The course of multiple sclerosis (MS) is influenced by sex, pregnancy and hormonal factors.
AimsTo analyse the influence of the above factors in order to clarify the aetiopathogenic mechanisms involved in the disease.
MethodsWe conducted a comprehensive review of scientific publications in the PubMed database using a keyword search for ‘multiple sclerosis’, ‘MS’, ‘EAE’, ‘pregnancy’, ‘hormonal factors’, ‘treatment’, and related terms. We reviewed the advances presented at the meeting held by the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) in March 2013 in London, as well as recommendations by international experts.
Results and conclusionsWe provide recommendations for counselling and treating women with MS prior to and during pregnancy and after delivery. Current findings on the effects of treatment on the mother, foetus, and newborn are also presented. We issue recommendations for future research in order to address knowledge gaps and clarify any inconsistencies in currently available data.
La esclerosis múltiple (EM) es una enfermedad en cuyo curso influyen el género, los factores hormonales y el embarazo.
ObjetivosRealizar un análisis de la influencia de esos factores para aportar información sobre los mecanismos etiopatogénicos involucrados en la enfermedad.
MétodosRevisión exhaustiva de publicaciones científicas (búsqueda en la base de datos PubMed utilizando los términos: esclerosis múltiple, EM, EAE, embarazo, factores hormonales, tratamiento y términos relacionados), de los avances presentados en una reunión organizada por el Comité Europeo para el Tratamiento e Investigación de Esclerosis Múltiple (ECTRIMS), celebrado en marzo de 2013 en Londres, así como de las recomendaciones de reconocidos expertos internacionales.
Resultados y conclusionesSe ofrecen recomendaciones para el asesoramiento y la gestión de personas con EM antes de la concepción, durante el embarazo y después del parto. Se comentan también los conocimientos actuales sobre el efecto del tratamiento en la madre, el feto y el recién nacido. Realizamos recomendaciones para investigaciones futuras a fin de subsanar deficiencias de conocimiento y aclarar incoherencias de los datos actualmente disponibles.
Multiple sclerosis (MS) is an autoimmune disease predominantly affecting women of childbearing age.1 Its higher prevalence among women is characteristic of most autoimmune diseases, which may reflect the potential impact of sex hormones on the nervous, endocrine, and immune systems.
MS is known to be more frequent among women and to be influenced by the hormonal changes women experience over their lifetimes.
This article reviews the available evidence regarding the impact of pregnancy and hormonal factors on MS. We discuss findings relating to the aetiology and pathophysiology of the disease from experimental models, summarise the results of clinical trials, comment on the implications for managing disease progression and the risk to the foetus, and propose a set of recommendations for the treatment of MS in women of childbearing age and in pregnant women.
Effects of sex hormones in experimental models of multiple sclerosisSeveral studies with both animal models of experimental allergic encephalomyelitis (EAE) and humans have shown the impact of sex hormones on disease expression, prognosis, and activity; these findings are relevant for pregnancy and for the development of treatments for MS.2 Studies with animal models of EAE have shown that oestrogens (17β-oestradiol [E2] and oestriol [E3]), progesterone, and testosterone have anti-inflammatory and neuroprotective effects during both the inductor and effector stages of the disease.3,4 These anti-inflammatory effects are mediated by oestrogen receptors alpha (ER-α) and beta (ER-β),5 which are expressed in CD4+ CD25+ regulatory T cells,6 regulatory B cells,7 and dendritic cells.8 The importance of B cells for E2-induced protection is demonstrated by the fact that the protective effect disappears when B cells are eliminated.9 B cells activate CD4+ Foxp3+ regulatory T cells by upregulating programmed death receptor-1.10,11 The protective effects of E2 in EAE also seem to be linked to the G-protein coupled receptor 30 (GPR30).10 Testosterone may act via androgen receptors12 or after it is converted into oestrogens via ER or GPR30. Experimental studies have shown that androgens may induce CNS remyelination after cuprizone-induced demyelination by acting on the neural androgen receptor.13
Some of the neuroprotective effects of oestrogens in EAE are mediated by ER-α expressed in astrocytes.14 ER-β ligands may prevent demyelination and stimulate remyelination15; treatment with ER-β ligands may have a protective effect on microglia, preventing CNS inflammation.16 Progesterone seems to be involved in axonal protection17 and remyelination,18 whereas testosterone may restore synaptic transmission in the hippocampus.19
Sex-specific incidence of multiple sclerosis: changes in temporal incidenceMultiple sclerosis predominantly affects women (70%); 90% of women with MS experience the first symptoms before the age of 50, and it is estimated that 20%-33% of these women will have children after diagnosis. Recent epidemiological studies report an increase in MS incidence in adult women, leading to an increase in the female-to-male ratio of patients from 2:1 to 3:1 in the last 30 years. According to a Danish registry, the incidence of MS among women has doubled since 1970, and the female-to-male ratio is higher in areas with greater incidence of MS.
