Recent years have seen considerable changes in the prevention and treatment of acute ischaemic stroke in adult patients. However, the low incidence of paediatric stroke makes the development of specific guidelines more challenging. This study aims to clarify the situation of these children in our region in order to establish a regional protocol to improve the care provided to these patients.
MethodsWe performed a regional incidence study of pediatric stroke (≤ 15 years of age) in Aragon, Spain (1308728 population, 15% aged ≤ 15 years) between 2008 and 2019. Data were obtained from hospital discharge records, including deaths, from the regional health service of Aragón, according to ICD codes for cerebrovascular disease. We analysed demographic, clinical, diagnostic/therapeutic, and prognostic variables.
ResultsA total of 21 events were recorded: 8 ischaemic (38.1%) and 13 haemorrhagic strokes (61.9%). The mean age (SD) was 9.3 years (1.0). The sample included 12 boys and nine girls. No statistically significant differences were found between ischaemic and haemorrhagic strokes, except in the chief complaint (language and motor impairment in ischaemic stroke and headache in haemorrhagic stroke). None of the patients with ischaemic stroke received reperfusion therapies. Including the 3 patients who died during hospitalisation, eight patients (42.1%) had modified Rankin Scale scores > 2 at 12 months. Motor deficits were the most common sequela (n=9).
ConclusionThough infrequent, paediatric stroke has an important functional impact. In Spain, Madrid was the first region to adapt the existing code stroke care networks for adult patients. In Aragon, this review has enabled us to work closely with the different stakeholders to offer a care plan for acute paediatric ischaemic stroke. Nevertheless, prospective national registries would be valuable to continue improving the care provided to these patients.
En los últimos años se han producido importantes cambios en la prevención y tratamiento del ictus isquémico agudo en el adulto. Sin embargo, la baja incidencia en edades pediátricas hace más difícil desarrollar protocolos y guías de asistencia específicas. Este trabajo busca conocer la situación de estos niños en nuestra región, con el objetivo de establecer un protocolo autonómico que mejore la atención a estos pacientes.
MétodosEstudio de incidencia de base hospitalaria del ictus infantil (≤ 15 años de edad) en Aragón (1.308.728 habitantes, 15% ≤ 15 años), desde 2008 a 2019. Los datos se extrajeron de los episodios de alta hospitalaria, incluidas las defunciones, del Servicio Aragonés de Salud, de acuerdo a los códigos de la Codificación Internacional de Enfermedades (CIE) definidos para enfermedad cerebrovascular. Se analizaron aspectos demográficos, clínicos, diagnóstico-terapéuticos y de pronóstico.
ResultadosSe recogieron un total de 21 eventos, ocho isquémicos (38,1%) y 13 hemorrágicos (61,9%). La media de edad fue 9,3 años (desviación típica 1,0). Hubo 12 hombres y nueve mujeres. No se encontraron diferencias estadísticamente significativitas entre ictus isquémicos y hemorrágicos, salvo por el síntoma guía (déficit motor o del lenguaje en isquémicos, y cefalea en hemorrágicos). Ninguno de los eventos isquémicos recibió terapias de reperfusión. Incluyendo los tres pacientes que fallecieron durante el ingreso, ocho (42,1%) tenían una puntuación en la escala modificada de Rankin (mRS) > 2 a los 12 meses. El déficit motor fue la secuela más común (n=9).
ConclusionesEl ictus infantil, aunque infrecuente, supone una importante limitación funcional. En España, Madrid fue pionera en adaptar las redes de asistencia existentes del Código Ictus en el adulto. En el caso de Aragón, esta revisión nos ha permitido trabajar de cerca con los diferentes actores implicados, para poder ofrecer un plan autonómico de atención al ictus isquémico infantil. Sin embargo, registros nacionales prospectivos ayudarían a avanzar en la atención a estos niños.
Over the past decade, changes and advances made in the prevention and acute treatment of stroke have enabled us to significantly improve outcomes in adult patients.1–7 In the case of the Spanish region of Aragon, the adoption of a regional strategy in 2009 has standardised the management of ischaemic stroke in adults, with mortality decreasing by 33% in men and 38% in women.8
In the paediatric population, the low incidence of cerebrovascular diseases (CVD) and their different aetiologies hinder the application of management guidelines and reperfusion treatments that, as in adult patients, help to decrease morbidity and mortality rates in these patients.9–12
Overall, the incidence of paediatric stroke is estimated at 2-3 cases per 100000 person-years between the ages of 0 and 15 years, with higher incidence in neonates (25-40 cases per 100000 person-years) and especially preterm neonates.10,13–16 In Spain, with a population of 47 million, these incidence rates translate to 140-270 children per year. In Aragon, with 1 308 728 inhabitants in 2020 and 15% of the population aged ≤ 15 years (196379 children, 51.41% boys), this would amount to 2-3 cases of paediatric stroke per year.
According to the published series, stroke risk factors in children are different to those observed in adult patients. Stroke predominantly affects male patients, and the type of presentation differs according to patient age.9,13–17 More than 50% of children present sequelae that will have an impact on their quality of life.18
Today, neurointerventional treatment is the option most likely to achieve favourable outcomes of ischaemic stroke in this age group.19 The real effectiveness of intravenous fibrinolysis has not been established due to recruitment issues, and recommendations are based on the results observed in adult patients.9,10,20–23 Furthermore, few studies have described the characteristics of children presenting ischaemic stroke, which makes it difficult to establish eligibility criteria for reperfusion treatment.
Given the need for the Health Department of Aragon to establish a paediatric stroke care plan including the implementation of a code stroke and the associated care pathways, we reviewed the cases of paediatric stroke in Aragon from the past decade. The current hypothesis is that increased knowledge of patient characteristics and of the susceptible target population is fundamental to the creation of care pathways and reduce unnecessary transfers and tests.
The aim of this descriptive study is to determine the incidence and epidemiological characteristics of paediatric stroke in Aragon, with a view to establishing a regional protocol to improve the care provided to these patients.
Material and methodsWe performed a retrospective study of paediatric stroke in the region of Aragon with data obtained from the electronic register of the Health Service of Aragon’s stroke strategy.
Aragon is an autonomous community located in north-eastern Spain with a low population density (28 inhabitants per km2) and uneven population distribution. Seventy-four percent of the population aged ≤ 15 years live in one of its 3 provinces, Zaragoza (with a population density of 55.8 inhabitants per km2). The capital of this province has the only reference centre for paediatric neurovascular disease, the Hospital Materno Infantil-Hospital Universitario Miguel Servet (HUMS).
