This study aimed to investigate the effectiveness and safety of endovascular revascularisation of intracranial artery occlusion and stenosis in moyamoya disease using stent angioplasty.
Materials and methodsWe recruited 12 patients (8 women and 4 men) with occlusion and stenosis of intracranial arteries in the context of moyamoya disease who underwent endovascular stent angioplasty. Clinical data, baseline conditions, lesion location, treatment outcomes, periprocedural complications, and follow-up outcomes were analysed.
ResultsThe occlusion was located at the M1 segment of the middle cerebral artery in 8 patients, at both the M1 and A2 segments in one patient, and at the C7 segment of the internal carotid artery in 3. Thirteen stents were deployed at the occlusion site, including the low-profile visualized intraluminal support (LVIS) device in 8 patients, an LVIS device and a Solitaire AB stent in one, and a Leo stent in 3, with a success rate of 100% and no intraprocedural complications. Plain CT imaging after stenting revealed leakage of contrast agent, which disappeared on the second day, resulting in no clinical symptoms or neurological sequelae. Follow-up angiography studies were performed in all patients for 6–12 months (mean, 8.8). Slight asymptomatic in-stent stenosis was observed in 2 patients (16.7%), and no neurological deficits were observed in the other patients. All preoperative ischaemic symptoms completely disappeared at follow-up.
ConclusionStent angioplasty is a safe and effective treatment for occlusion and stenosis of intracranial arteries in moyamoya disease.
Evaluamos la eficacia y la seguridad de la revascularización endovascular de oclusiones y estenosis de arterias intracraneales mediante angioplastia con stent en pacientes con enfermedad de moyamoya.
Materiales y métodosIncluimos a 12 pacientes (8 mujeres y 4 hombres) con oclusiones y estenosis de arterias intracraneales en el contexto de enfermedad de moyamoya que se sometieron a angioplastia con stent. Recogimos datos clínicos e información sobre enfermedades existentes, localización de la lesión, resultados terapéuticos, complicaciones periprocedimiento y resultados de seguimiento.
ResultadosLa oclusión afectaba al segmento M1 de la arteria cerebral media en 8 pacientes, a los segmentos M1 y A2 en un paciente, y al segmento C7 de la arteria carótida interna en los 3 restantes. Se colocaron 13 stents en el lugar de la oclusión: stents LVIS en 8 pacientes, un stent LVIS y un stent Solitaire AB en un paciente y stents Leo en 3 pacientes. La tasa de éxito fue del 100%, sin complicaciones durante el procedimiento. Una TC tras el procedimiento detectó fugas del medio de contraste, que desaparecieron al segundo día y no provocaron síntomas ni secuelas neurológicas. Se realizó un seguimiento de todos los pacientes con angiografía durante 6-12 meses (media: 8,8 meses). Dos pacientes (16,7%) presentaron una ligera reestenosis intrastent asintomática; el resto de los pacientes no presentaron déficits neurológicos. Todos los síntomas isquémicos previos desaparecieron durante el seguimiento.
ConclusionesLa angioplastia con stent es un tratamiento eficaz y seguro de las oclusiones y estenosis de arterias intracraneales en pacientes con enfermedad de moyamoya.