The fact that the female-to-male ratio has increased in many areas but at different rates20–24 may indicate that the disease's development involves epigenetic factors, that is, interactions between genes and such other factors as the environment,25 changes in lifestyle (contraception, diet, obesity, smoking, sunlight exposure, vitamin D deficiency),26 greater age at the birth of the first child,27 younger age at the first menstrual cycle, or fewer pregnancies during a woman's lifetime.28–30
Changes in the female-to-male ratio are also age-dependent: 1.5:1 in children younger than 10 years, 3:1 in adolescents and adults, and 1:1.5 in patients older than 50; the latter age group also shows a different clinical pattern, with a predominance of progressive forms of the disease.
Pregnancy planningWomen planning to conceive face numerous concerns, including the impact of MS on fertility, the risk of transmitting MS to the offspring, the effects of medication for MS on the foetus, the impact of pregnancy on disease progression, the impact of MS on the mother's ability to care for her child, and the socioeconomic burden on the family.31
Multiple sclerosis and fertilityMS has not been proven to affect female fertility.32 However, the proportion of women with no children is higher among women who eventually develop MS than among those who do not. Two epidemiological studies report lower incidence of MS among people (both women and men) who had children within the previous 5 years.28,31 The apparent protective effect of maternity/paternity was not seen in parents whose children had been born 10 years previously.28,31 This, combined with the fact that both women and men have a lower incidence of MS in the 5 years following a child's birth, suggests that pregnancy in itself is not a protective factor against MS. Alternative explanations for the apparently higher incidence of MS in people with no children may be preclinical MS acting to reduce fertility or influence family planning decisions.
Several studies have shown that fertility treatments increase the risk of relapses in women with MS, with relapse rates increasing during treatment and in the 3 months after in vitro fertilisation.33–36 This has been associated with use of gonadotropin-releasing hormone agonists.35,36 These drugs increase the number of cells producing IL-8, IL-12, IFN-γ, and TGF-β, which may have a proinflammatory effect and increase the levels of circulating vascular endothelial growth factor and chemokine CXCL-12, promoting transmission of peripheral blood mononuclear cells across the blood-brain barrier.36 This may explain the increased rate of MS relapses during fertility treatment. A role may also be played by sudden changes in oestrogen levels (as occurs during pregnancy and the postpartum period) and discontinuation of MS treatment during fertility treatment.
Risks of transmitting multiple sclerosis to offspringFamilial aggregation is an epidemiological feature of MS. Children with one parent with MS have been shown to have a 2% likelihood of developing MS at some point in their lives.37 The risk increases to 6%-12% when both parents have MS (conjugal MS),38,39 suggesting that the risk of developing MS is determined by genetic factors inherited from both parents.