Data were extracted from discharge reports, including deaths, from the different hospitals ascribed to the Health Service of Aragon. To identify patients, we used International Classification of Diseases (ICD) codes for CVD (both ischaemic and haemorrhagic) to consult records in the medical history database. We used ICD-9 codes (430 to 437) for cases recorded up to 2015, and ICD-10 (I60 to I67 and G45) for cases recorded since 2016, when the ICD-10 was implemented in the Spanish National Health System to record morbidity. Therefore, we did not include strokes that were exclusively attended at primary care centres or at private centres.
We consulted the available data on paediatric stroke for the last decade (2008-2019), considering this as stroke manifesting after the first 28 days of life. Due to their specific characteristics, we excluded episodes of perinatal stroke, defined as that occurring between week 28 of gestation and day 28 after birth,11 except in population incidence estimates. We also excluded children older than 15 years, as this is considered the upper limit of paediatric age for the Spanish National Health System at the hospital level.
We gathered patients’ baseline characteristics, including age, sex, place of residence, cerebrovascular risk factors or associated comorbidities, and previous functional status. Regarding stroke characteristics, we recorded symptoms at onset, time from symptom onset to arrival at the first healthcare centre and at the reference centre for paediatric neurovascular care, the type of neuroimaging study performed by the emergency department or during hospitalisation, stroke location, treatment during the acute phase, admission to the intensive care unit, stroke aetiology, and the total duration of hospitalisation. The Pediatric National Institutes of Health Stroke Scale (PedNIHSS) was applied retrospectively, as the scale was developed and implemented after its validation in 2012.24 Retrospective application of this instrument presents a high degree of validity and reproducibility and improves the quality of observational studies.25 For ischaemic stroke, we calculated the Alberta Stroke Program Early CT Score (ASPECTS) for the baseline neuroimaging study, recorded the presence or absence of haemorrhagic transformation, and classified stroke aetiology according to the Childhood AIS Standardized Classification and Diagnostic Evaluation criteria. For haemorrhagic stroke, we retrospectively used the modified Pediatric Intracerebral Hemorrhage (mPICH) score as a tool for prognostic stratification in the acute phase.26 To measure healthcare outcomes, we recorded in-hospital mortality, the type of sequelae at discharge, the need for outpatient rehabilitation, the presence of remote structural epilepsy, and the score on the modified Rankin Scale (mRS) at 12 months, according to data from follow-up reports.
We performed a descriptive statistical analysis using the SPSS statistics software for Mac (version 25.0, IBM Corp.; Armonk, NY, USA). Qualitative variables are expressed as total frequencies and percentages, whereas continuous quantitative variables are expressed as means and standard deviations (SD) or as medians and quartiles 1 and 3 (Q1-Q3), as appropriate.
The study was approved by the research ethics committee of the region of Aragon and authorised by the Healthcare Strategy Service of the General Directorate for Healthcare of the regional government of Aragon.
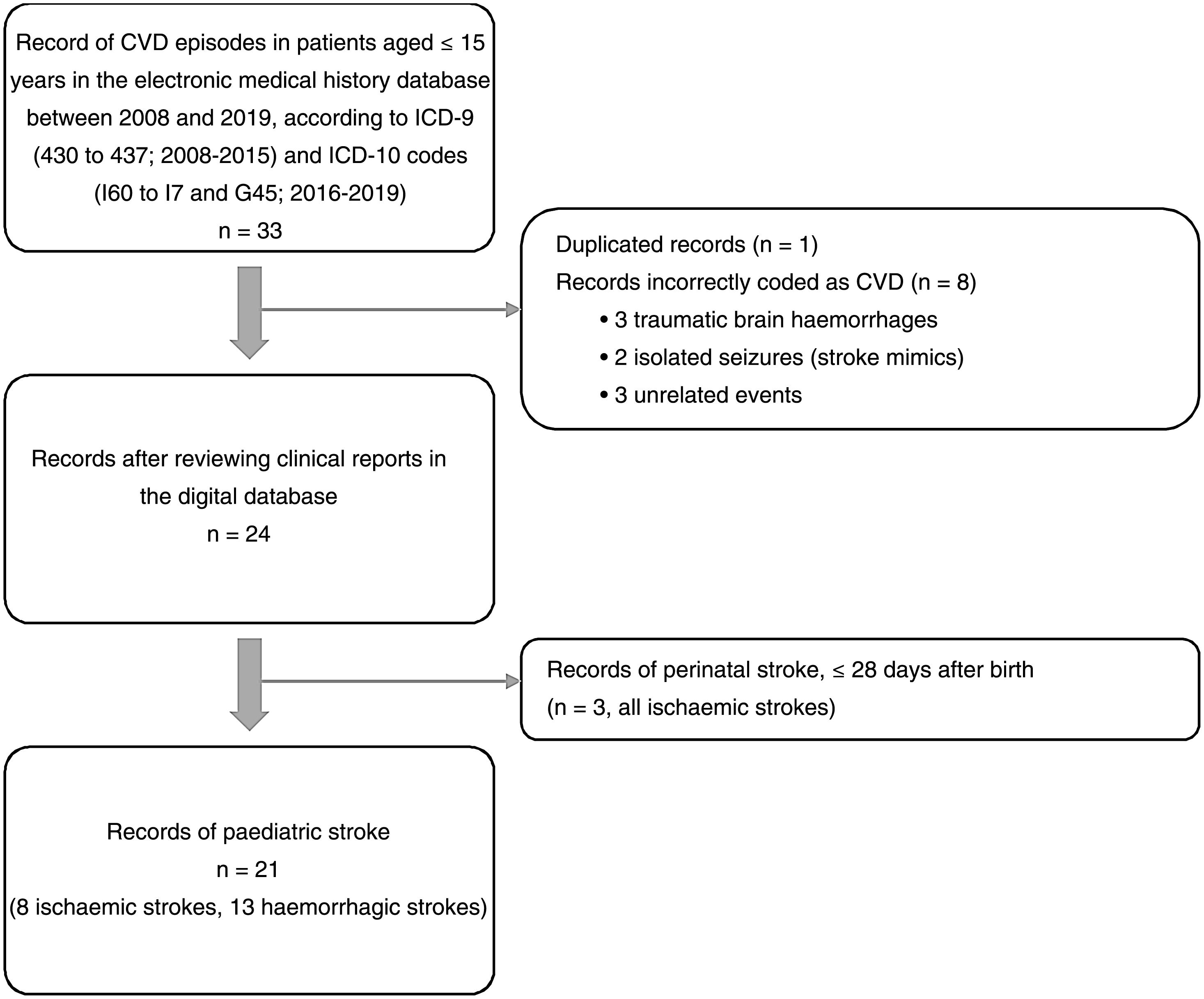
ResultsOf an initial record of 33 patients hospitalised with codes for CVD, 21 met all inclusion criteria and no exclusion criterion for paediatric stroke. Fig. 1 shows the patient selection process, according to the criteria mentioned above. Of 21 cases, 8 corresponded to ischaemic (38.1%) and 13 to haemorrhagic strokes (61.9%). No cases of transient ischaemic attack, cerebral venous sinus thrombosis, or spontaneous isolated subarachnoid haemorrhage were recorded.