As a chronic intracranial arterial occlusive disease with unknown reasons, Moyamoya disease has the following characteristics of stenosis and occlusion at the terminal segments of bilateral internal carotid artery (ICA) and formation of an abnormal vascular network at the base of the brain.1–4 Moyamoya disease is an important cause of nonatherosclerotic cerebrovascular disease, especially in East Asian where the prevalence is relatively high with the reported incidence of 0.54–6.03 per 100,000 in 2003 in Japan, 9.1 per 100,000 in 2008 in Korea, and 1.61 per 100,000 in 2011 in Taiwan.5,6 Isolated stenoses at the middle cerebral artery (MCA) may present in young people and may eventually progress into Moyamoya disease, which is the most important reason of transient ischemia attack and stroke in children in East Asian. Primary pathological findings of Moyamoya disease at the stenotic portion are idiopathic fibrocellular thickening of the arterial intima, thinned and weakened medial layer, and decreased arterial outer diameter.7,8 When Moyamoya disease has some causative reasons or conditions, it is referred to as Moyamoya syndrome.2,9,10 Moyamoya syndrome is also known as Moyamoya phenomena, which is characterized by progressive steno-occlusive changes in the distal internal carotid artery, proximal middle cerebral artery, and anterior cerebral artery, with associated collateral vascular formation.11,12 The baseline diseases of Moyamoya syndrome may include meningitis, autoimmune disease, atherosclerosis, brain tumors, cranialirradiation, and traumatic brain injury.2,9,13 Ischemic events are the most important presentation of Moyamoya disease and syndrome caused by progressive occlusion of major cerebral arteries, leading to repeated hemodynamic transient ischemic attacks and strokes in children and young people as well. Surgical revascularization with direct extracranial–intracranial bypass and indirect pial synangiosis can be performed to prevent ischemic attacks and strokes by improving cerebral blood flow. However, surgical recanalization is associated with a significantly increased morbidity and mortality rate even though it is effective in preventing ischemic events. Endovascular stent angioplasty has been successfully applied in treating patients with intracranial arterial stenosis, achieving a good clinical outcome.14–16 However, Gross et al. suggested against attempted endovascular management of symptomatic Moyamoya disease because of relatively high rates of complication and recurrence after reviewing 17 patients with Moyamoya disease or syndrome who had been treated with 28 endovascular procedures, including stenting (n=11) and angioplasty alone (n=17).17 The high recurrence rate in this study may be caused by use of angioplasty alone which is not effective in maintaining the arterial lumen because of elastic recoil of the treated artery. It was hypothesized that endovascular stent deployment in patients with Moyamoya syndrome would be safe and effective in maintaining the arterial lumen and preventing ischemic events in the long run, and this study was consequently performed to investigate the clinical effect and safety of endovascular stenting on occluded intracranial arteries in Moyamoya syndrome.
Materials and methodsThis retrospective study was approved by the ethics committee of our hospital, and all patients had given their signed informed consent to participate. Between February 2018 and June 2019, patients with Moyamoya syndrome but without cerebral hemorrhage who had been treated with endovascular stenting were enrolled. The inclusion criteria were patients with Moyamoya syndrome who had recurrent ischemic neurologic symptoms under regular medication, with underlying atherosclerosis, and normal vascular beds distal to the location of arterial occlusion. The following data were recorded and analyzed: age, sex, comorbidities, clinical symptoms, medications, arterial occlusion location, size of the stent deployed, balloon used for dilatation of the lesion before stenting, instent stenosis or restenosis, periprocedural complications, angiographic and clinical follow-up outcomes (duration, instent restenosis, stroke, myocardial infarction or death).
Before the procedure of endovascular stent angioplasty, all patients were treated with dual antiplatelet medication of aspirin (100mg per day) and clopidogrel (75mg daily) for five days, and computed tomography (CT) scan was performed in all patients to confirm no presence of cerebral hemorrhage. Then, the endovascular procedure was performed under general anesthesia, and the Seldinger technique was used to puncture the femoral artery. After percutaneous access, a 6F introducer sheath was inserted into the femoral artery, and heparin was administered intravenously at a bolus dose of 70U/kg to achieve an activated coagulation time of 300–350s. Oxygen saturation, blood pressure, heart rate, neurologic state, and urine output were monitored throughout the whole procedure. After a 5F Navien guiding catheter (Medtronic Inc., Minneapolis, MI, USA) was navigated into the distal cervical internal carotid artery through the 6F introducer sheath, a micro-guidewire was sent to the occlusion lesion via the guiding catheter and explored carefully to pass through the occlusion site. During exploration using the micro-guidewire, the tip of the micro-guidewire was directed downwards rather than upwards. If the tip was directed upwards, the micro-guidewire could easily enter proliferated “smoking” vessels. Once the micro-guidewire was navigated through the occluded arterial segment, a micro-catheter was sent along the micro-guidewire across the occlusion before gentle angiography through the microcatheter was performed to confirm the presence of both the micro-guidewire and the micro-catheter in the distal artery. Then, the micro-catheter was withdrawn, and a Gateway balloon catheter (Gateway, Stryker) was sent along the micro-guidewire to the occlusion location and expanded to dilate the artery to approximately the normal diameter of the adjacent arterial segment. After balloon angioplasty, a stent was navigated to the site of occlusion and deployed. Post-stenting angiography and intraprocedural XperCT scan were performed to confirm patency of the stented artery, presence of blood flow to the distal arterial segment, and possible contrast extravasation or exudation. Then, the patient gradually waked up 4–5h after the endovascular procedure. The blood pressure was decreased to 80% that before the procedure to prevent hyperperfusion hemorrhage which might be caused by revascularization of the occluded artery.