Some studies have found an association between the risk of developing MS and the month of birth (with risk of MS being slightly greater for people born in spring, and lower for those born in autumn), especially in cases of familial MS.40 This suggests that MS pathogenesis involves an interaction between genetic and climate-related environmental factors. However, subsequent studies have shown that these results were biased by geographical and seasonal differences in birth rates.41
Several studies suggest an inverse correlation between vitamin D levels and the risk of MS.42 Furthermore, women whose mothers consumed large amounts of vitamin D during pregnancy have been observed to have a lower risk of MS.43 Although the safety of vitamin D supplements has not been evaluated, these data suggest that pregnant women with low vitamin D levels should receive supplementation43 to maintain normal serum levels of 1,25-dihydroxyvitamin D (50-125nmol/L).44
Effects of pregnancy on the progression of multiple sclerosisThe Pregnancy In Multiple Sclerosis (PRIMS) study was the first prospective study to analyse the short-term impact of pregnancy on MS.45 The study included 254 women with MS (269 pregnancies), who were followed up for one year after delivery. The annual relapse rate decreased from a mean of 0.7 relapses before pregnancy to 0.2 relapses during the third trimester, increasing to 1.2 relapses during the first 3 months postpartum, in which period nearly 30% of patients experienced relapses. After this period, the relapse rate stabilised, showing similar values to those observed pre-pregnancy. Subsequent studies report similar findings.46
The lower number of relapses during pregnancy may be due to hormonal changes (increased levels of oestrogens, progesterone, prolactin, etc.), which have an anti-inflammatory effect. Pregnancy does not seem to affect long-term disease progression.47–49
Patient follow-up during pregnancyMRI has not been shown to have a negative impact on pregnant women with MS, although insufficient evidence is available to confirm the safety of the technique; MRI may in fact entail a certain risk due to the thermal effects of radiofrequency.50 MRI studies should therefore not be conducted during the first trimester. In the event of an emergency, MRI scans may be performed at any time if the clinical benefits of the technique clearly outweigh the risks. The use of such intravenous contrast agents as gadolinium should be avoided during pregnancy.51
Lumbar puncture and electrophysiological tests have no specific risks for either the mother or the foetus, but should not be performed frequently as they can cause discomfort in pregnant women.
Treatment before and during pregnancyPharmacological treatments for MS should be avoided during pregnancy. This usually involves discontinuing symptomatic and disease-modifying treatment during pregnancy planning and pregnancy, unless the benefits of the drugs outweigh the potential risks.52,53
Management of relapses during pregnancy and the postpartum periodCorticosteroids are classified under pregnancy risk category C by the USA Food and Drug Administration (FDA). These drugs should be avoided during the first trimester: corticosteroid use during the first 3 months of pregnancy has been associated with such foetal malformations as cleft palate.54 High-dose methylprednisolone is relatively safe for the treatment of MS relapses during the second and third trimesters, but should be limited to the treatment of disabling relapses. Although methylprednisolone is metabolised in the placenta before reaching the foetus, it may cause mild leukocytosis and immunosuppression in neonates.
Disease-modifying treatmentMS treatment during pregnancy should be adapted to each patient's needs, considering such variables as age, disease progression, clinical and radiological stability, previous relapses, disability, the risks of discontinuing treatment, the risks of maintaining treatment, and the patient's preferences.
The available information on the effects of disease-modifying treatment on pregnancy comes mainly from personal experiences. Due to the scarcity of data on the safety of this treatment for pregnant women, effective contraception during treatment is highly recommended.
Patients should follow the indications of the summary of product characteristics of each drug; patients are discouraged from using most of these drugs during pregnancy. In exceptional cases where treatment is maintained during pregnancy, the drug should be prescribed off-label and patients should sign informed consent forms.
IFN-β is classified under FDA pregnancy risk category C. In experimental animals receiving higher doses than humans, the abortive effects of the drug have shown to increase in a dose-dependent manner during the first trimester. Small amounts of IFN-β are excreted into breast milk. Contraception should be used during treatment with the drug. Women with MS who plan to become pregnant should discontinue treatment with IFN-β.
The literature includes around 1000 cases of patients taking IFN-β during pregnancy. However, in most cases the drug was administered only during the first weeks of the first trimester, since treatment was discontinued after pregnancy was confirmed.55–60 Two studies analysing a small number of patients with MS who became pregnant while receiving IFN-β-1a60 and IFN-β-1b55 report a higher than expected frequency of miscarriages. However, subsequent studies with larger samples have not been able to confirm these results.57 These studies report a low incidence of foetal malformations (similar rates to those found in the general population); the incidence of malformations did not follow a coherent pattern. However, insufficient data are available to rule out an association between treatment with IFN-β and rare foetal abnormalities.56,57
Some of the series of pregnant women treated with IFN-β show a slight increase in the number of preterm births and low birth weight.61 These findings have not been confirmed by the results of other series. IFN-β should not be introduced during pregnancy; treatment discontinuation is common practice among women planning to become pregnant.62
Glatiramer acetate is classified under FDA pregnancy risk category B. Its safety profile is more favourable, as no alterations associated with the drug have been observed in animal models. The molecule cannot cross the placenta or be excreted into breast milk due to its high molecular weight. Studies of pregnant women receiving glatiramer acetate, including over 400 patients, have shown the safety of the drug and report normal rates of foetal malformations.55,58,63,64 However, given the low number of pregnant women treated with the drug, the risk of foetal malformations cannot be ruled out; glatiramer acetate should therefore be used with caution.
