Many studies have described the presence of difficulty processing and generating social behaviour in patients who have suffered a traumatic brain injury (TBI). These difficulties in social cognition (SC) deteriorate personal relationships in the family, at work, or in the community. However, therapeutic programmes aiming to improve SC continue to be an outstanding issue in clinical practice. We performed a systematic review of the existing literature on the recovery of SC in patients with TBI, assessing the methodological quality of the included studies and the therapeutic effectiveness of the rehabilitation strategies used.
DevelopmentWe performed a bibliographic search of papers published before June 2018 in the Medline/PubMed, Google Scholar, PsycINFO, and ClinicalTrials.gov databases. Of the 198 potentially relevant articles, 10 met our eligibility criteria. Two of the authors independently and blindly assessed the methodological quality of these studies using the PEDro scale.
ConclusionsThe articles included in this systematic review essentially studied the effect of different interventions aimed at the rehabilitation of SC in patients with chronic TBIs. The analysis showed adequate methodological quality and an acceptable level of evidence. Future research should analyse the effect of these interventions in patients with TBIs in the sub- and post-acute phases.
Múltiples estudios han descrito la presencia de dificultades para procesar y generar conductas de tipo social en pacientes que han sufrido un traumatismo craneoencefálico (TCE). Tales dificultades, englobadas bajo el término genérico de cognición social (CS), provocan un deterioro en las relaciones personales, tanto a nivel familiar, como laboral o comunitario. No obstante, los programas terapéuticos dirigidos a la mejora de la CS continúan siendo una asignatura pendiente en la práctica clínica. El objetivo de este trabajo es realizar una revisión sistemática de la literatura existente sobre rehabilitación de la CS en pacientes con TCE, valorar su calidad metodológica y la efectividad terapéutica de las estrategias rehabilitadoras empleadas.
DesarrolloSe realizó una búsqueda bibliográfica hasta Junio de 2018 en las bases de datos Medline/PubMed, Google Scholar, PsycInfo y ClinicalTrials.gov. De los 198 artículos potencialmente interesantes, diez cumplieron los criterios de elegibilidad. Dos de los autores evaluaron, de forma independiente y ciega, la calidad metodológica de los estudios incluidos en la revisión mediante la escala PEDro.
ConclusionesLos artículos incluidos en esta revisión sistemática han estudiado esencialmente el efecto de diferentes intervenciones dirigidas a la rehabilitación de la CS en pacientes con TCEs en fase crónica. El análisis muestra que su calidad metodológica es adecuada y que el nivel de evidencia es aceptable. Se constata la necesidad de analizar el efecto de estas intervenciones en pacientes con TCEs en fase sub-aguda y post-aguda.
Humans are set apart from other animals by our capacity for social interaction. While other mammals (eg, bonobos, wolves, and dolphins) also exhibit this behaviour, its highest evolutionary expression is in humans. In this sense, the human brain areas linked to social interaction present considerable ontogenetic development.1 One factor explaining this development is the fact that throughout our evolutionary history humans have lived in communities and have needed to cooperate and compete with our peers to survive, which has led to increasingly complex interactions.2 This fundamentally social conception of humankind is nothing new: in classical Greece, Aristotle postulated the “natural sociability” of humans, whom he considered a social animal in that we need other members of our species in order to survive.3
This feature is highly characteristic of humans and constitutes a subject of interest in cognitive psychology. The construct of social cognition (SC) was proposed to define the processes responsible for the coding and decoding of social life.4 This difficult-to-define construct represents a specialised domain of general cognition, and is involved in solving social problems or processing social behaviour. The studies conducted in the late 1970s by the ethologists Premack and Woodruff5 constitute a significant development in this field of study. These researchers observed that chimpanzees are able to solve problems by making inferences from the behaviour of other individuals. Premack and Woodruff coined the term “theory of mind,” defined as the ability to attribute mental states to others and to predict their behaviour. Subsequently, Baron-Cohen et al.6 proposed that impaired theory of mind is the origin of the difficulties experienced by people with autism spectrum disorders when theorising about the minds of others and attributing external mental states.