After the endovascular stent angioplasty procedure, all patients were administered aspirin (100mg per day) and clopidogrel (75mg daily) for one year, and then, aspirin alone was taken orally for the whole life. Patients were followed up 3–6 months after the procedure for possible deaths, stroke, and transient ischemic attack (TIA). Cerebral angiographic follow-up using CT angiography, magnetic resonance imaging or digital subtraction angiography was conducted once between 3 and 12 months after stenting and once yearly afterwards to detect possible instent stenosis.
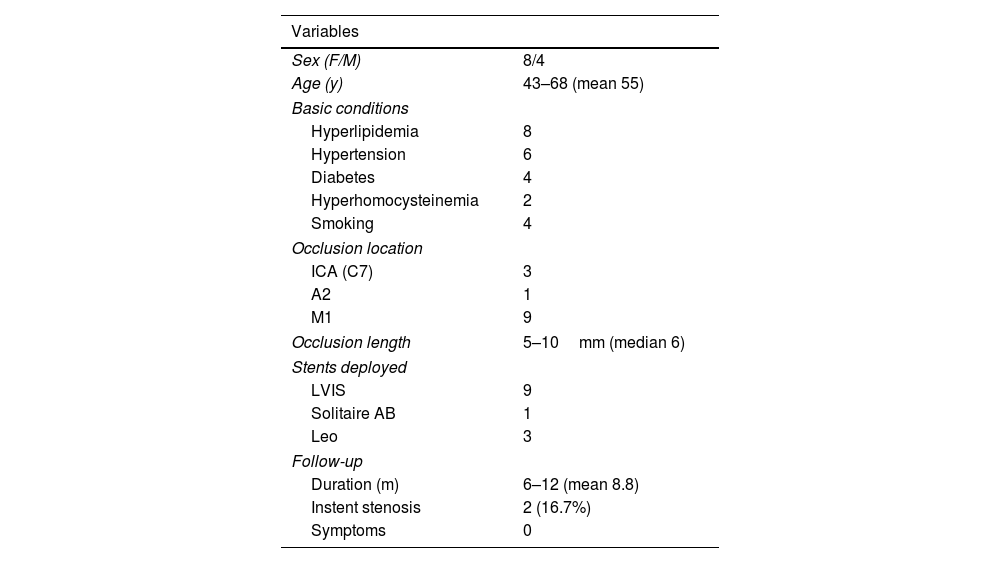
ResultsThere were 12 patients who were treated with stent deployment, including eight females and four males with an age range of 43–68 (mean 55) years (Table 1). The baseline diseases and conditions included hyperlipidemia in eight patients, hypertension in six, diabetes mellitus in four, hyperhomocysteinemia in two, and smoking in four. Two patients each had the following baseline conditions: hyperhomocysteinemia, hypertension, hyperlipidemia, and smoking.
Data of patients treated with stenting.
| Variables | |
|---|---|
| Sex (F/M) | 8/4 |
| Age (y) | 43–68 (mean 55) |
| Basic conditions | |
| Hyperlipidemia | 8 |
| Hypertension | 6 |
| Diabetes | 4 |
| Hyperhomocysteinemia | 2 |
| Smoking | 4 |
| Occlusion location | |
| ICA (C7) | 3 |
| A2 | 1 |
| M1 | 9 |
| Occlusion length | 5–10mm (median 6) |
| Stents deployed | |
| LVIS | 9 |
| Solitaire AB | 1 |
| Leo | 3 |
| Follow-up | |
| Duration (m) | 6–12 (mean 8.8) |
| Instent stenosis | 2 (16.7%) |
| Symptoms | 0 |
Note: ICA, internal carotid artery.