Little information is available on the use of other drugs, including natalizumab,65,66 fingolimod,67 dimethyl fumarate,68 and alemtuzumab,69 during pregnancy.
Natalizumab is classified in FDA pregnancy risk category C. The drug is associated with increased incidence of miscarriage in animals.70 Patients should discontinue natalizumab and allow a 3-month wash-out period before conception. The drug crosses the placenta during the second trimester and small amounts are excreted into breast milk. Natalizumab has occasionally been administered during the third trimester to patients with highly active MS, resulting in such haematological alterations as thrombocytopaenia and haemolytic anaemia in 8 of every 9 live births (Hellwig, personal communication).
Teriflunomide is classified in FDA pregnancy risk category X. The drug has been found to be teratogenic in animal models and is therefore contraindicated during pregnancy.71 It has a long plasma half-life, but colestyramine can be co-administered to accelerate elimination in women planning to conceive.52,71
Fingolimod is a pregnancy risk category C drug.72 Animal studies have shown that the drug crosses the placenta, has teratogenic effects, and is excreted into breast milk. Treatment discontinuation and a 2-month wash-out period are recommended.
Dimethyl fumarate is classified in pregnancy risk category C. It has been found to be toxic to the foetus and to cause testicular problems, low birth weight, and behavioural alterations in experimental animals.
Mitoxantrone, an FDA pregnancy risk category D drug, should be discontinued during pregnancy due to its potential teratogenicity.53 The drug may also cause infertility, premature ovarian failure, and amenorrhoea, especially in women older than 35.73
The disease should be stable before patients attempt to conceive. In patients with highly active relapsing-remitting MS, discontinuation of disease-modifying treatment prior to conception may be associated with an increased risk of relapses.74 Little is known about the effects of disease-modifying treatments for MS on male fertility and paternity.61 In a study by Hellwig et al.,75 MS treatment (mainly glatiramer acetate and IFN-β) was found to have no effect on patients’ children.
Teriflunomide can be detected at low concentrations in semen. Some regulatory agencies recommend that male patients receiving teriflunomide use contraception and follow an accelerated elimination procedure if they wish to conceive. According to the European Medicines Agency, the risk of embryo-foetal toxicity due to treatments administered to male patients is low.71
Symptomatic treatmentThe available information on symptomatic treatment for MS is insufficient to provide evidence-based guidance on the use of these drugs during pregnancy, except for antiepileptic drugs.76 Baclofen,77 oxybutynin,78 amantadine,79 and clonazepam,80 some of the symptomatic drugs frequently used in MS, are classified under pregnancy risk categories B, C, or D.81 Discontinuation of symptomatic treatment before conception is generally recommended. If treatment is not discontinued, patients should receive the minimum effective dose for the shortest possible time.
Delivery and postpartum periodDelivery and neonatal outcomesNo significant differences have been observed between women with MS and the general population in gestational age and birth weight, duration of the mother's hospital stay after delivery, frequency of assisted vaginal delivery, and frequency of caesarean delivery.82–85 The decision to use a specific technique during delivery remains with the obstetrician, although a higher rate of induced labours has been observed in women with a higher degree of disability.84 Anaesthesia is not contraindicated in patients with MS.86,87
Postpartum relapsesWhen MS is highly active, disease-modifying treatment should be resumed as early as possible to minimise occurrence of postpartum relapses. Methylprednisolone and IV immunoglobulins do not seem to be effective for preventing postpartum relapses, although further controlled studies with larger samples are necessary to confirm this hypothesis.88–90
BreastfeedingStudies into the progression of MS during the postpartum period suggest that breastfeeding has no impact on the relapse rate.43,91,92 Several recent studies have suggested that breastfeeding may have a protective effect against relapses.58,92 However, these findings result from a selection bias: the disease followed a more benign course in most of the study participants. Disease-modifying treatment is usually discontinued during breastfeeding since drugs may be excreted into breast milk. The decision to continue taking a disease-modifying drug immediately after delivery has to be weighed against the potential benefits of breastfeeding. For postpartum relapses requiring high-dose methylprednisolone, breastfeeding should be temporarily suspended until 12 to 48hours after administration of the last dose of methylprednisolone.53
Clinical trials of sex hormone therapyA clinical trial of oral oestriol including 10 women with relapsing-remitting MS showed improvements in gadolinium-enhancing lesions in MRI. These beneficial effects may be due to a hormone-induced decrease in Th1 responses and increase in Th2 response.93 A cross-over trial of transdermal testosterone administered to 10 men with relapsing-remitting MS found no changes in the frequency of gadolinium-enhancing lesions but did observe a decrease in cerebral atrophy.94 This may be due to increased production of neurotrophic factors by peripheral blood mononuclear cells.4 The results of these studies point to a neuroprotective and immunomodulatory effect of oestrogens in women and testosterone in men.