However, SC is not limited to theory of mind; rather, this cognitive construct encompasses various other processes. For Adolphs,7 SC is a complex construct involving the coexistence of mechanisms for perceiving, processing, and evaluating social stimuli, enabling representation of the social environment and equipping the individual to respond appropriately to the situation. Through SC, we are able to recognise another person’s emotional state, to empathise with them, to put ourselves in their position (taking their perspective), and to glimpse the “hidden” motives behind their actions.8 To summarise, SC involves 3 fundamental elements: affect recognition, mentalisation (or theory of mind), and emotional regulation. Therefore, SC is responsible for processing both the initial or basic elements of social interactions or exchange (eg, emotional perception and recognition), and more advanced or complex elements, such as attributional style (the intentionality or causality attributed to an individual’s behaviour).9
SC is also an area of interest in the field of cognitive neuroscience. An important example is the socio-emotional processing model proposed by Ochsner.10 According to this model, SC involves 5 constructs: 1) acquisition of social-affective values and responses from the stimuli processed (associative conditioning between stimulus and emotional value); 2) recognising and responding to social-affective stimuli (facial or prosodic affect recognition, etc); 3) low-level mental state/trait inference (empathy); 4) high-level mental state/trait inference (theory of mind); and 5) context-sensitive regulation systems (regulating one’s own judgements and behaviours). Each of these constructs would have a specific neuroanatomical and neurofunctional substrate.
SC impairment is common in numerous neurological and psychiatric disorders, including traumatic brain injury (TBI) and other forms of acquired brain injury (ABI),11,12 frontotemporal dementia,13,14 schizophrenia,15,16 and autism spectrum disorders, as mentioned above.6
Several studies have described facial affect recognition difficulties in patients with TBI.17,20 According to a meta-analysis by Babbage et al.,21 39% of patients with severe TBI present severe facial affect recognition difficulties, particularly with regard to negative facial expressions.22,23 These problems are not limited to facial cues: difficulties with perceiving emotional prosody are also reported.18,24 Patients with TBI also exhibit impairment of theory of mind. Martín-Rodríguez and León-Carrión25 conducted a meta-analysis focused on patients with ABI (TBI in approximately half of cases), corroborating the existence of these deficits. The authors report a significant correlation between presence of ABI and moderate or severe alterations in tasks assessing theory of mind. The fact that TBI is the leading cause of death in people younger than 45 years, and the main neurological cause of disability associated with long life expectancy,26 demonstrates the scale of the healthcare, economic, and social problem of the association between this type of injury and SC impairment.
At a functional level, it is indisputable that the difficulties discussed above affect the day-to-day lives and family environment of patients with TBI. According to McDonald,27 the affect recognition difficulties experienced by patients with severe TBI result in inability to respond appropriately to other people’s reactions, to interpret feedback from interlocutors on whether or not their behaviour is appropriate, and to fully understand communication with other people. The combination of these difficulties results in the deterioration of social, family, workplace, and community relationships, leading to distress on the part of the patient’s family members, unemployment, and social isolation.28 SC impairment worsens if patients with TBI remain untreated,29 resulting in significant (and increasing) economic and social problems. Despite the evident need to include interventions targeting SC in all neurorehabilitation programmes, this remains an outstanding issue in the treatment of patients with TBI. These interventions have yielded promising results in other clinical conditions, such as schizophrenia,30 autism spectrum disorders,31 and intellectual disability.32 In fact, a limited number of studies in the field of TBI have shown positive results with rehabilitation treatments for SC.33 These circumstances demonstrate the need to consider the rehabilitation of affect perception and recognition, as well as mentalisation, as an essential objective in the design of holistic neurorehabilitation programmes for these patients.
We deemed it important to conduct a systematic review of the literature on the rehabilitation of SC in patients with TBI, generating a summary of the different approaches used to date. Our objective is to identify which rehabilitative treatments have been used, the degree of evidence supporting them, and what types of patients may benefit from them, with a view to guiding the work of professionals involved in the treatment of these deficits.
Material and methodsLiterature search strategyWe searched for scientific articles on the Medline/PubMed, Google Scholar, PsycInfo, and ClinicalTrials.gov databases.
We used the following search terms, combined with the Boolean operators AND and OR, as follows: “social cognition” OR “affect recognition” OR “emotional recognition” OR “emotion perception” OR “social functioning” OR “emotional processing” OR “emotion recognition” OR “emotional prosody” AND “brain injury” AND “treatment.”
Results were not limited according to year of publication. Therefore, the period searched had no start date, and the end date was June 2018.
