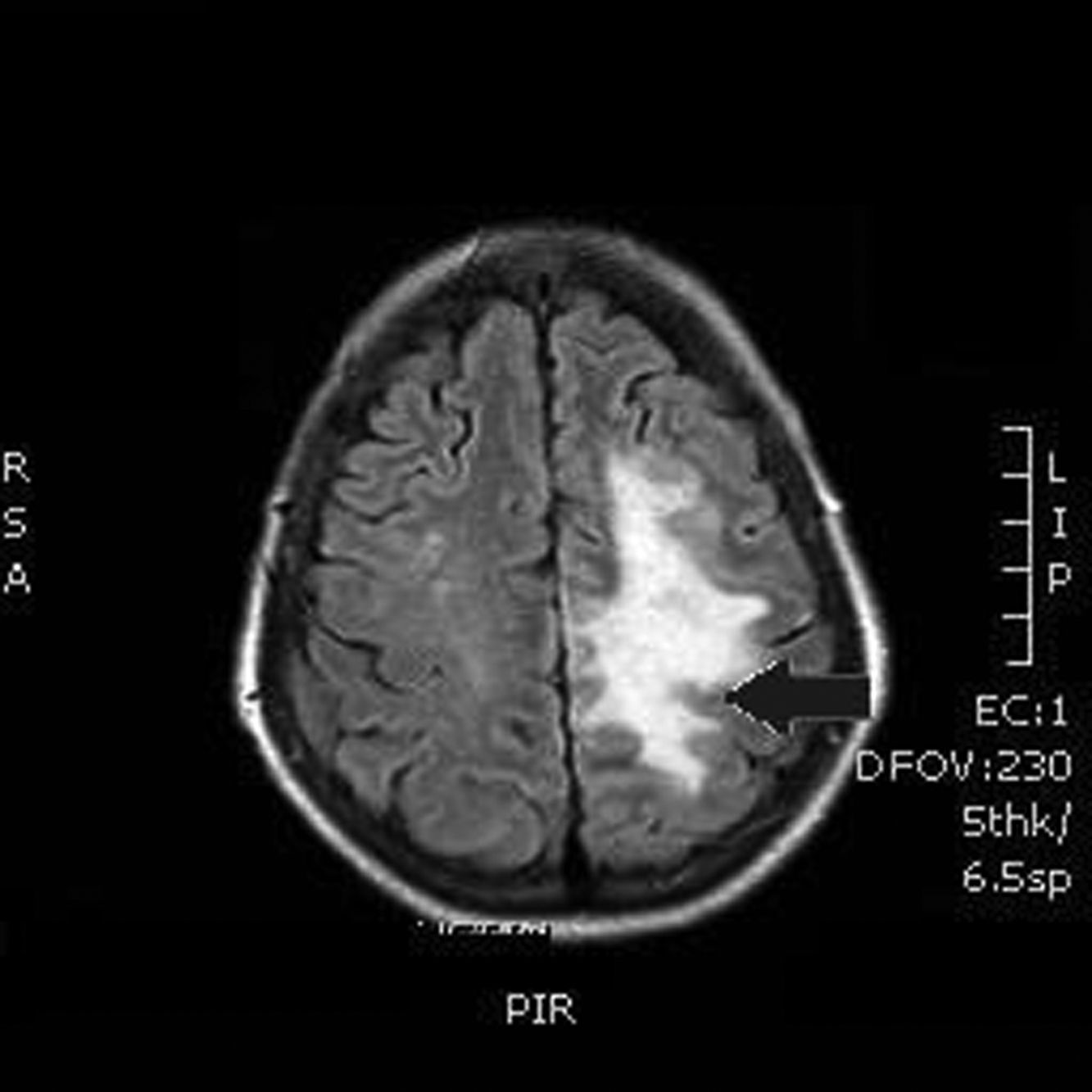
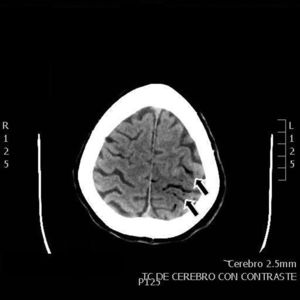
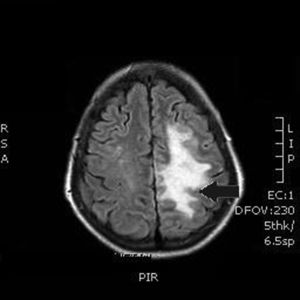
Our patient was a 55-year-old woman, a smoker (15 cigarettes/day) who presented chronic obstructive pulmonary disease, depressive disorder, and systemic lupus erythematosus (with lupus nephritis and antiphospholipid syndrome). The patient was receiving escitalopram, salbutamol, and acenocoumarol. She was admitted due to abdominal pain of 10hours’ progression, due to a duodenal perforation. She underwent emergency surgery, with suture and Graham patch of the anterior wall of the duodenal bulb by midline laparotomy. During the intervention, a CVC had to be placed in the right internal jugular vein (guided by ultrasound) to be used for parenteral nutrition in the immediate postoperative period. The procedure was uneventful and the patient was transferred to the recovery room, where she stayed for 12hours with no complications and started parenteral nutrition. She was subsequently transferred to an admission ward. At 48hours, immediately after replacement of the parenteral nutrition bag with the patient in a seated position, she presented an abrupt decrease in the level of consciousness, with quadriparesis, gaze deviation to the left, and facial droop. Arterial blood pressure, heart rate, and oxygen saturation values were normal. An emergency cranial CT scan (Fig. 1) revealed pneumocephalus with intraparenchymal air bubbles in the border zone of the middle cerebral artery and left anterior cerebral artery, and in arachnoid sulci of the left parietal convexity, compatible with air embolism. When her level of consciousness recovered, the patient presented right-sided hemiplegia and dysarthria, and was transferred to be treated with hyperbaric oxygen. An MRI study performed at 48hours (Fig. 2) revealed an extensive area of vasogenic cerebral oedema compatible with acute/subacute ischaemic lesions. An echocardiogram showed no evidence of heart disease, valvulopathy, or intracardiac shunts. Despite 4 sessions of treatment in a hyperbaric chamber with administration of 100% oxygen at 2.2 atmospheres for 60minutes, the patient showed no clinical improvement, with the right hemiplegia and dysarthria persisting. When she was discharged, the patient presented paresis of the right upper and lower limbs (3/5 in both) and hypoaesthesia, with altered positional sensitivity and inability to hold a standing position or walk, making her dependent in the activities of daily living. The Barthel Index score was 15/100.
The patient was assessed 2 months later in our clinic, and the examination found that right hemiparesis persisted, but that she could exert pressure and perform weak pinch grips with the right hand. She had started to walk with the help of a walker and a caregiver, needing help in the activities of daily living; Barthel Index score was 40/100.