The effects of oral contraceptives on inflammation have also been evaluated with MRI.95 One study included 149 women treated with subcutaneous IFN-β-1a who were randomly assigned to receive either IFN-β-1a alone, IFN-β-1a plus 20μg ethinyloestradiol and 150μg desogestrel (low-dose oestrogen group), or IFN-β-1a plus 40μg ethinyloestradiol and 125μg desogestrel (high-dose oestrogen group). The group treated with high doses of oestrogens showed a decrease in the number of new lesions during a 2-year follow-up period. These results indicate that oral contraceptives containing high doses of oestrogens may boost the effects of IFN-β. Epidemiological data have shown that oestrogen-containing oral contraceptives do not increase the risk of MS; rather, they delay onset of the disease.96 Another study of treatment with progesterone and oestriol in MS during the postpartum period (POPARTMUS) analysed the efficacy of sex hormone therapy, administering 10mg nomegestrol acetate and a placebo for 12 weeks and finding no difference between groups in relapse rates during the postpartum period (Confavreux, personal communication).97
MenopauseThe effects of hormonal changes occurring during menopause may be mistaken for age-related changes in the activity of MS and associated conditions. Late-onset MS (starting after the age of 50) seems to progress less favourably regardless of sex, rapidly progressing to secondary progressive MS.98 This underscores the complexity of MS pathogenesis, which involves the interaction between such factors as age, sex, genetics, and environmental factors.
Counselling for patients with multiple sclerosis who plan to conceiveBoth parents should receive counselling before, during, and after pregnancy. Helping families to evaluate their physical, economic, and emotional capacity to be parents and to understand the short- and long-term impact of pregnancy on women with MS and the risk of their children developing MS may be useful in ensuring that patients have realistic expectations (Table 1). Patients with MS who plan to conceive should be informed about the effects of the disease on fertility and conception, the effects of pregnancy on disease progression, the implications for symptomatic and disease-modifying treatment, and other issues related to pregnancy, delivery, and the postpartum period (Table 2). Physicians should also inform patients of the contents of the summary of product characteristics of each drug, enabling them to weigh the benefits and risks of discontinuing pharmacological treatment during pregnancy.
Factors to be considered when counselling MS patients/families at different stages of the reproductive cycle.
| Counselling should be provided in a language that the patient can easily understand, and should be evidence-based and adapted to each patient's clinical, psychological, and socioeconomic situation. Patients with MS may have several doubts and concerns during the reproductive cycle and family planning. |
| Before conception: Female and male sexual function, the effect of MS on fertility and the risks of fertility treatment, the use/effects of contraceptives, the potential risk of the offspring developing MS, the potential effects of previous and current treatments on pregnancy and the foetus, the impact of the month of birth on MS progression and on the offspring, and the short- and long-term effects of pregnancy and MS. |
| During pregnancy: The management of MS during pregnancy (use and effects of symptomatic and disease-modifying treatment and treatment for relapses), the use of MRI to evaluate the course of the disease, and the short- and long-term effects of pregnancy on MS. |
| After delivery: Prognosis of relapses and management of MS during the postpartum period, when to resume symptomatic or disease-modifying treatment, breastfeeding, parenting with a disability, and long-term planning. |
MRI: magnetic resonance imaging; MS: multiple sclerosis.