Selection criteriaInclusion criteria were as follows: 1) articles published in English or Spanish; 2) samples of patients aged over 18 years and diagnosed with TBI; 3) focus on the treatment of SC in patients with TBI (at least in the majority of participants); 4) randomised clinical trials, case reports, or cohort studies; and 5) completed, published studies (specifically for studies published on ClinicalTrials.gov). We excluded studies meeting the following criteria: 1) studies including mainly patients with non-traumatic brain injury (eg, stroke or tumours); 2) studies not focusing on the rehabilitation of SC; 3) narrative or systematic reviews and meta-analyses; 4) theoretical articles and chapters of books.
Assessment of methodological qualityWe evaluated the methodological quality and risk of bias of articles meeting the selection criteria using the validated Spanish-language version of the Physiotherapy Evidence Database (PEDro) scale.34
The PEDro scale includes 11 items evaluating various aspects related to internal and external validity and interpretability. We assessed all items but the first (selection criteria) to calculate the total PEDro score. Therefore, the maximum possible score was 10 points. Each study’s methodological quality was assessed using the classification proposed by the ERABI Research Group35 and rated as excellent (9-10 points), good (6-8), acceptable (4-5), or poor (< 4). We determined the level of evidence according to the system proposed by Sackett et al.36 (Table 1).
Levels of evidence.
| Level | Study design | Description |
|---|---|---|
| Level 1a | RCT | More than one RCT scoring ≥ 6 on the PEDro scale. Including comparisons between subjects in randomised conditions and cross-over designs. |
| Level 1b | RCT | RCT scoring ≥ 6 on the PEDro scale |
| Level 2 | RCT | RCT scoring < 6 on the PEDro scale |
| Prospective controlled trial | Prospective controlled trial (not randomised) | |
| Cohort study | Prospective longitudinal study using at least 2 similar groups, one of which is exposed to a specific condition | |
| Level 3 | Case-control study | Retrospective study comparing conditions, including historic cohorts |
| Level 4 | Pretest-posttest study | Prospective study with pre-treatment measurement, an intervention, and post-treatment measurement in one group of subjects |
| Posttest only | Prospective intervention study including one or more groups with post-treatment measurement (no pre-treatment measurements or measurements establishing a baseline) | |
| Case series | Retrospective study, generally collecting data from a review of clinical cases | |
| Level 5 | Observational study | Cross-sectional analysis to interpret associations |
| Clinical consensus statement | Expert opinion without explicit critical evaluation, based on physiology, biomechanics, or “basic principles” | |
| Case study | Pre-post study of a single case |
RCT: randomised controlled trial.