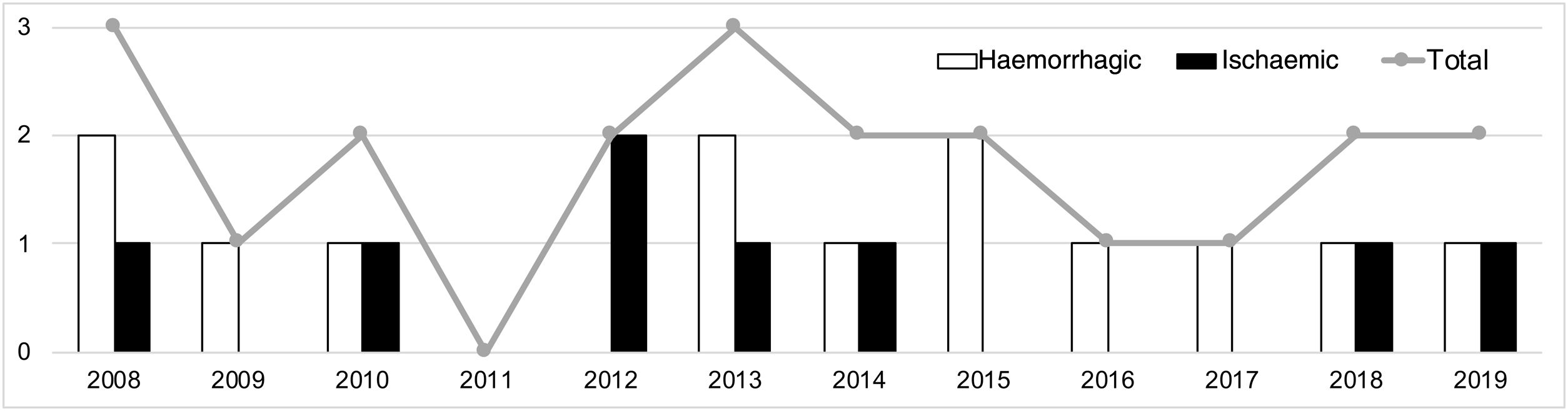
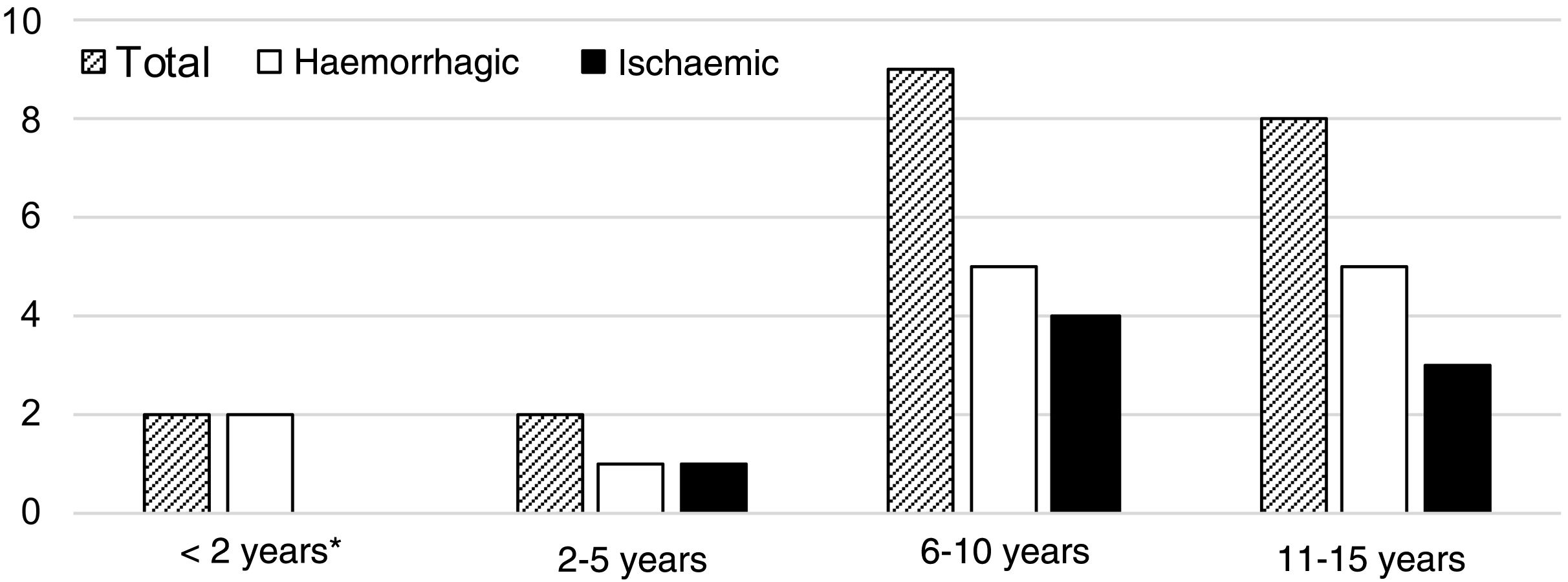
A median of 2 cases per year (range, 0-3) were recorded during the study period. Fig. 2 shows their distribution by year. Mean age was 9.3 years (SD: 1.0), and the sample included 12 boys (57.1%) and 9 girls (42.9%). Fig. 3 shows the distribution of strokes by age group, with 3 ischaemic events in patients younger than 8 years. Regarding functional status, all patients presented age-appropriate autonomy at baseline (mRS=0), with the exception of 2 children with ischaemic stroke with estimated mRS scores of 2 (congenital single ventricle heart defect that limited physical activity) and 3 (Down and West syndromes causing partial dependence).