Recommendations for managing MS during pregnancy.
| 1. Fertility treatment to increase the patient's chances of conceiving |
| Although one in every 5 fertility procedures in couples with MS is successful, mothers may experience relapses during fertility treatment. Further data are needed to understand and quantify this phenomenon; however, the available evidence suggests that the risk of relapse increases with use of fertility treatments. Couples considering fertility treatment should be informed of this. |
| 2. Use of MRI to monitor the disease during pregnancy |
| (a) Despite the lack of data supporting this hypothesis, monitoring MS during pregnancy with MRI is not essential; clinical assessment may be sufficient if increased disease activity is suspected. |
| (b) Gadolinium-enhanced MRI should be avoided during pregnancy as the effects of the contrast agent on pregnancy and breastfeeding have not been thoroughly evaluated. |
| (c) As a general rule, CT and X-ray imaging are not recommended during pregnancy due to the risk of exposing the foetus to radiation. |
| 3. Effects of previous use of disease-modifying treatment on foetal development |
| Animal studies suggest that teriflunomide is potentially teratogenic; a wash-out period is recommended before conceiving. |
| Little evidence is available on the impact of other disease-modifying drugs on fertility or the foetus, although a potential risk cannot be ruled out. Disease-modifying treatment is usually discontinued before conception, unless the benefits of treatment continuation outweigh the potential risks. |
| 4. Effects of pregnancy on short- and long-term disease progression |
| Multiple studies have observed a short-term decrease in the inflammatory activity of MS (reduction in the relapse rate) during the second and third trimesters of pregnancy, as well as an increase in the risk of relapses during the first trimester. Couples with MS should be informed of the implications of this before conception, during pregnancy, and after delivery. Pregnancy appears not to affect long-term disease progression. |
| 5. Effects of disease-modifying treatment on pregnancy |
| (a) IV methylprednisolone for treating acute relapses is associated with potential risks to the foetus and adverse reactions in the mother. The drug should be avoided, especially during the third trimester, and its use should be limited until delivery. |
| (b) Although IV immunoglobulins are probably safe during pregnancy, they cannot be recommended since evidence about their efficacy is lacking. |
| (c) The effects of IFN-β and glatiramer acetate on pregnancy have been extensively documented. Neither drug seems to increase the risk of miscarriage, although some animal studies of IFN-β do report miscarriages. The available evidence on subcutaneous IFN does not point to an increase in the frequency of foetal malformations. Data on intramuscular IFN and glatiramer acetate are limited. In clinical practice, these drugs are usually discontinued before and during pregnancy, unless the benefits of treatment outweigh the potential (yet unknown) risks to the foetus (high risk of increased inflammatory activity). |
| (d) Evidence about the effects of the recently approved disease-modifying drugs is limited. However: |
| (i) Natalizumab has been shown to increase the frequency of miscarriages in animal studies. The summary of product characteristics recommends discontinuation during pregnancy. The risks and benefits of treatment continuation during pregnancy are controversial, considering that patients who had highly active MS before treatment may experience relapses in MS activity after treatment discontinuation (during pregnancy). |
| (ii) Fingolimod should be discontinued 2 months before conception. |
| (iii) Teriflunomide should be discontinued and eliminated from the body before conception; in the event of unintended pregnancy during treatment, the drug should be eliminated from the body (combination treatment with colestyramine is recommended to accelerate elimination). |
| (iv) Evidence of the effects of dimethyl fumarate on pregnancy is scarce; the drug should be avoided until further information is available. |
| 6. Symptomatic treatment during pregnancy |
| (a) There is very little evidence on the effects of symptomatic treatment on pregnant women with MS. Nearly all symptomatic treatments for MS are classified into FDA pregnancy risk categories B, C, or D. Until further evidence is available, a conservative approach would involve: |
| (i) Discontinuing symptomatic treatment before conception; patients must understand and accept the effects of discontinuation on activities of daily living. |
| (ii) Using the minimum effective dose for the shortest time possible, if treatment is not discontinued. |
| (iii) Avoiding drugs for which no data on the risks for pregnancy are available. |
| (b) Pregnant women with MS should take folic acid supplements to reduce the likelihood of neural tube defects in the foetus. Vitamin D supplementation should be considered in women with low vitamin D levels. |
| 7. Caesarean delivery and epidural anaesthesia |
| (a) There is no evidence that caesarean delivery or epidural anaesthesia have a negative impact on patients with MS: both techniques may be used if necessary. The decision to choose vaginal or caesarean delivery remains with the obstetrician. |
| 8. Effects of breastfeeding on newborns and on mothers with MS |
| (a) Breastfeeding is recommended to women whose disease has low inflammatory activity and who can therefore postpone the reintroduction of the disease-modifying drug. Breastfeeding should be discontinued if the treatment has to be resumed after delivery. There is no evidence that breastfeeding is harmful to women with MS. Little is known about the administration of symptomatic or disease-modifying treatment to breastfeeding women. |
CT: computed tomography; FDA: USA Food and Drug Administration; IFN-β: interferon beta; IV: intravenous; MRI: magnetic resonance imaging; MS: multiple sclerosis.