For an air embolism to be located in the brain artery tree, air must enter the circulatory system and pass to the left system, skipping the lung, which acts as a filter for these air bubbles. These bubbles occlude small arteries, causing reduced perfusion distal to the occlusion. The passage of air to the left system, skipping the lung filter, is known as paradoxical embolism5 and results from the passage of air from the venous system to the arterial system through a vascular anomaly; patent foramen ovale is the most frequent,6 presenting in up to 30% of the population. In our case, the presence of a right-to-left communication was ruled out; the mechanism causing the air embolism was the retrograde ascent of air through the CVC in the venous system, moving against the normal flow of venous blood and ultimately reaching the cerebral venous system, facilitated by the central venous pressure being lower than atmospheric pressure, as occurs in the superior vena cava when the patient's thorax is elevated or during Valsalva manoeuvres. This is an underdiagnosed entity of unknown prevalence, as few cases have been published.7–9 A study analysing strokes of unusual cause over a period of time of 10 years, with a total of 70 patients recruited from a hospital clinical series of 2000 consecutive patients with stroke, reported no cases of air embolism in the context of CVC.10 Its aetiology seems to be related to the size of the air bubbles, the diameter of the catheter, and the ejection fraction.11 Ischaemia due to air embolism is caused by the obstruction of blood flow by embolisms, vasospasm, and formation of thrombi by platelet activation. When cerebral vessels are affected, air embolism causes neuronal death by ischaemia and gives rise to an inflammatory response by endothelial irritation, leading to vasogenic cerebral oedema.
Diagnosis is based on neuroimaging studies, which should be performed immediately to detect gas, and clinical symptoms including confusion, amnesia, seizures, cerebral ischaemic vasculopathy, and coma.
When air embolism is suspected, the patient should be placed in the Trendelenburg position or left lateral decubitus position to favour the return of air to the central venous circulation. Administration of 100% oxygen in a hyperbaric chamber is recommended to treat hypoxia and hypoxaemia and to remove air bubbles from the brain, using a diffusion gradient favouring air exit.12,13
Air embolism should be suspected in patients with CVC who present unexplained neurological symptoms after manipulation or insertion of the catheter; early diagnosis and treatment are essential to prevent irreversible sequelae.
Please cite this article as: España Fuente L, Méndez Redondo RE, Gutiérrez Corral N, Fernández Martínez D. Ictus isquémico tras el recambio de una nutrición parenteral en un paciente postoperado. Neurología. 2020;35:341–343.