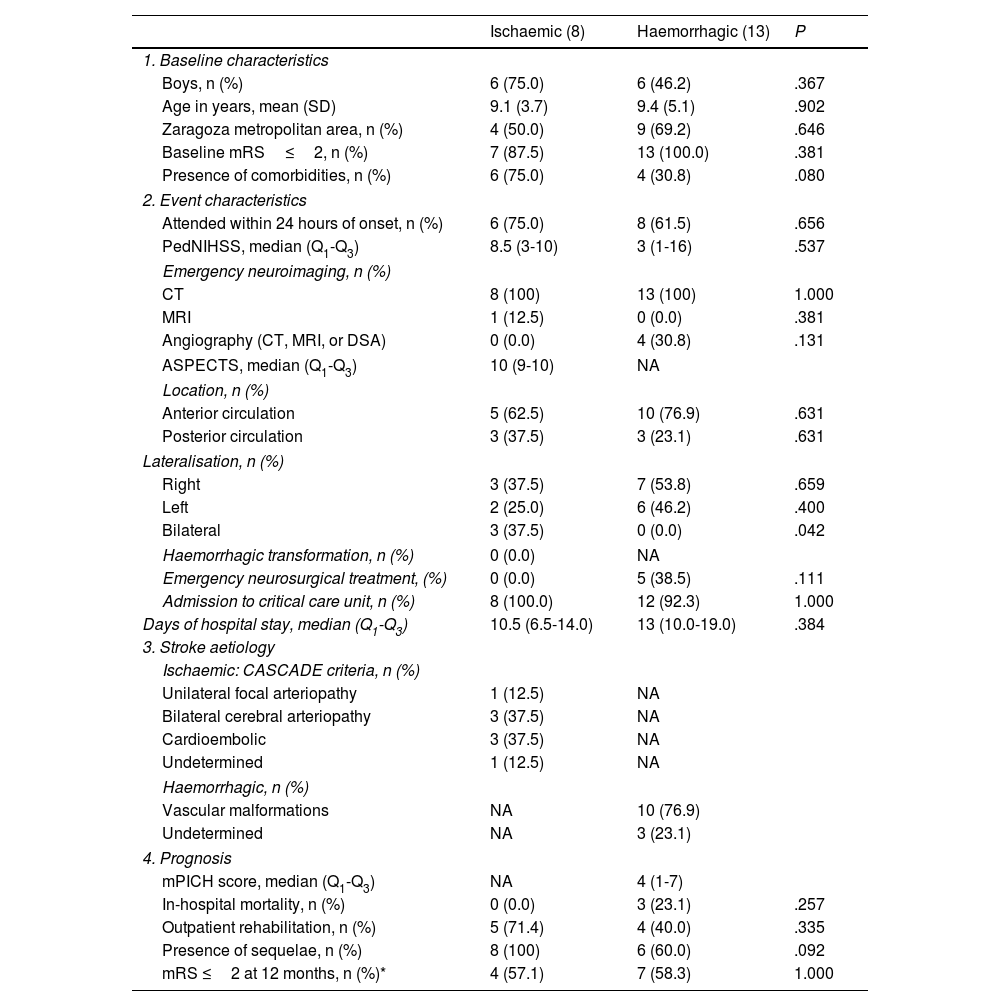
Tables 1 and 2 show demographic, clinical, and prognostic characteristics grouped by type of stroke (ischaemic or haemorrhagic). We observed no statistically significant differences between groups for the variables analysed, except for the clinical reason for consultation: patients with ischaemic stroke more frequently presented language alterations (37.5% [n=3], vs 0.0% in patients with haemorrhagic stroke; P=.042) and motor deficits (62.5% [n=5] vs 0.0%; P=.003), whereas headache was more frequently reported in patients with haemorrhagic stroke (0.0% vs 69.2% [n=9]; P=.002). We also observed a non-significant trend towards increased presence of comorbidities (75.0% vs 30.8%; P=.080) and increased presence of sequelae at discharge (100% vs 60%; P=.092) in patients presenting ischaemic stroke as compared with those with haemorrhagic stroke.
Characteristics of paediatric patients admitted due to acute (ischaemic or haemorrhagic) stroke in Aragon, between 2008 and 2019 (n=21).
| Ischaemic (8) | Haemorrhagic (13) | P | |
|---|---|---|---|
| 1. Baseline characteristics | |||
| Boys, n (%) | 6 (75.0) | 6 (46.2) | .367 |
| Age in years, mean (SD) | 9.1 (3.7) | 9.4 (5.1) | .902 |
| Zaragoza metropolitan area, n (%) | 4 (50.0) | 9 (69.2) | .646 |
| Baseline mRS≤2, n (%) | 7 (87.5) | 13 (100.0) | .381 |
| Presence of comorbidities, n (%) | 6 (75.0) | 4 (30.8) | .080 |
| 2. Event characteristics | |||
| Attended within 24 hours of onset, n (%) | 6 (75.0) | 8 (61.5) | .656 |
| PedNIHSS, median (Q1-Q3) | 8.5 (3-10) | 3 (1-16) | .537 |
| Emergency neuroimaging, n (%) | |||
| CT | 8 (100) | 13 (100) | 1.000 |
| MRI | 1 (12.5) | 0 (0.0) | .381 |
| Angiography (CT, MRI, or DSA) | 0 (0.0) | 4 (30.8) | .131 |
| ASPECTS, median (Q1-Q3) | 10 (9-10) | NA | |
| Location, n (%) | |||
| Anterior circulation | 5 (62.5) | 10 (76.9) | .631 |
| Posterior circulation | 3 (37.5) | 3 (23.1) | .631 |
| Lateralisation, n (%) | |||
| Right | 3 (37.5) | 7 (53.8) | .659 |
| Left | 2 (25.0) | 6 (46.2) | .400 |
| Bilateral | 3 (37.5) | 0 (0.0) | .042 |
| Haemorrhagic transformation, n (%) | 0 (0.0) | NA | |
| Emergency neurosurgical treatment, (%) | 0 (0.0) | 5 (38.5) | .111 |
| Admission to critical care unit, n (%) | 8 (100.0) | 12 (92.3) | 1.000 |
| Days of hospital stay, median (Q1-Q3) | 10.5 (6.5-14.0) | 13 (10.0-19.0) | .384 |
| 3. Stroke aetiology | |||
| Ischaemic: CASCADE criteria, n (%) | |||
| Unilateral focal arteriopathy | 1 (12.5) | NA | |
| Bilateral cerebral arteriopathy | 3 (37.5) | NA | |
| Cardioembolic | 3 (37.5) | NA | |
| Undetermined | 1 (12.5) | NA | |
| Haemorrhagic, n (%) | |||
| Vascular malformations | NA | 10 (76.9) | |
| Undetermined | NA | 3 (23.1) | |
| 4. Prognosis | |||
| mPICH score, median (Q1-Q3) | NA | 4 (1-7) | |
| In-hospital mortality, n (%) | 0 (0.0) | 3 (23.1) | .257 |
| Outpatient rehabilitation, n (%) | 5 (71.4) | 4 (40.0) | .335 |
| Presence of sequelae, n (%) | 8 (100) | 6 (60.0) | .092 |
| mRS ≤2 at 12 months, n (%)* | 4 (57.1) | 7 (58.3) | 1.000 |
ASPECTS: Alberta Stroke Program Early Computed Tomography Score; CASCADE: Childhood AIS Standardized Classification and Diagnostic Evaluation; CT: computed tomography; DSA: digital subtraction angiography; IQR: interquartile range; mPICH: modified Pediatric Intracraneal Cerebral Hemorrhage; MRI: magnetic resonance imaging; NA: not applicable; PedNIHSS: Pediatric National Institutes of Health Stroke Scale; SD: standard deviation.
