Nutritional deficiencies are frequent in Alzheimer disease (AD), even in early stages. Nutritional impairment (NI) may be associated with faster disease progression. The objective of this study was to describe the frequency of NI and the associated risk factors at the time of diagnosis and to analyse its influence on subsequent progression.
MethodsWe performed a prospective, multicentre, observational study of patients recently diagnosed with prodromal AD (pAD) or dementia due to AD (ADd). Two clinical assessments were conducted over a period of 18 months. The Mini Nutritional Assessment test (MNA; score range, 0-30; cut-off point for NI, < 24) was used to estimate nutritional status. Progression was defined as an increase of ≥ 3 points on the Clinical Dementia Rating-sum of boxes test.
ResultsThe sample included 50 patients with pAD (mean [standard deviation] age, 76.1 [5.3] years; 68% women), and 127 with ADd (80 [5.9] years; 72.4% women). A total of 141 (79.7%) completed both evaluations. The prevalence of NI was 28.2% (24% for pAD, 29.9% for ADd; P = .43), with the majority (92%) at risk of malnutrition. NI was associated with female sex (odds ratio [OR]: 4.2; 95% confidence interval [CI]: 1.7-10.5; P < .001) and greater behavioural involvement (OR: 5.8; 95% CI: 2.6-12.7; P < .001). A larger proportion of patients with progression was observed among those with NI than among those with normal nutritional status (50% vs 28.7%, P < .05; ADd: 53.6% vs 31.8%, P < .05; pAD: 41.7% vs 22.9%, P = .21). Greater cognitive impairment (OR: 2.1; 95% CI: 1.03-4.4; P < .05) and NI (OR: 2.4; 95% CI: 1.1-5.1; P < .05) were independent risk factors for disease progression.
ConclusionsNI is highly prevalent in patients with AD. Assessing nutritional status at the time of diagnosis may enable identification of patients at greater risk of disease progression.
Las deficiencias nutricionales son frecuentes en la enfermedad de Alzheimer (EA), incluso en fases iniciales. El deterioro nutricional (DN) puede asociarse con una progresión más rápida de la enfermedad. El objetivo fue describir la frecuencia y los factores de riesgo asociados a DN en el momento del diagnóstico y analizar su influencia en la evolución posterior.
MétodosEstudio observacional, multicéntrico, prospectivo. Se incluyeron sujetos recién diagnosticados de EA prodrómica (EAp) o demencia por EA (EAd). Se realizaron dos evaluaciones en un periodo de 18 meses. Para estimar el estado nutricional se empleó el Mini Nutritional Assessment Test (MNA, rango 0-30; DN: MNA < 24). El criterio de progresión fue un incremento en la Clinical Dementia Rating-sum of boxes ≥ 3.
ResultadosSe incluyeron 50 sujetos con EAp (edad 76,1 ± 5,3 años; 68% mujeres) y 127 con EAd (edad 80 ± 5,9 años; 72,4% mujeres); 141 (79,7%) completaron las dos evaluaciones. La prevalencia de DN fue del 28,2% (EAp 24%, EAd 29,9%; p = 0,43), la mayoría (92%) en riesgo de desnutrición. El DN se asoció con el sexo femenino (OR: 4,2; IC 95%: 1,7-10,5; p < 0,001) y mayor afectación conductual (OR: 5,8; IC 95%: 2,6-12,7; p < 0,001). Se observó mayor proporción de sujetos con progresión entre los que tenían un DN respecto a estado nutricional normal (50% vs 28,7%, p < 0,05; EAd 53,6% vs 31,8%, p < 0,05; EAp 41,7% vs 22,9%; p = 0,21). Una mayor afectación cognitiva (OR: 2,1; IC 95%: 1,03-4,4; p < 0,05) y un DN (OR: 2,4; IC 95%: 1,1-5,1; p < 0,05) fueron factores de riesgo independientes de progresión.
ConclusionesLa prevalencia de DN en la EA es elevada. La evaluación del estado nutricional en el momento del diagnóstico puede permitir identificar pacientes con mayor riesgo de progresión de la enfermedad.
Due to the lack of an effective treatment for Alzheimer disease (AD), it is essential to conduct research into modifiable factors affecting disease progression. Progressive weight loss and the appearance of clinical signs of malnutrition are frequent manifestations of the disease. Numerous studies published in recent years have demonstrated the relationship between weight loss, nutritional status, and AD1–5 or dementia.6 Furthermore, malnutrition has been linked to faster disease progression7,8 and increased severity and mortality rates.9 Some studies evaluating the influence of different dietary patterns on mild cognitive impairment and dementia underscore the importance of nutrition in disease progression.10
Among institutionalised elderly individuals, weight loss is more frequent in patients with dementia than in their cognitively healthy peers; this effect is particularly marked in women, and is independent of nutritional status.11 Weight loss may be detected in up to 40% of patients with AD, at any stage of the disease, even several years before diagnosis.3,6,12,13 The prevalence of malnutrition and risk of malnutrition is higher among patients with AD or dementia in general, with rates ranging from 14% to 80%, depending on the methodology used.2,5,14,15 An “obesity paradox” has been described in this context: on the one hand, overweight during middle age constitutes a risk factor for dementia, whereas on the other, higher body mass index (BMI) at older age seems to be a protective factor.16,17
The mechanism of interaction between nutritional status and AD is unknown. Weight loss may be provoked by reduced caloric intake. However, some studies have found higher caloric intake among patients with AD than in healthy controls.4 Some biological phenomena, such as subcortical lesions to the hypothalamus18 and medial temporal lobe,19 may affect appetite control centres. The apraxia and agnosia inherent to AD may affect feeding patterns or the preparation and intake of foods in patients who do not receive adequate care. Additionally, behaviour disorders or agitation in some patients may, in turn, increase energy expenditure.20,21
The DEMDIAG prospective study (named for its Spanish abbreviation: “Evaluation of Alzheimer-type dementia at the time of diagnosis”) was designed to assess different clinical, sociodemographic, and nutritional characteristics of AD at the time of diagnosis and to analyse their influence on disease progression, identifying factors associated with good and poor prognosis. This study presents the results of our analysis of dietary variables addressed in the study, reporting the frequency of impaired nutritional status, as well as factors predisposing to this condition and its influence on disease progression.