The methodological quality of the studies reviewed was independently evaluated by 2 blinded researchers (PRR and DLC). Inter-rater agreement was evaluated by determining the intraclass correlation coefficient, calculated with version 18 of the MedCalc statistics package (https://www.medcalc.org/).
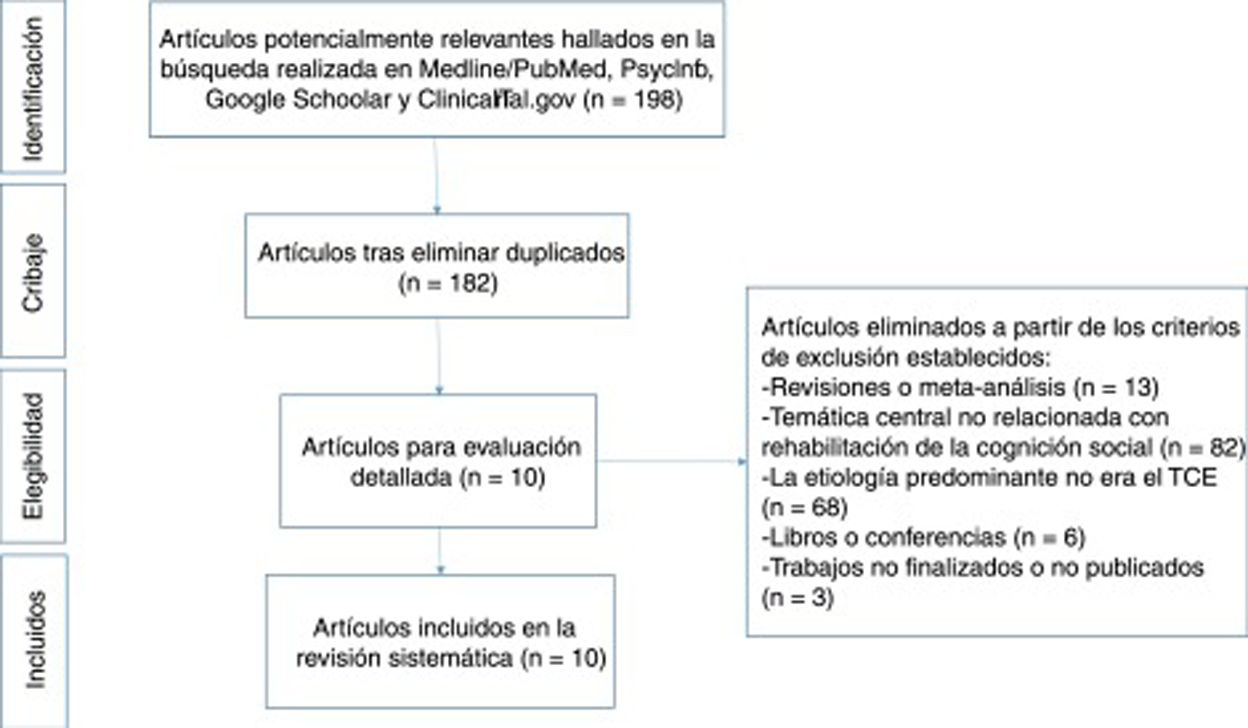
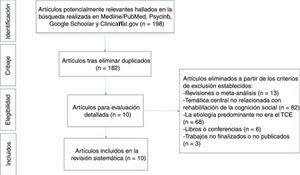
ResultsDescription of studiesThe literature search of the 4 databases cited above yielded a total of 198 results meeting the search criteria. After eliminating duplicate results, 182 articles remained, 10 of which met the eligibility criteria and were read and analysed in full text. This final sample included 6 randomised clinical trials (RCT),33,37–41 one prospective controlled trial (PCT),42 and 3 case studies.43–45Fig. 1 shows the review process followed, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) model.46
The studies reviewed include a total of 260 participants, 146 of whom were included in experimental groups and 107 in control groups (75 patients with ABI [mainly TBI] and 32 healthy controls). The 7 remaining patients were described in case studies.43–45Table 2 provides details of study design, sample characteristics, and the duration of interventions in each article.
Characteristics of the studies reviewed.
| Study | Design | Sample | Age, years (SD) | Progression time, years (SD) | Treatment intensity and duration |
|---|---|---|---|---|---|
| Westerhof-Evers et al.37 | RCT | N=59 TBIEG (TScEmo): 30 (72% M, 28% W)CG (CogniPlus): 29 (93% M, 7% W) | 43.2 (13)43.8 (13)42.3 (14) | 8.1 (8.2)7.1 (7.1)9.0 (9.2) | 16-20 sessions; 1h/week |
| Winegardner et al.43 | CS | 1 TBI (M)1 stroke (M) | 3746 | 2411 | 1 weekly 1-h session (6 weeks) |
| Neumann et al.38 | RCT | N=71 TBIEG (FAR): 24 (97% M, 3% W)EG (stories): 23 (78% M, 22% W)CG: 24 (67% M, 33% W) | 39.8 (12.0)41 (11.6)41.5 (11.6)39.5 (10.3) | 10.3 (8.9)12.3 (1.7)13.2 (2.8)12.6 (2.7) | 3 weekly 1-h sessions (3 weeks) |
| Williamson and Isaki44 | CS | 1 TBI (M)1 TBI (M) | 5344 | 137 | 2 weekly 1-h sessions (3 weeks)1 weekly 1-h session (3 weeks) |
| McDonald et al.39 | RCT | N=20 (16 TBI, 3 stroke, 1 other)EG: 10 (60% M, 40% W)CG: 10 (90% M, 10% W) | 42.6 (11.2)44.5 (12.7)46.6 (10.1) | 9.4 (7.7)10.4 (7.7)8.3 (8.0) | 3 2-h sessions |
| McDonald et al.42 | PCT | N=54 (22 TBI)EG: 22 TBI (73% M, 27% W)CG: 32 healthy controls (65% M, 35% W) | 42.2 (12.6)45.4 (12.8) | 10.8 (11.3) | Single 1-h session per condition (spontaneous/focus/mimic); 3h total |
| Radice-Neumann et al.40 | RCT | N=19 ABI (17 TBI, 2 other)EG (FAR): 10 (90% M, 10% W)EG (stories): 9 (33% M, 67% W) | 4347 (6.3)38 (14.3) | 1216 (8.5)8 (7.3) | 3 weekly 1-h sessions; 6 or 9 sessions total |
| Bornhofen and McDonald41 | RCT | N=18 TBIEG (EL): 6EG (SIT): 6CG (WL): 6 | 43.7 (12.3)35.4 (14.0)31.2 (16.8) | 5 (4.0)6.63 (4.6)12.35 (11.4) | 1 weekly 2.5-h session (10 weeks) |
| Bornhofen and McDonald33 | RCT | N=12 TBI (91% M, 9% W)EG: 5 (1 drop-out)CG (WL): 6 | 35.83 (13.0)29.2 (4.49)43.5 (14.43) | 7.8 (6.01)6.6 (5.9)9.7 (6.2) | 2 weekly 1.5-h sessions (8 weeks) |