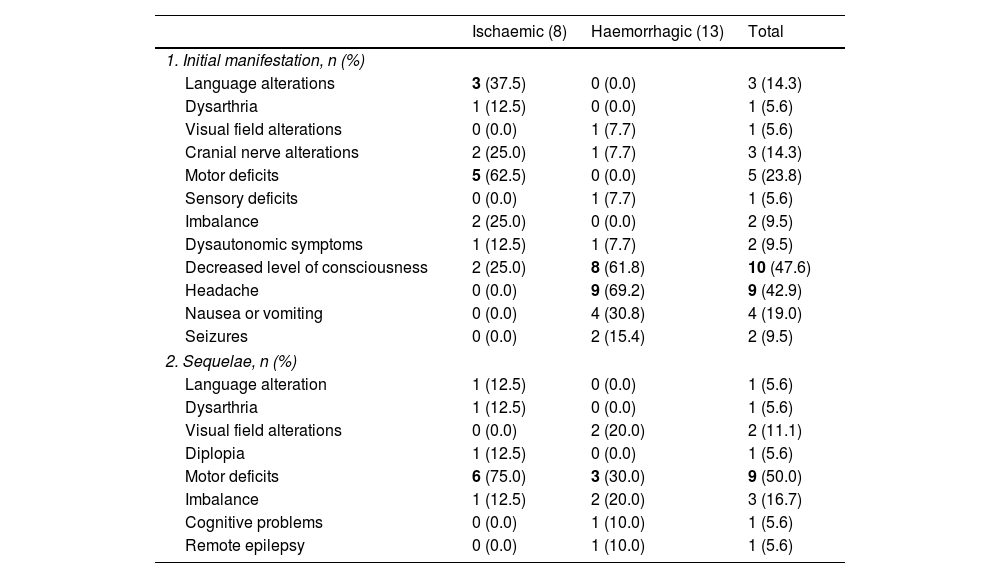
Initial symptom recorded as the reason for consultation and sequelae at hospital discharge of patients with acute paediatric stroke (2008-2019), overall and by stroke type (ischaemic or haemorrhagic).
| Ischaemic (8) | Haemorrhagic (13) | Total | |
|---|---|---|---|
| 1. Initial manifestation, n (%) | |||
| Language alterations | 3 (37.5) | 0 (0.0) | 3 (14.3) |
| Dysarthria | 1 (12.5) | 0 (0.0) | 1 (5.6) |
| Visual field alterations | 0 (0.0) | 1 (7.7) | 1 (5.6) |
| Cranial nerve alterations | 2 (25.0) | 1 (7.7) | 3 (14.3) |
| Motor deficits | 5 (62.5) | 0 (0.0) | 5 (23.8) |
| Sensory deficits | 0 (0.0) | 1 (7.7) | 1 (5.6) |
| Imbalance | 2 (25.0) | 0 (0.0) | 2 (9.5) |
| Dysautonomic symptoms | 1 (12.5) | 1 (7.7) | 2 (9.5) |
| Decreased level of consciousness | 2 (25.0) | 8 (61.8) | 10 (47.6) |
| Headache | 0 (0.0) | 9 (69.2) | 9 (42.9) |
| Nausea or vomiting | 0 (0.0) | 4 (30.8) | 4 (19.0) |
| Seizures | 0 (0.0) | 2 (15.4) | 2 (9.5) |
| 2. Sequelae, n (%) | |||
| Language alteration | 1 (12.5) | 0 (0.0) | 1 (5.6) |
| Dysarthria | 1 (12.5) | 0 (0.0) | 1 (5.6) |
| Visual field alterations | 0 (0.0) | 2 (20.0) | 2 (11.1) |
| Diplopia | 1 (12.5) | 0 (0.0) | 1 (5.6) |
| Motor deficits | 6 (75.0) | 3 (30.0) | 9 (50.0) |
| Imbalance | 1 (12.5) | 2 (20.0) | 3 (16.7) |
| Cognitive problems | 0 (0.0) | 1 (10.0) | 1 (5.6) |
| Remote epilepsy | 0 (0.0) | 1 (10.0) | 1 (5.6) |
The most frequent reasons for consultation in each group are shown in bold. Statistically significant differences were observed in language alterations (P=.042), motor deficits (P=.003), and headache (P=.002).