Patients and methodsStudy designWe conducted an observational, prospective, multicentre, analytical, closed-cohort epidemiological study at the outpatient neurology clinics of Hospital Universitario Río Hortega de Valladolid and Complejo Hospitalario de Segovia. Each patient was evaluated twice. For the baseline evaluation, patients were prospectively recruited over a period of 16 months. The second assessment was performed 18 ± 1 months after the baseline evaluation. Patients underwent no intervention outside of everyday practice.
Inclusion criteriaInclusion criteria were as follows:
- a)
Any age and sex.
- b)
Living in the community.
- c)
Patients consulting specialists for the first time due to symptoms of cognitive impairment of any degree of severity and presenting a cortical profile.
- d)
Subsequent diagnosis of AD dementia, according to the NINCDS-ADRDA criteria for the diagnosis of probable AD,22 or prodromal AD, according to the IWG-1 criteria.23
- e)
Availability of a reliable informant or caregiver.
- f)
Written informed consent to participate in the study, either from the patient or from their legal guardian, if the patient did not have legal competence.
Exclusion criteria were as follows:
- a)
Previous diagnosis of any type of dementia.
- b)
Results of complementary tests, neuropsychological assessment, or follow-up confirming the absence of dementia, or presence of a different disease.
Patients underwent 2 neurological and neuropsychological assessments (at baseline and at 18 months). The neurologists responsible for the study (MFRS and MATA), specialists in the assessment and treatment of dementia, followed up the patients during the period between the 2 assessments.
The baseline assessment included a structured interview aimed at gathering different sociodemographic variables and data on education and years of schooling. We identified the main initial symptom and recorded medical history as well as specific information on vascular risk factors, toxic substance use, and physical exercise. The differential diagnosis of secondary dementias included a complete blood analysis with ions, renal function, thyroid hormones, and vitamin B12. APOE genotype was determined. All patients underwent neuroimaging studies, in most cases brain MRI with T1-weighted coronal sequences, and hippocampal atrophy was estimated with the visual scale proposed by Scheltens et al.24 Medial temporal lobe atrophy scoring ≥ 2 on the scale was considered significant for diagnosis of prodromal AD. When MRI was not possible, patients underwent head CT scans.
Measurement instrumentsNutritional status was assessed with the Spanish-language version of the Mini Nutritional Assessment (MNA), a tool for identifying elderly individuals with or at risk of malnutrition.25 It comprises 6 screening questions and 12 evaluation questions. The MNA includes:
- 1
Anthropometric measurements: BMI, weight loss, mid–upper arm circumference, and calf circumference.
- 2
Global evaluation: lifestyle, everyday medications, and mobility.
- 3
Diet questionnaire: number of meals per day, fluid and solid food intake, and independence when eating.
- 4
Subjective health and nutritional status
The instrument typically takes 10 minutes to administer. Scores range from 0 to 30, and are classified as follows: 24-30, normal nutritional status; 17-23.5, at risk of malnutrition; < 17, malnourished. This classification has been validated against a comprehensive nutritional assessment, showing sensitivity of 96%, specificity of 98%, and positive predictive power of 97%.25 For the purposes of this study, impaired nutritional status was defined as MNA score < 24. As well as administering the MNA, we also measured biochemical markers of nutritional status: total cholesterol, albumin, prealbumin, and transferrin.
To assess cognitive status, we used the Spanish-language version of the Cambridge Cognitive Examination-Revised (CAMCOG-R; range, 0-105),26 which includes Folstein’s Mini–Mental State Examination (MMSE). Significant episodic amnesia for the IWG-1 criteria was defined as a memory subscale score (range, 0-27) more than 2 standard deviations below the mean established in normative studies of individuals without dementia. We used the Spanish-language version of the Rapid Disability Rating Scale-2 (RDRS-2; range, 18-72)27 for the functional evaluation. To evaluate behaviour, we used the Spanish-language version of the Neuropsychiatric Inventory Questionnaire (NPI-Q), which measures the presence and severity of 12 behavioural disorders, as well as the distress caused to caregivers.28 The NPI-Q severity score ranges from 0 to 36 and the NPI-Q caregiver distress score ranges from 0 to 60. Caregiver burden was evaluated using the Zarit Burden Interview (range, 22-110).29 We used the Clinical Dementia Rating scale (CDR) as an overall measurement of dementia severity.30 Additionally, we calculated the CDR sum of boxes (CDR-SOB) as the sum of the individual scores for each of the 6 domains evaluated in the scale.31 AD progression between the 2 evaluations was defined as an increase of ≥ 3 points on the CDR-SOB.32
Statistical analysisClinical and sociodemographic characteristics are expressed as absolute and relative frequencies for qualitative variables and as measures of central tendency and dispersion for quantitative variables. We tested for a normal distribution using the Kolmogorov-Smirnov test, to determine whether a parametric or a non-parametric test was appropriate. We used the chi-square test to compare proportions. For hypothesis testing, we used the t test for normal variables (independent or paired data). For ordinal and non-normal quantitative variables, we used the Mann-Whitney U test or Kruskal-Wallis H test for independent variables, and the Wilcoxon signed rank test for paired data. For the bivariate correlation analysis we calculated the Spearman correlation coefficient (ρ).
To analyse predictor variables of nutritional status, we categorised baseline MNA scores as < 24 or ≥ 24. In the analysis of predictor variables of disease progression, we classified the increase in CDR-SOB scores at 18 months as < 3 or ≥ 3. In both cases, a forward stepwise multivariate logistic regression analysis was conducted. We classified all the quantitative variables included in the model according to their median value in the baseline evaluation. We calculated crude (bivariate analysis) and adjusted (multivariate analysis) odds ratios with 95% confidence intervals. Statistical analysis was conducted using the SPSS software, v. 22.0 (IBM Corp; Armonk, NY, USA). The significance level was established at P < .05.
Ethical considerationsThe study was approved by the Clinical Research Ethics Committees at Hospital Universitario Río Hortega de Valladolid and Complejo Hospitalario de Segovia. Informed consent forms were signed by all patients or their legal representatives or next of kin if patients were not legally competent.