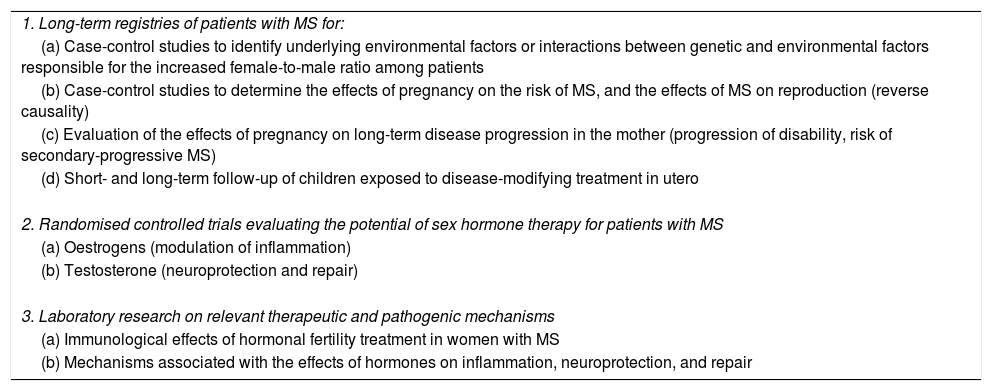
The recent epidemiological changes in MS incidence require further research; new clinical data may result in novel strategies for MS prevention and treatment (Table 3).
Recommendations for future research into the topic.
| 1. Long-term registries of patients with MS for: |
| (a) Case-control studies to identify underlying environmental factors or interactions between genetic and environmental factors responsible for the increased female-to-male ratio among patients |
| (b) Case-control studies to determine the effects of pregnancy on the risk of MS, and the effects of MS on reproduction (reverse causality) |
| (c) Evaluation of the effects of pregnancy on long-term disease progression in the mother (progression of disability, risk of secondary-progressive MS) |
| (d) Short- and long-term follow-up of children exposed to disease-modifying treatment in utero |
| 2. Randomised controlled trials evaluating the potential of sex hormone therapy for patients with MS |
| (a) Oestrogens (modulation of inflammation) |
| (b) Testosterone (neuroprotection and repair) |
| 3. Laboratory research on relevant therapeutic and pathogenic mechanisms |
| (a) Immunological effects of hormonal fertility treatment in women with MS |
| (b) Mechanisms associated with the effects of hormones on inflammation, neuroprotection, and repair |
Multiple studies have evaluated the effects of pregnancy on MS. However, results are not always coherent and are occasionally contradictory. This may reflect methodological problems, such as small samples, heterogeneous populations, and confounding factors (comorbidities, co-treatments, family history, and age of the mother). Future research should address the effects of pregnancy and MS treatment during pregnancy on the course of the disease, using patient registries obtained from independent national databases, and analyse the long-term impact on mothers and their children. One issue with MS registries is the difficulty of combining datasets to create large international cohorts with shared demographic and clinical information.
Further research is necessary to determine the mechanisms of action and the potential positive and negative effects of different pharmacological treatments for the disease.
Conflicts of interestThe authors have received no funding from any pharmaceutical company and have no conflicts of interest to declare.
Please cite this article as: Mendibe Bilbao M, Boyero Durán S, Bárcena Llona J, Rodriguez-Antigüedad A. Esclerosis múltiple, maternidad y cuestiones relacionadas con el género. Neurología. 2019;34:259–269.