The occlusion was located at M1 segment of MCA in eight patients, at both M1 and A2 segments in one patient, and at the C7 segment of ICA in three. The median length of occlusion was 6mm (range 5–10mm). Thirteen stents were deployed at the occlusion location, including the low-profile visualized intraluminal support (LVIS) device (MicroVention, Tustin, CA, USA) in eight patients, a LVIS device and a Solitaire AB stent (ev3, Irvine, CA, USA) in one, and a Leo stent (Balt, Montmorency, France) in three, resulting in the success rate of 100% (Figs. 1–5). No intraprocedural complications were present. However, in one patient with occlusion of the left M1 segment, CT angiographic imaging after stenting revealed contrast exudation, which disappeared on the second day after stenting (Fig. 5), resulting in no clinical symptoms or sequela.
A patient in their 40s with diabetes and hypertension experienced intermittent weakness of right limbs and unclear speech 15 months ago, and the symptoms were deteriorated two months before admission. The muscle strength on the right limbs was level 4. (A) Cerebral angiography revealed that right middle cerebral artery (MCA) was occluded at the M1 segment with smoke-like collaterals which connected distal MCA segments. (B) A micro-guidewire was navigated to the occluded M1 segment for exploring the distal arterial segment. (C) The micro-guidewire was navigated through the occlusion site into the distal segment. (D) A balloon was sent along the micro-guidewire to the occluded location for dilatation. (E) A LVIS stent was deployed at the M1 segment. (F) Immediately after stenting, angiography revealed unobstructed M1 segment with good display of distal arterial branches.
Post-stenting and follow-up angiography in the same patient as in Fig. 1. (A&B) Computed tomography (CT) perfusion imaging showed improved blood perfusion in the left hemisphere immediately following stenting (A) compared with that before stenting (B). (C&D) CT angiography two months later demonstrated that the stent was unobstructed (C) at the left M1 segment compared with that before stenting (D). (E&F) Six months later, angiography revealed no obstruction nor stenosis at the left M1 segment.
A patient in their 50s with hypertension, hyperlipemia, hyperhomocysteinemia, and a smoking habit with 40 cigarettes/day experienced right limb weakness and dizziness two years ago and unclear speech one week before admission. The right hand had the muscle strength of level 4. (A) Cerebral angiography showed occlusion of the M1 segment of the left middle cerebral artery (MCA) with smoke-like arteries connecting distal branches. (B) A micro-guidewire was navigated through the occluded M1 segment into the distal segment. (C) After balloon dilatation, a LEO baby stent was deployed at the M1 segment. (D) After stenting, the occlusion was revascularized with no stenosis at M1 segment. (E&F) At six-month follow-up, the stent remained patent at the M1 segment of left MCA.
A patient in their 50s with hyperlipidemia had arterial occlusion at the M1 segment of the left middle cerebral artery (MCA) and the A2 segment of the left anterior cerebral artery (ACA). (A) Arterial occlusion was shown at left M1 and A2 segments. (B) A micro-guidewire was navigated through the A2 occlusion site into distal branches. (C) After angioplasty, a Solitaire AB stent was deployed. (D) After stent deployment, the A2 segment was recanalized, but the M1 segment remained occluded. (E) A micro-guidewire was navigated across the M1 occlusion into the distal branch. (F) After balloon angioplasty, a LVIS stent was deployed. (G) After stenting, the M1 segment was revascularized. (I) At one year, the A2 and M1 segments remained patent.
A patient in their 50s with hypertension and hyperlipidemia experienced occlusion of the M1 segment of the left middle cerebral artery (MCA). (A&B) Cerebral angiography revealed occlusion of the left M1 segment. (C) A micro-guidewire was navigated to explore the occluded M1 segment. (D) The micro-guidewire successfully passed through the occluded M1 segment. (E&F) After balloon angioplasty and stenting with a LVIS stent, the left M1 segment was successfully recanalized. (G) Immediately after the stenting procedure (Fig. G1), computed tomography angiography showed contrast exudation which disappeared on the second day (Fig. G2). (I) Six months later, cerebral angiography revealed slight asymptomatic instent stenosis (20%, arrow) compared with that immediately after stenting (E, arrow).