ResultsDuring the recruitment period, we selected 204 individuals who consulted due to cognitive impairment presenting a cortical profile. We excluded 27 whose diagnosis of AD was not confirmed during follow-up. Therefore, the initial population who completed the baseline assessment included 177 patients: 50 with prodromal AD and 127 with AD dementia. A total of 141 participants (79.7% of the baseline population) completed the second evaluation: 47 with prodromal AD and 94 with AD dementia. Twenty-four patients were lost to follow-up, 9 died, and 3 were excluded due to severe disease. Compared to patients who completed both evaluations, the patients lost to follow-up were older (81.5 vs 78.2 years; P < .01), included a higher percentage of women (86% vs 67%; P < .05), and achieved poorer scores on the MMSE (18.3 vs 21.3; P < .01), CAMCOG-R (54.3 vs 64.7; P < .01), and CDR-SOB (6 vs 5; P < .01). No differences were observed in MNA score, BMI, or biochemistry variables.
Table 1 shows the main sociodemographic characteristics, lifestyle habits, and medical history of the population assessed at the baseline visit. We should emphasise the higher proportion of individuals living in urban settings or living alone and the higher level of schooling in the prodromal AD group than in the group of patients with AD dementia. Table 2 shows the clinical, biochemical, and genetic characteristics of the baseline population, and their results on the assessment scales. A total of 48.8% of patients carried the APOE ε4 allele. According to MNA scores, 4 patients were malnourished (2.3%; 1 with prodromal AD and 3 with AD dementia) and 46 were at risk of malnutrition (26%; 11 with prodromal AD and 35 with AD dementia).
Sociodemographic, lifestyle, and clinical characteristics at baseline.
| Complete sample (n = 177) | pAD (n = 50) | ADd (n = 127) | P (pAD vs ADd) | |
|---|---|---|---|---|
| Age, years (mean ± SD; range) | 78.9 ± 6.0; 55.5-91.0 | 76.1 ± 5.3; 58.6-84.6 | 80.0 ± 5.9; 55.5-91.0 | < .001a |
| Sex (n [%]) | ||||
| Men | 51 (28.8%) | 16 (32%) | 35 (27.6%) | .56b |
| Women | 126 (71.2%) | 34 (68%) | 92 (72.4%) | |
| Place of residence (n [%]) | ||||
| Urban | 123 (69.5%) | 44 (88%) | 79 (62.2%) | < .01b |
| Rural | 54 (30.5%) | 6 (12%) | 48 (37.8%) | |
| Lives alone | 38 (21.5%) | 13 (26%) | 25 (19.7%) | .36b |
| Marital status (n [%]) | ||||
| Married | 96 (54.2%) | 29 (58%) | 67 (52.8%) | |
| Widowed | 71 (40.1%) | 19 (38%) | 52 (40.9%) | .85b |
| Single or separated | 10 (5.7%) | 2 (4%) | 8 (6.3%) | |
| Years of schooling (median [Q1-Q3]) | 8 (6-8) | 8 (5-11) | 8 (6-8) | < .05c |
| Level of schooling (n [%]) | ||||
| Illiterate or primary study not completed | 95 (53.7%) | 21 (42%) | 74 (58.3%) | < .01b |
| Primary study | 54 (30.5%) | 13 (26%) | 41 (32.3%) | |
| Secondary or further education | 28 (15.8%) | 16 (32%) | 12 (9.4%) | |
| Current activity (n [%]) | ||||
| Retired and inactive | 88 (49.7%) | 25 (50%) | 63 (49.6%) | .91b |
| Homemaker | 84 (47.5%) | 23 (46%) | 61 (48%) | |
| Active employment | 5 (2.8%) | 2 (4%) | 3 (2.4%) | |
| Medical history and lifestyle habits (n [%]) | ||||
| Arterial hypertension | 92 (52%) | 26 (52%) | 66 (52%) | .99b |
| Diabetes mellitus | 35 (19.8%) | 8 (16%) | 27 (21.3%) | .43b |
| Stroke | 15 (8.5%) | 3 (6%) | 12 (9.4%) | .46b |
| Family history of dementiad | 66 (37.3%) | 22 (44%) | 44 (34.6%) | .25b |
| Alcohol consumptione | 39 (22%) | 18 (36%) | 21 (16.5%) | < .01b |
| Current or previous smoking | 40 (22.6%) | 16 (32%) | 24 (18.9%) | .06b |
| Sedentary lifestyle | 32 (18.1%) | 6 (12%) | 26 (20.5%) | < .01b |
ADd: dementia due to Alzheimer disease; pAD: prodromal Alzheimer disease; Q1: first quartile; Q3: third quartile; SD: standard deviation.
Clinical, biochemical, genetic, and nutritional characteristics at baseline.
| Complete sample (n = 177) | pAD (n = 50) | ADd (n = 127) | P (pAD vs ADd) | |
|---|---|---|---|---|
| Main symptom at onset (n [%]) | ||||
| Memory loss | 151 (85.3%) | 46 (92%) | 105 (82.7%) | .11a |
| Other | 26 (14.7%) | 4 (8%) | 22 (17.3%) | |
| Clinical scales | ||||
| MMSE (mean ± SD) | 20.7 ± 4.7 | 24.9 ± 2.7 | 19 ± 4.2 | < .001b |
| CAMCOG-R (mean ± SD) | 62.6 ± 15.4 | 78.8 ± 6.2 | 56.2 ± 13.1 | < .001b |
| CAMCOG-R memory (mean ± SD) | 10.2 ± 4.8 | 14.6 ± 3.7 | 8.5 ± 4.1 | < .001b |
| RDRS-2 (median [Q1-Q3]) | 25 (21-31) | 22 (20-25) | 28 (22-35) | < .001c |
| NPI-Q severity (median [Q1-Q3]) | 6 (3-10) | 4 (2-6.25) | 7 (3-11) | < .01c |
| NPI-Q caregiver distress (median [Q1-Q3]) | 7 (3-13) | 4 (1-8.25) | 8 (4-14) | < .001c |
| Zarit Burden Interview (mean ± SD) | 27.4 ± 17.2 | 18.2 ± 13.3 | 31 ± 17.3 | < .001b |
| CDR, n (%) | ||||
| CDR 0.5 (n [%]) | 50 (28.2%) | 50 (100%) | – | – |
| CDR 1 (n [%]) | 97 (54.8%) | – | 97 (76.4%) | – |