| Guercio et al.45 | CS | 1 TBI (M)1 TBI (M)1 encephalitis (M) | 192719 | 6 months3 months2 months | 1 session of 15-30min duration per condition (4 conditions:label original pictures/label updated pictures/match updated to original picture/match original to updated picture) |
ABI: acquired brain injury; CG: control group; CS: case study; EG: experimental group; EL: errorless learning; FAR: facial affect recognition; M: man; PCT: prospective controlled trial; RCT: randomised controlled trial; SIT: self-instruction training; TBI: traumatic brain injury; W: woman; WL: wait-list.
Inter-rater agreement (intraclass correlation coefficient) for total PEDro scale score was 0.99 (95% confidence interval, 0.96-0.99). Tables 3 and 4 show total and item PEDro scores for each study according to both raters, the level of evidence, and the effect of the treatment applied. The methodological quality of 3 RCTs37,38,41 was excellent; the remaining 3had good methodological quality.33,39,40 The only PCT42 reviewed also showed good methodological quality. The methodological quality of the case studies43–45 could not be assessed with the PEDro scale: given the considerable risk of bias inherent to this type of study, administration of the scale is not recommended. None of the studies analysed is registered on the clinicalTrials.gov database or any other registry enabling the risk of bias to be checked.
Methodological quality (PEDro scale scores) of the studies reviewed.
| Study | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PEDro score | Westerhof-Evers et al.37 | Winegardner et al.43 | Neumann et al.38 | Williamson and Isaki44 | McDonald et al.39 | McDonald et al.42 | Radice-Neumann et al.40 | Bornhofen and McDonald41 | Bornhofen and McDonald33 | Guercio et al.45 |
| 1. Eligibility criteria specified | 1 | NA | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA |
| 2. Random allocation | 1 | NA | 1 | NA | 1 | 0.5 | 1 | 1 | 1 | NA |
| 3. Concealed allocation | 1 | NA | 1 | NA | 1 | 0.5 | 1 | 1 | 1 | NA |
| 4. Groups similar at baseline | 1 | NA | 0 | NA | 0 | 1 | 0 | 1 | 1 | NA |
| 5. Participant blinding | 1 | NA | 1 | NA | 1 | 0.5 | 1 | 1 | 0 | NA |
| 6. Therapist blinding | 0 | NA | 0.5 | NA | 0 | 0 | 0 | 0 | 0 | NA |
| 7. Assessor blinding | 0.5 | NA | 1 | NA | 1 | 0.5 | 0 | 1 | 0 | NA |
| 8. Less than 15% drop-outs | 1 | NA | 1 | NA | 1 | 1 | 1 | 0.5 | 1 | NA |
| 9. Intention to treat analysis | 1 | NA | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA |
| 10. Between-group statistical comparisons | 1 | NA | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA |
| 11. Point measures and variability data | 1 | NA | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA |
1: both raters considered the study to meet this criterion; 0.5: disagreement between raters as to whether the study met the criterion; 0: both raters considered the study not to meet the criterion.
NA: not applicable.