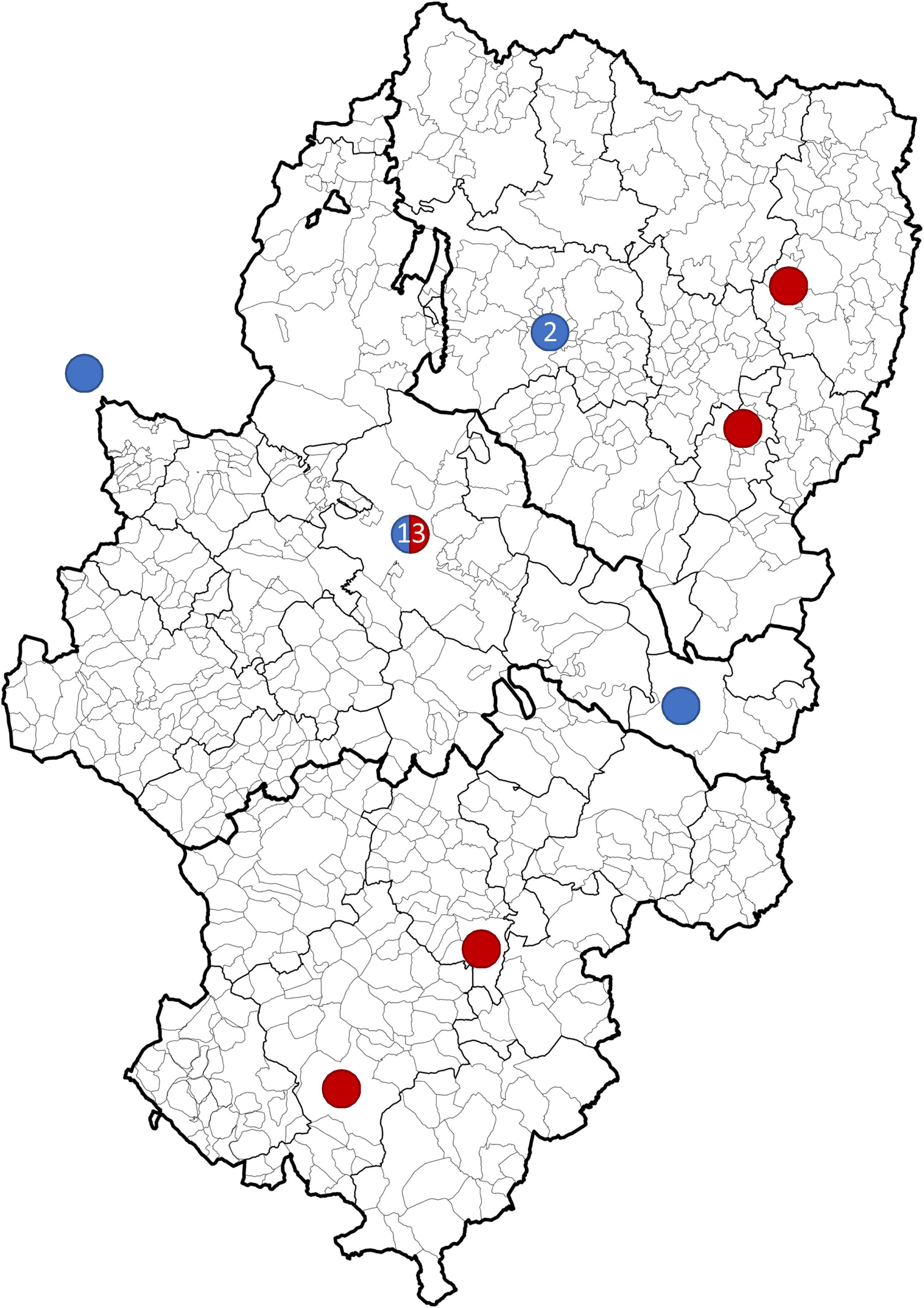
Overall, 61.9% of strokes (n=13) occurred in Zaragoza or its metropolitan area (Fig. 4). Twenty patients (95.8%) were attended at the HUMS. These patients were admitted to critical care units: 18 to the paediatric intensive care unit and 2 to adult stroke units. Seven patients (33.3%) did not attend hospital within 24hours of symptom onset. The patient who was directly admitted to an inpatient ward presented symptoms less than 72hours before admission, with no worsening. The median hospital stay was 12 days (8-17).
Geographical distribution of strokes between 2008 and 2019 in Aragon. Ischaemic strokes (blue) in Huesca (2008, 2012), Zaragoza (2010, 2012, 2018, 2019), Caspe (2013), and La Rioja (2014). Haemorrhagic strokes (red) in Zaragoza (2008, 2013×2, 2014, 2015×2, 2017, 2018, 2019), Graus (2008), Aliaga (2009), Monzón (2010), and Teruel (2016). The Hospital Materno Infantil - Hospital Universitario Miguel Servet was previously also the reference centre for emergency paediatric neurovascular disease in La Rioja.
Regarding management during the acute phase, no patient with ischaemic stroke received intravenous fibrinolysis or mechanical thrombectomy. The baseline neuroimaging study was performed at the first hospital in 19 cases (90.5%). An emergency artery study was performed in 4 patients (17.4%), all of whom presented haemorrhagic strokes. During admission, a brain MRI scan was performed in the first 24hours in 50% (n=4) of patients with ischaemic stroke; all of these patients also underwent an angiography study of the brain and supra-aortic trunks. The following types of angiography were performed: MRI angiography (5 patients), digital subtraction angiography (2), neurosonology (2), and CT angiography (1). Among patients with haemorrhagic strokes, only 2 (15.4%) did not undergo artery studies during admission, in both cases due to a clinical situation leading to the patient’s death in the first days.
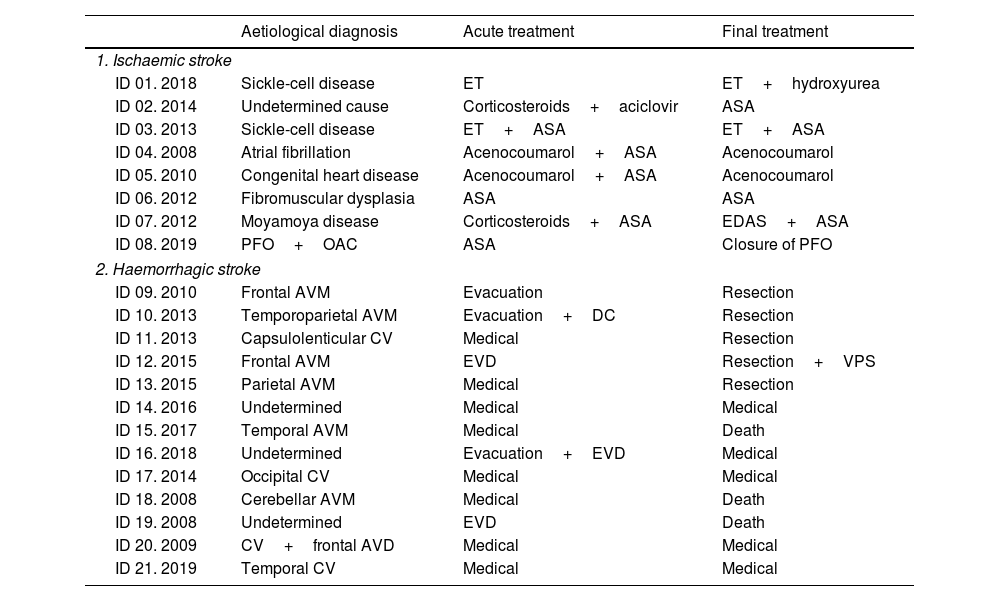
Table 3 shows the aetiological diagnosis and treatments for acute stroke and the cause of stroke in each patient. Aetiology was not determined in 4 cases (17.4%, one ischaemic and 3 haemorrhagic).