| CDR 2 (n [%]) | 26 (14.7%) | – | 26 (20.5%) | – |
| CDR 3 (n [%]) | 4 (2.3%) | – | 4 (3.1%) | – |
| CDR-SOB (median [Q1-Q3]) | 5 (4-8) | 3 (2.5-3.625) | 6 (5-9) | < .001c |
| Nutritional evaluation | ||||
| MNA score (median [Q1-Q3]) | 25.5 (23.5-27) | 26 (23.875-27.625) | 25.5 (23-26.5) | .11c |
| INS (n [%]) | 50 (28.2%) | 12 (24%) | 38 (29.9%) | .43a |
| BMI (mean ± SD) | 26.1 ± 4.1 | 27.7 ± 4.1 | 25.4 ± 3.9 | < .01b |
| Albumin (mean ± SD; sample evaluated) | 4.1 ± 0.3; 169 | 4.1 ± 0.3; 49 | 4.2 ± 0.4; 120 | .09b |
| Prealbumin (mean ± SD; sample evaluated) | 24 ± 5.7; 153 | 24.2 ± 5.8; 43 | 23.9 ± 5.7; 110 | .80b |
| Transferrin (mean ± SD; sample evaluated) | 255.2 ± 40.7; 155 | 258 ± 42.4; 45 | 254 ± 40.2; 110 | .58b |
| Total cholesterol (mean ± SD; sample evaluated) | 215.3 ± 40.5; 177 | 220.5 ± 40.6; 50 | 213.3 ± 40.4; 127 | .30b |
| Positive APOE ε4 genotype (n [%]; sample evaluated) | 79 (48.8%); 162 | 24 (51.1%); 47 | 55 (47.8%); 115 | .71a |
CAMCOG-R: cognitive section of the Cambridge Cognitive Examination-Revised; CDR: Clinical Dementia Rating scale; CDR-SOB: CDR-sum of boxes; INS: impaired nutritional status (MNA < 24); ADd: dementia due to Alzheimer disease; pAD: prodromal Alzheimer disease; BMI: body mass index; MMSE: Mini–Mental State Examination; MNA: Mini Nutritional Assessment; NPI-Q: Neuropsychiatric Inventory Questionnaire; Q1: first quartile; Q3: third quartile; RDRS-2: Rapid Disability Rating Scale-2.
Table 3 shows patients’ sociodemographic and clinical characteristics as a function of nutritional status (normal or impaired) in the baseline evaluation. Female sex, more severe functional impairment, and higher CDR-SOB scores were more prevalent in the impaired nutritional status group. No differences were observed in BMI or biochemical parameters. Table 4 shows the changes in clinical scales and nutritional parameters between the 2 evaluations. Most patients (n = 118; 83.7%) who were evaluated twice showed no changes in nutritional status over the study period. However, 23 patients did present changes in nutritional status: 11 moved from the normal nutrition to the risk of malnutrition group, and 12 moved from the risk of malnutrition to the normal nutrition group. BMI remained stable.
Clinical and sociodemographic characteristics as a function of nutritional status (normal or impaired) in the baseline evaluation.
| Normal nutritional status (MNA > 23.5) (n = 127) | Impaired nutritional status (MNA < 24) (n = 50) | P | |
|---|---|---|---|
| pAD (n [%]) | 38 (29.9%) | 12 (24%) | .43a |
| ADd (n [%]) | 89 (70.1%) | 38 (76%) | |
| Sociodemographic characteristics | |||
| Age at baseline evaluation, years (mean ± SD) | 78.7 ± 5.9 | 79.3 ± 3.2 | .60b |
| Women (n [%]) | 83 (65.4%) | 43 (86%) | < .01a |
| Living alone (n [%]) | 27 (21.3%) | 11 (22%) | .91a |
| Rural setting (n [%]) | 41 (32.3%) | 13 (26%) | .41a |
| No studies, n (%) | 71 (55.9%) | 24 (48%) | .34a |
| Clinical assessment | |||
| MMSE (mean ± SD) | 21 ± 4.6 | 19.7 ± 4.7 | .07b |
| CAMCOG-R (mean ± SD) | 62.9 ± 15.2 | 61.6 ± 16.2 | .61b |
| CAMCOG-R memory (mean ± SD) | 10.1 ± 4.7 | 10.4 ± 5.1 | .78b |
| RDRS-2 (median [Q1-Q3]) | 23 (21-28) | 30 (24.75-38) | < .001c |
| NPI-Q severity (median [Q1-Q3]) | 5 (2-8) | 9.5 (6-15.25) | < .001c |
| NPI-Q caregiver distress (median [Q1-Q3]) | 5 (2-10) | 12 (6-19.25) | < .001c |
| Zarit Burden Interview (mean ± SD) | 25.8 ± 16.6 | 31.3 ± 18.4 | .06b |
| CDR | |||
| CDR 0.5 (n [%]) | 38 (29.9%) | 12 (24%) | .62a |
| CDR 1 (n [%]) | 73 (57.5%) | 24 (48%) | .25a |
| CDR 2 (n [%]) | 14 (11%) | 12 (24%) | < .05a |
| CDR 3 (n [%]) | 2 (1.6%) | 2 (4%) | .36a |
| CDR-SOB (median [Q1-Q3]) | 5 (4-7) | 6.75 (4.5-10) | < .05c |
| Nutritional parameters | |||
| MNA score (median [Q1-Q3]) | 26.5 (25-26.5) | 21.5 (19.875-23) | – |
| BMI (mean ± SD) | 26.2 ± 3.7 | 25.7 ± 4.8 | .52b |
| Albumin (mean ± SD; sample evaluated) | 4.1 ± 0.4; 122 | 4.2 ± 0.3; 47 | .17b |
| Prealbumin (mean ± SD; sample evaluated) | 24.1 ± 5.1; 112 | 23.6 ± 6.3; 41 | .58b |
| Transferrin (mean ± SD; sample evaluated) | 256.1 ± 42.7; 113 | 252.6 ± 35.3; 42 | .63b |
| Total cholesterol (mean ± SD; sample evaluated) | 214.4 ± 39.9; 127 | 217.8 ± 42.1; 50 | .61b |
| Positive APOE ε4 genotype (n [%]; sample evaluated) | 59 (50.9%); 116 | 20 (43.5%); 46 | .40a |
CAMCOG-R: Cambridge Cognitive Examination-Revised; CDR: Clinical Dementia Rating scale; CDR-SOB: CDR-sum of boxes; ADd: dementia due to Alzheimer disease; pAD: prodromal Alzheimer disease; BMI: body mass index; MMSE: Mini–Mental State Examination; MNA: Mini Nutritional Assessment; NPI-Q: Neuropsychiatric Inventory Questionnaire; Q1: first quartile; Q3: third quartile; RDRS-2: Rapid Disability Rating Scale-2.