Angiographic follow-up was performed in all patients for 6–12 months (mean 8.8). Slight asymptomatic instent stenosis (20–30% stenosis, Fig. 5) was present in two patients (16.7%), resulting in an instent stenosis rate of 16.7%, and no new neurologic deficits were present in the other patients. All preoperative ischemic symptoms completely disappeared at follow-up.
DiscussionIn this study, the safety and effect of endovascular stent angioplasty for cerebral arterial occlusion in Moyamoya syndrome was investigated, and all occlusions or stenoses of cerebral arteries were safely and successfully recanalized. Followed up for 6–12 months, slight asymptomatic instent stenosis was present in two patients, no new neurologic deficits presented, and all preoperative ischemic symptoms completely disappeared.
In this study using endovascular stent deployment for cerebral arterial occlusion of stenoses in Moyamoya syndrome, the instent restenosis rate was low with only two patients harboring slight asymptomatic instent restenosis (20–30%%) at follow-up. This is quite different from that (70–90%) of Moyamoya disease treated with endovascular approaches,18 and the difference in the instent restenosis rate is primarily caused by varied pathologies in these two conditions. In a report using angioplasty and stenting for preventing ischemia in five patients with Moyamoya disease,18 two patients were treated with ICA balloon angioplasty and Wingspan stenting, one patient underwent ICA-M1 angioplasty alone without use of stenting, and the other two patients had M1 angioplasty and Wingspan stenting. After the endovascular treatment, all patients experienced repeated transient ischemic attacks in the vascular territories of treatment, and instent stenoses of 70–90% were revealed in these patients. Consequently, open surgical revascularization was performed in all these patients. Moyamoya disease and Moyamoya syndrome have different pathological presentations, which is probably the primary reason why a higher instent stenosis rate was present in Moyamoya disease after angioplasty with or without stenting, resulting in failure of treatment. The primary pathology of Moyamoya disease at the stenotic segment is idiopathic fibrocellular thickening of the intima, thinned and weakened medial layer, and decreased outer diameter of the artery, resulting in arterial stenosis.7,8 Moyamoya disease is increasingly demonstrated to be a proliferative disease of the intima, and histopathological research in the distal ICA with this disease revealed hyperplasia of endothelia and smooth muscle cells, with arterial stenosis or occlusion being related to progressive fibrocellular thickening of the intimal layer.19–21 For Moyamoya syndrome, the artery affected at the stenosis or occlusion has arteriosclerotic or inflammatory changes caused by the basic diseases or conditions rather than by persistent idiopathic fibrocellular thickening or proliferation of the intima and smooth muscle cells as in Moyamoya disease.17,20,22 The outer diameter of the stenotic or occluded artery does not decrease in Moyamoya syndrome, and stent angioplasty is thus effective in maintaining the arterial lumen after deployment, preventing further stenosis and ischemic events afterwards. In our study, we did not use balloon angioplasty alone which may cause a higher restenosis rate, because the expanded artery has no further support once the balloon is withdrawn, resulting in restenosis caused by recoil of the artery. All patients underwent stent deployment at the occlusion site, and the stent continued to support and prevent the artery from collapse afterwards. Another reason for a low instent stenosis rate in our study may be related to the shorter follow-up duration.
The indications for endovascular revascularization of intracranial arterial occlusion or stenoses in Moyamoya syndrome were atherosclerotic occlusion of arteries with proliferation of “smoking” vessels, existence of distal M1 stump, and distal normal vascular bed which is displayed via collateral circulations. However, the operators of endovascular treatment should have rich experience to revascularize occluded arteries, and high-resolution magnetic resonance imaging should be present to demonstrate the cross section of the occluded arterial segment. Arterial occlusion in Moyamoya syndrome may be caused by atherosclerosis-related diseases or conditions like diabetes mellitus, hyperlipidemia, hypertension, hyperhomocysteinemia, and smoking. Patients with Moyamoya syndrome may have neurological symptoms. If no symptoms exist, collateral circulations must be sufficient to compensate for the occluded artery with sufficient blood perfusion to the corresponding area, thus eliminating the necessity of endovascular revascularization of the occluded artery. After the procedure, all patients gradually obtained their consciousness 4–5h later, and the blood pressure was decreased to 80% that before the procedure to prevent hyperperfusion hemorrhage which may be caused by revascularization of the occluded artery. If the patient had acute cerebral infarction, revascularization of the occluded artery in Moyamoya syndrome should be performed three months later so as to prevent possible cerebral bleeding. These measures were effective to prevent cerebral bleeding caused by hyperperfusion after revascularization.