Aetiological diagnosis and therapeutic management of each case of paediatric stroke (ischaemic and haemorrhagic).
| Aetiological diagnosis | Acute treatment | Final treatment | |
|---|---|---|---|
| 1. Ischaemic stroke | |||
| ID 01. 2018 | Sickle-cell disease | ET | ET+hydroxyurea |
| ID 02. 2014 | Undetermined cause | Corticosteroids+aciclovir | ASA |
| ID 03. 2013 | Sickle-cell disease | ET+ASA | ET+ASA |
| ID 04. 2008 | Atrial fibrillation | Acenocoumarol+ASA | Acenocoumarol |
| ID 05. 2010 | Congenital heart disease | Acenocoumarol+ASA | Acenocoumarol |
| ID 06. 2012 | Fibromuscular dysplasia | ASA | ASA |
| ID 07. 2012 | Moyamoya disease | Corticosteroids+ASA | EDAS+ASA |
| ID 08. 2019 | PFO+OAC | ASA | Closure of PFO |
| 2. Haemorrhagic stroke | |||
| ID 09. 2010 | Frontal AVM | Evacuation | Resection |
| ID 10. 2013 | Temporoparietal AVM | Evacuation+DC | Resection |
| ID 11. 2013 | Capsulolenticular CV | Medical | Resection |
| ID 12. 2015 | Frontal AVM | EVD | Resection+VPS |
| ID 13. 2015 | Parietal AVM | Medical | Resection |
| ID 14. 2016 | Undetermined | Medical | Medical |
| ID 15. 2017 | Temporal AVM | Medical | Death |
| ID 16. 2018 | Undetermined | Evacuation+EVD | Medical |
| ID 17. 2014 | Occipital CV | Medical | Medical |
| ID 18. 2008 | Cerebellar AVM | Medical | Death |
| ID 19. 2008 | Undetermined | EVD | Death |
| ID 20. 2009 | CV+frontal AVD | Medical | Medical |
| ID 21. 2019 | Temporal CV | Medical | Medical |
ASA: acetylsalicylic acid at 100mg; AVD: anomalous venous drain; AVM: arteriovenous malformation; CV: cavernoma; DC: decompressive craniectomy; EDAS: encephaloduroarteriosynangiosis; ET: exchange transfusion; EVD: external ventricular drain; ID: identification number of the patient and year of the event; OAC: oral anticoagulants; PFO: patent foramen ovale; VPS: ventriculoperitoneal shunt.
Among haemorrhagic strokes, patients 12, 15, and 21 presented intraventricular haemorrhage, patients 10, 14, 16, 18, and 19 presented a subarachnoid haemorrhage component, and patient 10 a subdural haematoma.
Medical treatment of intraparenchymal haemorrhage refers to the standardised care provided for clinical and haemodynamic control in patients not requiring interventional therapies.
Of the resections of vascular malformations, 3 of the 4 AVMs were previously secured by endovascular embolisation and one patient received subsequent radiation therapy. Radiosurgery was used to excise the resected cavernoma.
Regarding patient outcomes, 3 patients (14%) died during the acute phase, all of whom presented haemorrhagic strokes. Two died due to intracranial hypertension secondary to posterior fossa haematoma (a girl aged 1.8 years and a boy aged 10.2 years, both with mPICH scores of 9) and the remaining patient due to refractory status epilepticus secondary to right temporal haematoma with ventricular spillover (a boy aged 8.5 years, mPICH score of 1). We found no statistically significant differences in functional prognosis, location of the haemorrhage, or mPICH score.
Sequelae at discharge for the 18 surviving patients are listed in Table 2, with outpatient rehabilitation prescribed in 55.6% of cases (n=10). Motor deficits were the most frequent sequela (50%, n=9). Six patients presented epileptic seizures secondary to stroke, haemorrhagic in all cases. Of these, 5 presented acute symptomatic seizures during admission, and only one presented remote epilepsy (a boy aged 12.8 years with history of complex febrile seizures).
Sixteen patients (88.9%) were followed up; follow-up data were not available for the other 2 cases as they moved outside Aragon. No recurrent strokes were observed during follow-up, and at the 12-month visit, no new deaths were reported, with 5 patients (31.3%) presenting an mRS score>2 (including the patient with a baseline mRS score of 3). Including the 3 patients who died during admission, 42.1% (n=8) presented a poor functional prognosis in the medium to long term (mRS>2).
DiscussionDue to the low incidence of paediatric stroke, few series have analysed the profile of these patients.15,27,28 The incidence of paediatric stroke in our sample is lower than that expected for the population of our region, with 0.89 cases per 100000 children aged ≤ 15 years, with a predominance of haemorrhagic strokes (61.9%) vs ischaemic stroke (38.1%) in the 12-year study period. This lower incidence rate is more patent for ischaemic stroke, with 0.47 cases per 100000 children, including neonates in this case (vs 1.7-2.7 in recent series)15,27,28; and a low number of cases in the group frequently reported to present higher prevalence, that is, children aged < 2 years. This may be explained by several reasons, such as a difficulty in the identification of stroke in the acute phase, but also problems with coding episodes. As in other studies, ischaemic stroke was more frequent among boys (75%).15,27
Unlike in adults, the incidence of paediatric stroke in Aragon has remained stable over the past 12 years. This finding is also reported in another population series,28 which also observed greater incidence of ischaemic stroke (2.7/100000) than haemorrhagic stroke (1.7/100000). The stable incidence rate may be due to the lack of substantial changes in the prevention of paediatric stroke in recent years, with the exception of management of sickle-cell anaemia.29
As in most series, most of our patients with ischaemic stroke presented some known risk factor for CVD (75%, 6 out of the 8 cases). Noteworthy examples include a patient with Emery-Dreifuss muscular dystrophy who presented stroke due to de novo atrial fibrillation, one of the heart diseases most frequently associated with this type of dystrophy30; and 2 patients with sickle-cell disease, both of whom were black, which increases the risk of stroke associated with the condition up to 200 times with regard to the remaining paediatric population.31 Our patient with Down syndrome was diagnosed with moyamoya disease. This arterial disease presents more frequently in patients with Down syndrome and is responsible for 6%-10% of all paediatric strokes and transient ischaemic attacks.32,33
The presence of comorbidities and known risk factors before the ischaemic event highlights the relevance of close monitoring of this type of patients to prevent CVD. Given the limited number of aetiologies of paediatric ischaemic stroke, further efforts should be made in primary prevention in selected patients (eg, those with heart or vascular disease), as has been the case with sickle-cell disease (achieving a significant decrease in the incidence of these events). If this is not possible and stroke eventually occurs, an established protocol should be in place to promote early identification and treatment.