Progression of clinical scale scores and nutritional parameters between evaluations.
| Baseline visit (n = 141) | Follow-up visit (n = 141) | P | |
|---|---|---|---|
| Age in years (mean ± SD) | 78.2 ± 6.2 | 79.7 ± 6.2 | – |
| Clinical scales | |||
| MMSE (mean ± SD) | 21.3 ± 4.4 | 19.0 ± 5.7 | < .001a |
| CAMCOG-R (mean ± SD) | 64.7 ± 14.7 | 57.3 ± 18.9 | < .001a |
| CAMCOG-R memory (mean ± SD) | 10.8 ± 4.8 | 9.3 ± 5.4 | < .001a |
| RDRS-2 (median [Q1-Q3]) | 24 (21-30) | 29 (23.5-36) | < .001b |
| NPI-Q severity (median [Q1-Q3]) | 5 (3-10) | 7 (3-11) | < .001b |
| NPI-Q caregiver distress (median [Q1-Q3]) | 7 (3-12) | 8 (4-13) | < .001b |
| Zarit Burden Interview (mean ± SD) | 26.1 ± 16.5 | 32.7 ± 18.1 | < .001a |
| CDR (median [Q1-Q3]) | 1 (0.5-1) | 1 (1-2) | < .001b |
| CDR-SOB (median [Q1-Q3]) | 5 (3.5-7) | 7 (5-10.5) | < .001b |
| Nutritional parameters | |||
| MNA (median [Q1-Q3]) | 25.5 (23.5-27) | 25.5 (23.5-27) | .08b |
| BMI (mean ± SD) | 26.2 ± 4.1 | 26.1 ± 4.7 | .62a |
| Albumin (mean ± SD; sample evaluated) | 4.2 ± 3.6; 75 | 4.0 ± 0.4; 75 | < .001a |
| Prealbumin (mean ± SD; sample evaluated) | 24.4 ± 5.5; 63 | 22.9 ± 5.3; 63 | < .001a |
| Transferrin (mean ± SD; sample evaluated) | 257.5 ± 38.8; 66 | 252.3 ± 40.3; 66 | .26a |
| Total cholesterol (mean ± SD; sample evaluated) | 215.4 ± 38.4; 110 | 209.3 ± 40.2; 110 | .12a |
CAMCOG-R: Cambridge Cognitive Examination-Revised; CDR: Clinical Dementia Rating scale; CDR-SOB: CDR-sum of boxes; BMI: body mass index; MMSE: Mini–Mental State Examination; MNA: Mini Nutritional Assessment; NPI-Q: Neuropsychiatric Inventory Questionnaire; Q1: first quartile; Q3: third quartile; SD: standard deviation; RDRS-2: Rapid Disability Rating Scale-2.
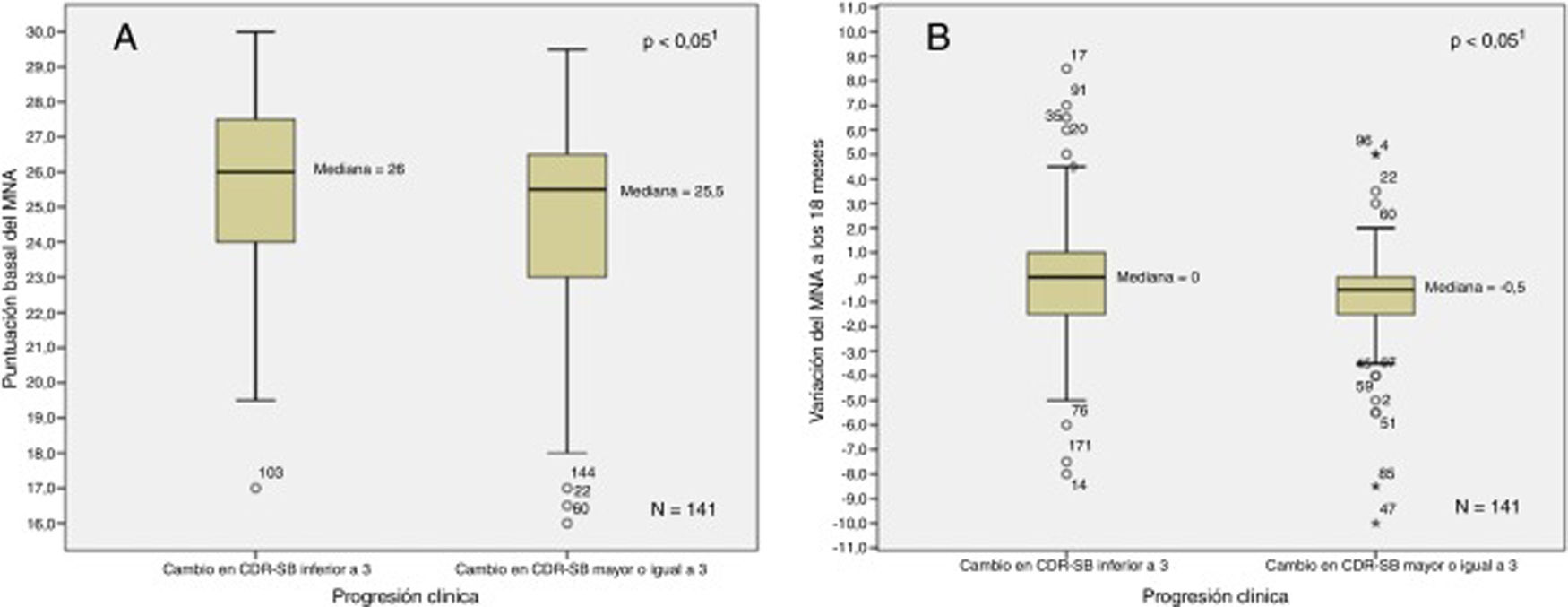
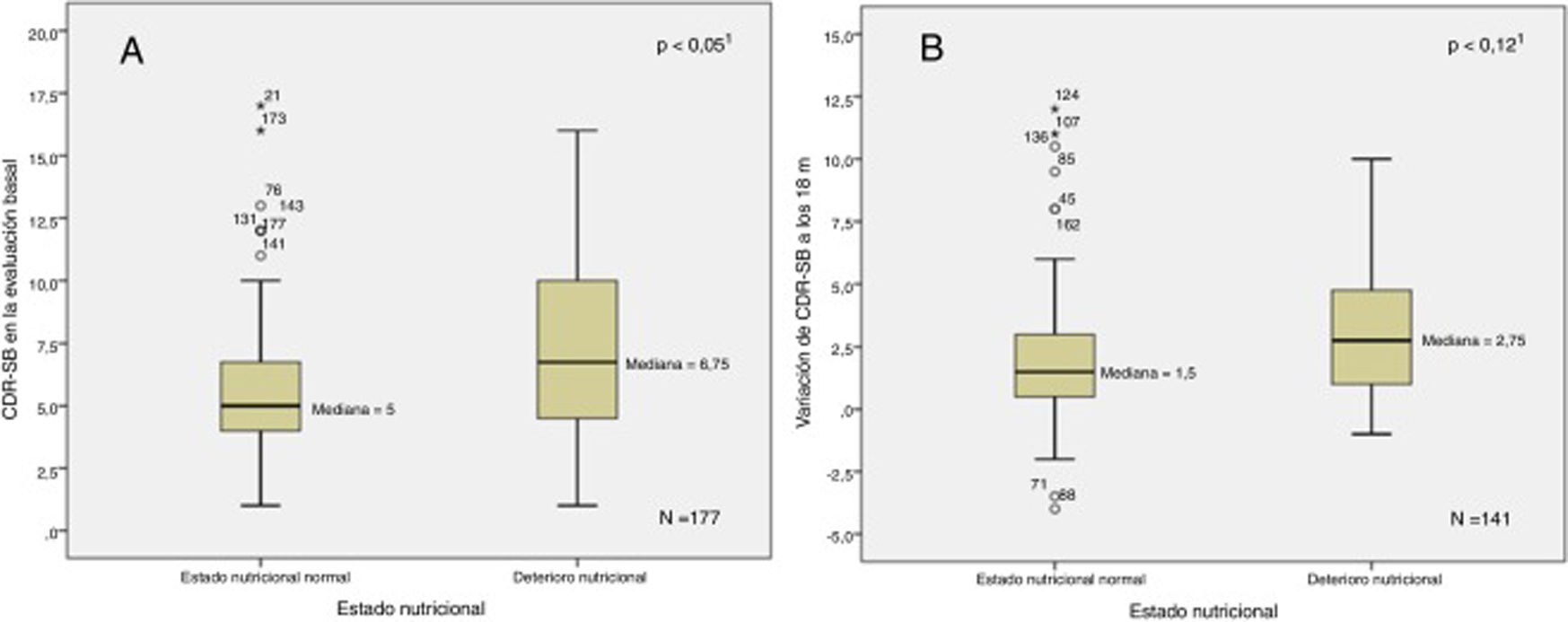
Fig. 1 shows the relationship between baseline and follow-up MNA scores and disease progression. Fig. 2 shows the relationship between baseline and follow-up CDR-SOB scores and nutritional status. In the bivariate analysis, we observed a positive correlation between MNA and MMSE scores at baseline (ρ = 0.15; P < .05) and negative correlations between MNA score and NPI-Q severity score (ρ = –0.35; P < .001), NPI-Q caregiver distress score (ρ = –0.34; P < .001), RDRS-2 score (ρ = –0.37; P < .001), and baseline CDR-SOB score (ρ = –0.17; P < .05). We observed negative correlations between variation in MNA classification at 18 months and variation in RDRS-2 (ρ = 0.23; P < .01) and CDR-SOB scores (ρ = –0.18; P < .05) during the study period.
Association between baseline (A) and follow-up (B) MNA scores and clinical progression.
1Mann-Whitney U test.
Association between baseline (A) and follow-up (B) CDR-SOB scores and impaired nutritional status.