Methodological quality, level of evidence, and effect of treatment for each study.
| Study | PEDro score (rater 1/rater 2) | Methodological quality (PEDro) | Level of evidence | Effect of treatment |
|---|---|---|---|---|
| Westerhof-Evers et al.37 | 9/8 | Excellent/good | 1a | Positive |
| Winegardner et al.43 | NA/NA | NA | 5 | Partially positive |
| Neumann et al.38 | 8/9 | Good/excellent | 1a | Partially positive |
| Williamson and Isaki44 | NA/NA | NA | 5 | Positive |
| McDonald et al.39 | 8/8 | Good | 1a | Partially positive |
| McDonald et al.42 | 6/6 | Good | 2 | Negative |
| Radice-Neumann et al.40 | 7/7 | Good | 1a | Positive |
| Bornhofen and McDonald41 | 9/8 | Excellent/good | 1a | Partially positive |
| Bornhofen and McDonald33 | 7/7 | Good | 1a | Positive |
| Guercio et al.45 | NA/NA | NA | 5 | Partially positive |
Treatment effects. Positive: treatment shows benefits; partially positive: treatment shows relative benefits; negative: treatment shows no benefits.
NA: not applicable.
The interventions applied in the different studies involved a combination of different treatments, including: focused attention (7 studies37–40,42,44,45), mimicry (4 studies37,38,40,42), errorless learning (3 studies33,39,41), self-instruction (3 studies33,37,41), massed and distributed practice (2 studies33,39), perspective-taking (2 studies37,43), vanishing cues (one study38), psychoeducation (one study37), and social skills training (one study37). Table 5 summarises the treatments used in each study.
Summary of the interventions used in each study.
| Focused attention | Mimicry | Errorless learning | Self-instruction | Massed and distributed practice | Perspective-taking | Vanishing cues | Psychoeducation | Social skills training | |
|---|---|---|---|---|---|---|---|---|---|
| Westerhof-Evers et al.37 | X | X | X | X | X | X | |||
| Winegardner et al.43 | X | ||||||||
| Neumann et al.38 | X | X | X | ||||||
| Williamson and Isaki44 | X | ||||||||
| McDonald et al.39 | X | X | X | ||||||
| McDonald et al.42 | X | X | |||||||
| Radice-Neumann et al.40 | X | X | |||||||
| Bornhofen and McDonald41 | X | X | |||||||
| Bornhofen and McDonald33 | X | X | X | ||||||
| Guercio et al.45 | X |
Westerhof-Evers et al.37 performed a study evaluating the effects of a multifaceted approach (Treatment for Social Cognition and Emotion Regulation; T-ScEmo) in patients with TBI. T-ScEmo aims to treat SC through strategies to improve perception of social information, social understanding or the ability to mentalise, and the ability to regulate social behaviour. The group receiving T-ScEmo (n=30) was compared to a control group (n=29) of patients who received computerised non-social cognitive training (CogniPlus). According to the results of the study, the multifaceted treatment is effective for improving aspects of facial affect recognition and theory of mind, and increased societal participation and the level of empathy in social relationships.
Winegardner et al.43 trained 2 patients with anger and irritability problems in perspective-taking. The authors hypothesise that the irritability problems in their patients, and in patients with ABI in general, are largely explained by a hostile attributional style in their interpretation of the intentionality of others’ behaviour. Treatment involves training in cognitive reappraisal strategies and perceptual positioning through video and role-playing. The authors compared Aggression Questionnaire-Short Form47 and Interpersonal Reactivity Index48 scores from before and after treatment against normative data. Both patients showed reduced aggressiveness after the intervention.
In the RCT by Neumann et al.,38 71 patients with moderate or severe TBI were allocated to receive either facial affect recognition training (focused attention and mimicry) (FAR group; n=24); emotional inferencing training through the use of stories (stories group; n=23); or online tasks addressing other aspects of cognition (control group; n=24). Patients were assessed immediately after completing treatment and at 3 and 6 months. Patients in the FAR group scored significantly better than controls on measures of facial affect recognition. No differences were detected between the control group and the stories group.
Williamson and Isaki44 reported the cases of 2 patients with chronic TBI who received rehabilitation focused on improving facial affect recognition. The treatment involved several exercises: identifying emotions through static facial expressions, reflecting on personal experience of emotions, identifying sarcasm and emotions in social stories, and role-playing. The intervention was applied over the telephone; this was the only study we identified that used telemedicine to treat SC. After treatment, both patients showed improvements in the assessment measures used.