In the case of haemorrhagic stroke, our series shows similarities with others reported in the literature. Seventy-seven percent of these strokes (10 out of 13 patients) were caused by a structural lesion, in most cases an arteriovenous malformation or cavernoma. In 3 of these patients (23%), aetiology was undetermined; this was the case for 10% of patients in other series.34
The type of CVD presentation differs according to age. Perinatal stroke frequently presents with diffuse symptoms, which are usually associated with haemorrhagic stroke, or epileptic seizures in all types of stroke.15 In paediatric strokes, symptoms are similar to those observed in adults. However, some series report that up to one-third of cases (as compared to 15%-25% of adult patients35) may be classified as stroke mimics in the acute phase,11 which illustrates the difficulty in correctly identifying the clinical symptoms at early ages. In our series, however, it is difficult to assess the presence of stroke mimics in the acute phase, as we used diagnoses established at hospital discharge, when most stroke mimics have been ruled out.
Overall, in our series, headache and decreased level of consciousness were the most frequent reasons for consultation in patients with haemorrhagic stroke (69.2% and 61.8%, respectively). In those with ischaemic stroke, the most frequent reason for consultation was presence of focal symptoms, as reported in other series.11,15 Focal symptoms included unilateral motor deficits (62.5%), present in older age groups (4 out of the 5 patients were aged 6-15), and language alterations (37.5%). Seizures were only observed in 2 of the 21 confirmed strokes (9.5%), a lower rate than that reported in the literature (15%-25%)11; both patients had haemorrhagic strokes, and were different ages (1.8 and 10.8 years). Seizures in paediatric patients more frequently affect children younger than 7 years, and their presence depends on the aetiology. The mean age in our sample was 9.3 years, and only 3 patients presented cardioembolic stroke, one of the aetiologies most frequently associated with seizures in paediatric stroke (seizures in more than 40% of the cases).11
It is noteworthy that 33% of the children in our series consulted more than 24hours after symptom onset. According to the literature, although the majority of children are attended during the first hours, the median time between symptom onset and parents seeking medical care is highly variable, ranging from 1.7 to 21hours.36,37 A study into the behaviour of parents in case of stroke revealed that 24% did not recognise the severity of the symptoms, taking a “wait and see” approach.37,38 This shows that symptoms in children are not always recognised by parents as a severe situation requiring immediate care. Raising awareness, as in the adult population, is fundamental.
Regarding the use of resources in these patients, 95.8% were attended at a critical care unit. Unlike adults (in whom management at semicritical care units such as stroke units is widespread), these children are predominantly admitted to the paediatric intensive care unit at the HUMS, with the associated economic cost (Aragon has no paediatric intermediate care units for these conditions). Median duration of hospitalisation was 10.5 days for ischaemic and 13 days for haemorrhagic stroke, with a trend towards longer stays in patients with haemorrhagic strokes; this association was not significant, perhaps due to the small sample size. Furthermore, no reperfusion treatment was performed in any children attended due to ischaemic stroke. As a result of the low incidence of paediatric stroke, reperfusion is less likely to be used in these patients; furthermore, neurointerventional treatment (considered the most effective option in paediatric age) was not included in the Aragon healthcare services portfolio, nor had paediatric code stroke been implemented (the protocol was approved in October 2020). In children with haemorrhagic stroke, the limited use of emergency artery studies may be due to the lack of an established protocol (only 4 of the 13 patients underwent an artery study, despite a final diagnosis of vascular malformation in 10 of them). An emergency neurosurgical intervention was performed in 38.5% of patients with haemorrhagic stroke, and 46.8% required subsequent surgical treatment of the cause of the bleeding.
Lastly, our results confirm the high rate of functional dependence following stroke in children. In our series, 42.1% of patients (8 of 19) died or presented functional dependence or lack of autonomy (mRS>2 at 12 months). Our findings are consistent with those of other series, such as the Canadian registry (Canadian Pediatric Ischemic Stroke Registry, 1992-2001), in which only half of the children with ischaemic stroke presented favourable outcomes.15 However, the use of such functional scales as the mRS, designed for the adult population, may have limitations for the proper assessment of sequelae in children; therefore, the use of such specific instruments as the Pediatric Stroke Outcome Measure is becoming increasingly frequent.39
In the light of this functional dependence, it is essential to have effective treatments for the acute phase. However, the low incidence of paediatric stroke results in slow recruitment in randomised clinical trials,23 limiting the available evidence on the treatments indicated for this age group.
To overcome these limitations and to improve outcomes, consensus statements have been proposed in recent years, such as that published by the American Heart Association in 2019,11 which combines information from such specific studies as the Thrombolysis in Pediatric Stroke study23 with the available experience with reperfusion therapies in adults with ischaemic stroke.
In Spain, the region of Madrid was the first to adapt code stroke networks for the care of paediatric patients. In the case of Aragon, this review has enabled us to align the positions of the different professionals involved and to offer a paediatric ischaemic stroke care plan in the autonomous community of Aragon, which was finally approved in late 2020.40 The plan seeks to offer the best available treatment, using all the existing resources and networks for adults, and promoting training and the early identification of symptoms. Therefore, multidisciplinary teams including physicians specialising in paediatric neurology are essential. The experience obtained from the adult code stroke system may contribute to this training and further the skills of other professionals, such as paediatricians, in using objective tools to monitor activities in order to continuously improve outcomes.
Lastly, as this is an infrequent disease with a significant impact on quality of life, it may be interesting to document patients in national registries to establish their characteristics, in order to subsequently adapt care to children in a specific healthcare system.
LimitationsThe main limitation of this study is its retrospective, observational design. Coding errors may also have led to some cases being missed. Furthermore, though less likely due to the organisation of paediatric care in Aragon (the HUMS is the only centre with a paediatric stroke care unit), there is also a risk of underdiagnosis due to mild cases exclusively attended by primary care or private health centres. Furthermore, given the low incidence of the disease and the small sample size, the comparative analysis is limited and its results may not be extrapolated to other regions.
We also observed limited use of semiquantitative clinical (mRS, PedNIHSS) or radiological (ASPECTS, mPICH) scales in the initial assessment and follow-up of these patients and of aetiological classification criteria for ischaemic stroke, as occurs in adults. Therefore, we needed to perform a retrospective assessment of this type of variables, which also served as an opportunity to offer training on this type of assessment for the professionals responsible for the care of paediatric patients in the future.
ConclusionThough rare, paediatric stroke results in a significant functional limitation for at least half of patients, with an increased level of disability. The advancements observed in recent years in acute ischaemic stroke management in adult patients have led to the creation of new action guidelines and consensus statements.18 In our case, they have led to the approval of the paediatric ischaemic stroke care plan of the region of Aragon, which aims to detect patients at risk and to offer the best available treatments in the event of stroke.40 We encourage other communities to follow this pathway and to create common databases enabling us to advance together in the care of these children.
FundingNo funding was received for this study.
Conflicts of interestThe authors have no conflicts of interest to declare.