1Mann-Whitney U test.
Table 5 shows the differences in demographic and clinical variables between the 34.8% of patients presenting significant worsening between the 2 assessments and the 65.2% who remained stable according to CDR-SOB. The proportion of patients with impaired nutritional status was significantly higher among patients with worsening according to the CDR-SOB, who presented significantly lower MNA scores and BMI. A total of 85.9% of the sample were being treated with acetylcholinesterase inhibitors, with a similar percentage in both groups. In the cohort of patients who were evaluated twice (n = 141), a higher percentage of patients with nutritional impairment than those with normal nutritional status at baseline presented significant worsening (50% vs 28.7%; P < .05). The same was true for the group of patients with AD dementia (53.6% vs 31.8%; P < .05). The prodromal AD group presented a similar trend, although this difference was not statistically significant (41.7% vs 22.9%; P = .21).
Demographic and clinical characteristics as a function of disease progression.
| CDR-SOB worsening < 3 (n = 92) | CDR-SOB worsening ≥ 3 (n = 49) | P | |
|---|---|---|---|
| Sociodemographic characteristics | |||
| Age (mean ± SD) | 77.6 ± 6 | 79.2 ± 6.5 | .19a |
| Women (n [%]) | 59 (64.1%) | 36 (73.5%) | .26b |
| No studies (n [%]) | 49 (53.3%) | 24 (49%) | .63b |
| Clinical assessment | |||
| MMSE (mean ± SD) | 21.3 ± 4.6 | 21.1 ± 4 | .76a |
| CAMCOG-R (mean ± SD) | 66.2 ± 15.2 | 61.8 ± 13.3 | .09a |
| CAMCOG-R memory (mean ± SD) | 11.2 ± 4.7 | 9.8 ± 4.9 | .09a |
| RDRS-2 (median [Q1-Q3]) | 24 (21-30) | 25 (22-30) | .28c |
| NPI-Q severity (median [Q1-Q3]) | 5 (2-9) | 6 (3.5-10.5) | .20c |
| NPI-Q caregiver distress (median [Q1-Q3]) | 7 (2-11.75) | 6 (4-13) | .40c |
| Zarit Burden Interview (mean ± SD) | 24.6 ± 16.8 | 28.8 ± 15.6 | .15a |
| CDR-SOB at baseline (median [Q1-Q3]) | 5 (3-6) | 5 (3.5-6.5) | .34c |
| Nutritional parameters | |||
| INS (MNA < 24) (n [%]) | 20 (21.7%) | 20 (40.8%) | < .05b |
| MNA (median [Q1-Q3]) | 26.0 (24-27.5) | 25.5 (22.75-26.5) | < .05c |
| BMI (mean ± SD) | 26.7 ± 3.8 | 25.2 ± 4.4 | < .05a |
| Positive APOE ε4 genotype (n [%]; sample evaluated) | 43 (49.4%); 87 | 20 (41.7%); 48 | .39b |
| AChEI treatment (n [%]) | 79 (85.9%) | 42 (85.7%) | .98b |
CAMCOG-R: Cambridge Cognitive Examination-Revised; CDR-SOB: Clinical Dementia Rating-sum of boxes; INS: impaired nutritional status (MNA < 24); AChEI: acetylcholinesterase inhibitor; BMI: body mass index; MMSE: Mini–Mental State Examination; MNA: Mini Nutritional Assessment; NPI-Q: Neuropsychiatric Inventory Questionnaire; Q1: first quartile; Q3: third quartile; SD: standard deviation; RDRS-2: Rapid Disability Rating Scale-2.