In the review of endovascular treatment of symptomatic Moyamoya disease or syndrome by Gross et al who suggested against attempted endovascular treatment for this disease or condition,17 instent restenosis was demonstrated in 66% for angioplasty alone and 70% for stenting. Angioplasty alone is not suitable for Moyamoya disease which has idiopathic fibrocellular thickening of the intimal layer, causing progressive arterial stenosis or occlusion.17,20,22 In nine patients with stenting in the ICA (n=4) and M1 segment (n=5) in the study by Gross et al.,17 eleven stents were deployed, including ten Wingspan and one AVEINX3 coronary stent. These stents have greater radial force and expanding strength and may cause greater damage to the vessel wall, resulting in intimal hyperplasia and subsequent instent restenosis. Even though these stents were an effective option for intracranial atherosclerotic stenosis, a high rate (31%) of instent restenosis and perioperative complications had been reported in stent angioplasty for intracranial atherosclerotic stenosis at angiographic and clinical follow-up.23–26 In our study, we used LVIS, LEO and Solitaire AB stents which are all self-expandable stents designed specifically for intracranial vasculature and cause less damage to the vascular wall. These stents were initially used for treating cerebral aneurysms, and the use for stent angioplasty in revascularizing occluded arteries in our study is not the nominal application. Most of the occlusions were located at M1 segment, and the LVIS stent was used in this location because it was supposed to have a good effect on repair of the arterial wall. In thinner arteries, the Solitaire stent was used because of its good trafficability to pass through thin arteries. The Leo stent was used without specific significance. In application of stent angioplasty for recanalizing occluded arteries, the balloon chosen for dilatation should be 0.1–0.2mm smaller than the diameter of adjacent normal artery for undersized dilatation, and a slightly oversized self-expandable stent should be chosen for treatment as suggested by Bose et al.27 These measures will effectively prevent damage to the vascular wall while maintaining the arterial lumen.
In our study, no periprocedural complications occurred even though contrast exudation took place in one patient on CT angiographic imaging immediately after stenting. The reasons for contrast exudation may include increased vascular permeability associated with arterial injury, which may be caused by ischemia or endovascular manipulation, sudden increase in local intraarterial pressure associated with contrast injection, and slow emptying of contrast media in the local area with bad collateral circulation. Dual antiplatelet therapy was used in our study. Clopidogrel is an antiplatelet drug commonly used to reduce the rate of procedure-related thrombotic ischemic complications. It has been reported that the response to clopidogrel varied among different patients.28 Although the benefit of dual antiplatelet therapy has been demonstrated in different studies, some patients still experienced repeated atherothrombotic events, with the rate of non-response to clopidogrel being 5–30%.28 In our study, we did not test the response of clopidogrel, however, no patients experienced ischemic events during and after the procedure or at follow-up, probably because there were only twelve patients enrolled. At follow-up, only slight asymptomatic instent stenosis occurred in two patients. The clinical outcome may also be related to follow-up duration, and in our study, the mean follow-up period was only 8.8 months. Longer follow-up duration is needed to monitor necessary changes in the stent after deployment to treat steno-occlusive arteries in Moyamoya syndrome.
There were some limitations in this study. The small cohort of patients limited its generalization. The single center study, retrospective nature, Chinese patients enrolled, short follow-up duration, and no control should all be resolved in the future with a prospective, controlled, and randomized study involving a larger cohort of patients and multiple centers for better outcomes.
In conclusion, Stent angioplasty can be applied safely and effectively to treat occlusion and stenosis of intracranial arteries in Moyamoya syndrome.
Ethical approvalAll procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consentInformed consent was obtained from all individual participants included in the study.
FundingNo funding was received for this study.
Conflict of interestThe authors declare that they have no conflict of interest.