Alemtuzumab is a monoclonal antibody targeting the CD52 receptor, and is approved for treating active relapsing-remitting multiple sclerosis (RRMS). It depletes T and B cells by binding to the CD52 receptor.
Adverse effects of alemtuzumab include infections, with the most frequent being nasopharyngitis, urinary tract infection, upper respiratory tract infection, and herpesvirus infection.1,2 Opportunistic infections after treatment have also been described, such as tuberculosis reactivation, listeria meningitis, and cerebral nocardiosis.3 Recent studies report 2 cases of cytomegalovirus (CMV) reactivation4 and a case of coinfection with CMV and Pneumocystis jirovecii.5 Another case of CMV infection was reported in the group receiving high-dose alemtuzumab (24 mg/kg) in a phase III clinical trial.6
We report the case of a patient with pneumonia due to coinfection with CMV and P. jirovecii after the first cycle of alemtuzumab.
The patient was a 39-year-old woman who was diagnosed with RRMS in 2009. She was initially treated with interferon beta 1b, which was switched to fingolimod after 9 months due to ineffectiveness. In 2014, the patient started treatment with natalizumab due to persistent disease activity. In 2016, she presented John Cunningham virus seroconversion, and treatment was changed to alemtuzumab for safety reasons.
Eight weeks before starting treatment with alemtuzumab, a complete blood count and a serology test including HIV, hepatitis, and varicella zoster viruses yielded normal results. A CMV IgG and IgM test showed negative results.
After a wash-out period of 7 weeks, a first cycle of alemtuzumab was administered over 5 consecutive days without event. Aciclovir at 200 mg/12 hours was started for prophylactic treatment of herpesvirus infection, and maintained for one month. Dietary recommendations to avoid listeriosis were given. A one-month follow-up blood analysis showed 3370 leukocytes/mm3, with 540 lymphocytes/mm3 and 6 CD4 + T cells/mm3. The remaining parameters, including platelets, biochemistry, thyroid hormones, and urine test results, were within normal ranges.
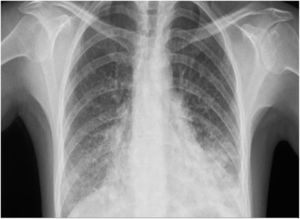
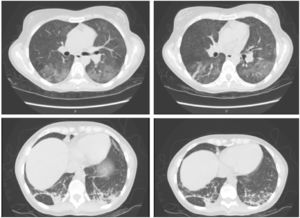
Five weeks after starting treatment with alemtuzumab, the patient attended the emergency department due to fever of up to 38 °C occurring in the evenings since the previous week, and dyspnoea, with a blood oxygen saturation of 93%. Physical examination revealed hypoventilation. A blood analysis revealed elevated transaminase and acute-phase reactant levels (aspartate transaminase 169 IU/L, alanine aminotransferase 163 IU/L, alkaline phosphatase 281 IU/L, gamma-glutamyl transpeptidase 513 IU/L, C-reactive protein 46 mg/dL). Leukocyte count was normal (1310 lymphocytes/mm3). A chest radiography revealed bilateral ground-glass opacification in the lungs (Fig. 1), which was subsequently confirmed by a chest computed tomography scan that also revealed left basal pneumonia (Fig. 2). Results from blood and urine cultures and an abdominal ultrasound were normal. A serology study returned positive results for CMV IgG and IgM antibodies, with the remaining values being normal.
Polymerase chain reaction (PCR) quantification of CMV DNA revealed 3893 copies/mL; the patient was admitted and started on treatment with valganciclovir dosed at 900 mg/12 hours and intravenous piperacillin/tazobactam dosed at 4/0.5 g/8 hours.
A culture of bronchoalveolar lavage was positive for P. jirovecii, and trimethoprim-sulfamethoxazole (TMP-SMX) dosed at 160/800 mg was added. Symptoms resolved after 10 days of treatment, but secondary prophylactic treatment with valganciclovir and TMP-SMX was maintained until the level of CD4 + T cells increased above 200/mm3. Quantification of CMV DNA was performed weekly, with positive results persisting for 7 weeks.
The patient currently presents no respiratory symptoms and is neurologically stable. The second cycle of alemtuzumab was also administered without event.
In addition to the case described above, a further 4 cases of patients presenting CMV infection after alemtuzumab treatment have been published.4,5,7 To our knowledge, this is the first case of primary infection with CMV, with positive results for CMV IgG and IgM antibodies approximately one month after onset of treatment with alemtuzumab. This is unusual since the prevalence of CMV seropositivity is approximately 60% in immunocompetent adults from developed countries.8,9
Coinfection with Pneumocystis spp. is frequent in these patients, since CMV infection enhances the adhesion and replication of other microorganisms.10
Cases have previously been reported of CMV reactivation in patients under treatment with alemtuzumab due to chronic lymphocytic leukaemia (CLL), with 4% to 29% of these patients being affected.11 Guidelines for the management of CMV infection in patients with CLL recommend that a serology test and PCR for CMV be performed before treatment with alemtuzumab is started, and weekly after treatment. In these patients, if PCR results for CMV are positive for 2 consecutive weeks, or if a single assessment with positive results is associated with clinical symptoms, it is recommended to start treatment with valganciclovir for 14 to 21 days (in symptomatic patients) or 7 to 14 days (asymptomatic patients), or until 2 CMV PCR tests return negative results.11
CMV infection is a potentially severe complication of treatment with alemtuzumab. With a view to its prevention or early diagnosis, we suggest quantifying CMV IgG and IgM antibodies before administering the treatment and subsequently in patients developing symptoms compatible with the infection. In our opinion, seronegativity for CMV should not rule out the administration of alemtuzumab if the risk/benefit ratio favours treatment; however, clinical vigilance should be increased to detect possible symptoms of primary infection, and serology tests should be performed in case of suspicion. PCR quantification of CMV DNA should be conducted in patients with seroconversion. As recommended by Clerico et al.,4 patients with positive PCR results should switch from aciclovir to ganciclovir or valganciclovir, as both of these treatments are effective against CMV. In these cases, the addition of prophylactic treatment with TMP-SMX should be considered to prevent coinfection with pneumocystis.
Please cite this article as: Eichau S, López Ruiz R, Castón Osorio JJ, Ramírez E, Domínguez-Mayoral A, Izquierdo G. Primoinfección por citomegalovirus en un paciente con esclerosis múltiple recurrente-remitente tratado con alemtuzumab. Neurología. 2020;35:440–443.