The analyses of independent risk factors for impaired nutritional status and worsening at 18 months are shown in Table 6. The multivariate logistic regression models found female sex and more severe behavioural involvement to be independently associated with impaired nutritional status. Lower scores on the CAMCOG-R and MNA scales were associated with worsening at 18 months.
Analysis of factors associated with impaired nutritional status and disease progression at 18 months.
| Dependent variable: baseline MNA < 24 (INS)a (n = 177) | Dependent variable: CDR-SOB ≥ 3 at 18 monthsb (n = 141) | |||||||
|---|---|---|---|---|---|---|---|---|
| Independent variable | Crude OR (95% CI) | P | Adjusted OR (95% CI) | P | Crude OR (95% CI) | P | Adjusted OR (95% CI) | P |
| Age (> 78.9) | 1.20 (0.62-2.32) | .59 | Excluded | 1.18 (0.59-2.36) | .64 | Excluded | ||
| Years of schooling (< 8) | 1.26 (0.65-2.45) | .49 | – | 1.07 (0.53-2.16) | .86 | – | ||
| No formal study | 1.37 (0.71-2.65) | .34 | – | 1.19 (0.59-2.38) | .63 | Excluded | ||
| Female sex | 3.26 (1.35-7.84) | < .01 | 4.17 (1.65-10.51) | < .001 | 1.55 (0.72-3.33) | .26 | Excluded | |
| MMSE (< 21) | 1.97 (0.99-3.90) | .051 | Excluded | 1.28 (0.64-2.57) | .48 | – | ||
| CAMCOG-R (< 65) | 1.49 (0.76-2.93) | .24 | – | 2.24 (1.09-4.59) | < .05 | 2.13 (1.03-4.43) | < .05 | |
| CAMCOG-R memory (< 11) | 1.23 (0.62-2.43) | .55 | – | 1.58 (0.78-3.20) | .20 | – | ||
| NPI-Q severity (> 5) | 4.95 (2.32-10.55) | < .001 | 5.79 (2.64-12.66) | < .001 | 1.59 (0.79-3.19) | .20 | – | |
| NPI-Q caregiver distress (> 7) | 4.11 (2.03-8.31) | < .001 | Excluded | 1.10 (0.55-2.21) | .79 | – | ||
| Zarit Burden Interview (> 24) | 1.54 (0.80-2.99) | .20 | – | 1.53 (0.76-3.07) | .23 | – | ||
| RDRS-2 (> 24) | 4.28 (2.05-8.96) | < .001 | Excluded | 1.19 (0.59-2.38) | .63 | – | ||
| CDR global score (> 1) | 2.70 (1.20-6.06) | < .05 | – | 1.01 (0.38-2.73) | .98 | – | ||
| CDR-SOB (> 5.0) | 2.00 (1.03-3.91) | < .05 | – | 1.47 (0.73-2.95) | .28 | – | ||
| MNA (< 25.5) | – | – | – | 1.22 (0.60-2.47) | .58 | – | ||
| BMI (< 25.6) | – | – | – | 1.34 (0.67-2.69) | .41 | – | ||
| Positive APOE ε4 genotypec | 0.74 (0.37-1.48) | .40 | – | 0.73 (0.36-1.49) | .39 | – | ||
| INS (MNA < 24) | – | – | – | 2.48 (1.17-5.28) | < .05 | 2.36 (1.1-5.09) | < .05 | |
CAMCOG-R: Cambridge Cognitive Examination-Revised; CDR: Clinical Dementia Rating scale; CDR-SOB: CDR-sum of boxes; CI: confidence interval; INS: impaired nutritional status; BMI: body mass index; MMSE: Mini–Mental State Examination; MNA: Mini Nutritional Assessment test; NPI-Q: Neuropsychiatric Inventory Questionnaire; OR: odds ratio; RDRS-2: Rapid Disability Rating Scale-2.
This study reflects the high frequency of nutritional deficiencies among patients with AD and confirms the negative influence of these deficiencies on disease progression. Our findings suggest an association, but are not sufficient to establish a causal relationship. In the DEMDIAG cohort, nutritional assessment started after diagnosis of AD, either in the prodromal or in the dementia phase. Other studies using this reference timeframe include the EACE study, a study into the clinical stage at which AD was diagnosed in 1700 Spanish patients. Fifty-two percent of patients showed mild dementia (CDR score of 1), mean age was 78 years, and 65% of patients were women.33 Our study did not include a group of patients with mild cognitive impairment. The AD dementia group in the DEMDIAG study included patients at a similar stage of disease progression, with a slightly older mean age and a higher percentage of women.
Given the very low prevalence of clear malnutrition in our sample (below 3%), we decided to treat malnutrition and risk of malnutrition as a single variable (“impaired nutritional status”) in our analysis; this condition was observed in 28.2% of the baseline population. While this prevalence figure seems objectively high, various studies of the general elderly population have reported even higher figures, which raises the question of how numerous factors may influence nutritional status in the elderly population. In 2005, a Spanish observational study used the MNA to evaluate the nutritional status of 22 007 community-dwelling individuals aged > 65 years, finding impaired nutritional status in 29.7% of patients, and associations between nutritional impairment and female sex, older age, and residence in the south of Spain.34 On the other extreme, a more recent Dutch study found much higher rates of impaired nutritional status in a non-institutionalised elderly population, with prevalence of up to 75% among individuals with a mean age of 80 years.35 These differences are probably at least partially explained by other reasons including cultural and lifestyle factors, and influenced by the level of institutionalisation in the elderly population. Taking a broader view, accounting for numerous countries and clinical scenarios, a meta-analysis of individuals older than 60 years found impaired nutritional status in 29.6% of those living in the community and 65% of those living in nursing homes. The same study found that 30% of community-dwelling patients with dementia presented impaired nutritional status; this is very similar to our own results.36 The NutriAlz trial, which included 940 community-dwelling elderly patients with dementia syndromes (AD and other types of dementia) at any stage of progression, reports a prevalence of impaired nutritional status of 47.8%, with higher prevalence observed in older patients and those presenting Lewy body dementia, greater cognitive and functional impairment, and more severe behavioural disorders.15 It is difficult to compare these studies with our own, as they were conducted in a range of different contexts, with differences in inclusion criteria and whether or not institutionalised populations were included.
It may be more beneficial to analyse the results of the DEMDIAG study in the context of other studies with largely similar methodologies. A retrospective study of Dutch patients with AD found low prevalence of impaired nutritional status (14%), and identified an association with more severe cognitive impairment, and particularly with poorer functional status in recently diagnosed patients.14 The REAL.FR study is a prospective multicentre study that included 579 patients diagnosed with AD in France and followed up for at least 4 years.37 Impaired nutritional status was associated with more severe behavioural alterations, and was recorded in 25.8% of participants at the baseline assessment. In the first year, patients with nutritional deficiencies showed poorer functional and cognitive progression and, surprisingly, better response to acetylcholinesterase inhibitors.38,39 Analysis of a subgroup of 160 participants from the REAL.FR study with very mild AD (CDR score 0.5) identified greater cognitive impairment and risk of malnutrition as independent risk factors for progression at one year40; this finding is consistent with our own results for the entire sample. The Cache County Dementia Progression Study also identified a clear association between malnutrition and faster cognitive and functional decline, as well as higher mortality rates.7,9
The analysis of factors associated with malnutrition and disease progression included numerous clinical, demographic, social, and cultural variables. As reported in previous studies, behavioural disorders were more prevalent in the group of patients with impaired nutritional status.15,38 This may reflect an unknown biological factor common to both conditions; in simpler terms, the nutritional deficiency may be the result of “hyperactive” behaviours. We also found that impaired nutritional status was independently associated with female sex, as reported in the extensive community study by Cuervo et al.34 From a cultural perspective, in the elderly population in our setting, women are typically responsible for cooking and food preparation, which may contribute to this effect in female patients. However, according to the same reasoning, we would expect to observe a similar effect among individuals living alone, but this was not the case. We also observed no association with level of schooling or residence in urban/rural settings. BMI was higher in the group of patients with prodromal AD, and barely changed after 18 months of follow-up; however, patients with clinical worsening did present significantly lower BMI. More detailed assessment of nutritional status, using the MNA, was much more sensitive. Cognitive impairment was not associated with nutritional status, contrary to reports from other studies, but was associated with progression, as observed in the subgroup of patients with very mild AD in the REAL.FR study40; this reflects the greater extent of neurodegeneration at onset and the probable subsequent depletion of cognitive reserve.41
The DEMDIAG epidemiological study presents several noteworthy methodological characteristics. Firstly, the observation period began at the time of diagnosis of AD. This time point is not relevant from a biological perspective, but is of undeniable practical interest, as it represents the point of first contact with the patient and marks the start point for therapeutic decision-making and communication on the prognosis of the disease, which patients and their family members generally want to know. Another essential characteristic is that our study reflects the current clinico-biological understanding of AD, including both patients with dementia and those in the prodromal phase. Progression was defined according to the increase in CDR-SOB scores; this score is more sensitive to change and has been proposed in recent years as an efficacy endpoint in clinical trials and observational studies.32 Finally, we analysed the frequency of the APOE ε4 allele in more than 90% of study participants, establishing the frequency at 49%, higher than the mean values reported in white patients with AD.42 While it did not contribute relevant information for the subgroup analysis, this test reflects that the patient selection process was appropriate.
The main limitation of our study is the small number of patients with prodromal AD, which probably prevented us from achieving sufficient statistical power in the analysis of prognostic differences between patients with normal or impaired nutritional status; however, these differences were clear and are consistent with those observed in patients with AD dementia. The 18-month interval between diagnosis and the second evaluation may also be insufficient for identifying differences between subgroups. Furthermore, 20% of patients were lost to follow-up after the baseline evaluation; this group presented greater predominance of older individuals, women, and patients with more severe cognitive and overall impairment, which may have introduced some bias to our results. While we studied biochemical markers of nutritional status, these did not contribute any relevant information. A broader multimodal evaluation with bioelectrical impedance vector analysis, a common technique in clinical nutrition units, would have provided valuable information to complement MNA scores, probably improving sensitivity for detecting malnutrition.
In conclusion, we should underscore the high prevalence of impaired nutritional status in patients with AD at the time of diagnosis, particularly among women and patients with more pronounced behavioural disorders. In addition to cognitive evaluation, a simple, rapid nutritional screening test may enable us to identify patients at greater risk of clinical progression after diagnosis and to design potential therapeutic interventions.
FundingThe DEMDIAG study received public funding from the health department of the regional government of Castile-Leon(project code GRS 556/A/10). The sponsor was not involved in study design; data collection, analysis, or interpretation; or drafting the study.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank the staff of the neurology departments at Complejo Hospitalario de Segovia and Hospital Universitario Río Hortega de Valladolid for their help in the selection of patients.
Please cite this article as: Izquierdo Delgado E, Gutiérrez Ríos R, Andrés Calvo M, Repiso Gento I, Castrillo Sanz A, Rodríguez Herrero R, et al. Evaluación del estado nutricional en la enfermedad de Alzheimer y su influencia en la progresión tras el diagnóstico. Neurología. 2022;37:735–747.
Currently, Sección de Neurología, Hospital Nuestra Señora de Sonsoles, Complejo Asistencial de Ávila, Ávila, Spain.
This work was partially presented as an oral communication at the 65th Annual Meeting of the Spanish Society of Neurology, Barcelona, November 2013 and at the 66th Annual Meeting of the Spanish Society of Neurology, Valencia, November 2014.