The RCT by McDonald et al.39 addresses the recognition of affect in verbal communication. In that study, the experimental group included 10 patients with ABI (mainly TBI), and the control group included 10 patients with similar characteristics who were on the wait list for rehabilitative treatment. The authors aimed to assess the effectiveness of a brief treatment for the rehabilitation of prosodic emotion recognition. The treatment involved 3 group tasks of increasing difficulty and individual exercises for home practice. The activities focused on establishing a common emotional vocabulary by discussing the meaning of the 6 basic emotions described by Ekman49 and addressing in greater detail the adjectives and behaviours associated with each. Subsequently, the treatment aimed to improve patients’ ability to distinguish between prosodic patterns associated with the different emotions through modelling, audio analysis, role-play, and production games. While no statistically significant differences were observed between groups, individual analysis revealed significant improvement in prosody recognition.
Another study by McDonald et al.42 had less promising results. In this PCT, 22 patients with TBI underwent 2 interventions that have previously shown good results for patients with other conditions.50 Specifically, the researchers instructed patients to focus their attention on or to mimic relevant facial expressions. Both techniques were applied to 22 patients with TBI and 32 healthy controls. Two surprising findings were reported: no significant differences in facial affect recognition were observed between groups prior to treatment, and the TBI group showed no benefit with either of the interventions. The authors suggest that the findings may be explained by additional “consumption” of attentional resources arising from the use of these strategies in patients with severe cognitive deficits, which could have a detrimental effect on and interfere with affect recognition. This is the only study to report that the treatment applied to the experimental group had no beneficial effects.
Radice-Neumann et al.40 randomly allocated 21 patients with ABI (19 with TBI and 2 with other causes) to 2 experimental groups. The first group (FAR; n=10) received a treatment aiming to improve facial affect recognition by training patients to focus attention on relevant visual information and to analyse and understand their own emotional experiences. The other group (stories of emotional inference group; n=9) received specific training to identify emotional responses through contextual cues in stories, and were asked to relate these stories to emotional events from their own lives. Patients in the FAR group showed significant improvements in facial affect recognition, ability to describe how they or others would feel in a hypothetical scenario, and socio-emotional behaviour (as reported by family members). Members of the stories group showed less pronounced improvements, although they did show an increase in the ability to make emotional inferences about how they would feel in a given context or scenario. The results suggest that patients with ABI are able to relearn affect recognition skills.
Bornhofen and McDonald41 conducted an RCT assessing the usefulness and effectiveness of 2 types of treatment strategies in the rehabilitation of affect recognition impairment in patients with TBI. Participants were allocated to 3 intervention groups: errorless learning (n=6), self-instruction training (n=6), and a wait-list control group (n=6). The effectiveness of the treatments was evaluated according to patients’ ability to identify emotions through visual cues, using both static and audiovisual (video) materials, and through social inferences based on emotional behaviour. After the intervention, patients in both the errorless learning and the self-instruction training groups showed improved ability to recognise emotions in photographs of faces, although the improvement in social inferences was only significant in patients receiving self-instruction training. Caution should be exercised in the interpretation of these results due to the small sample size.
The same researchers conducted another RCT with 12 patients with chronic TBI.33 Patients were allocated either to the wait-list control group (n=6) or to receive an intervention involving the application of several rehabilitation techniques to improve affect recognition (n=5; 1 drop-out). The treatment involved training participants in the perception and interpretation of static and dynamic emotional cues. The intervention was structured as follows: interpreting conventional emotional contexts; interpreting static visual cues; interpreting dynamic uni- or multimodal cues; and training in social inferences based on emotional behaviour and situational cues. The treatment was based on errorless learning, self-instruction, and massed and distributed practice in emotional discrimination tasks. The experimental group showed significant improvements with respect to the control group both in accuracy in judging dynamic cues related to basic emotions and in the ability to make emotional inferences to determine whether a person was being sarcastic, sincere, or deceptive.
Guercio et al.45 trained 3 patients (2 with TBI) in affect recognition through matching images of equivalent facial expressions. The cues used were photographs of faces with angry, sad, and happy expressions. Patients’ ability to label facial affect and to match expressions was evaluated before and after the training. The results show an improvement in patients’ facial affect recognition skills. One of the main methodological issues in this study is that the treatment phase used the same cues as the pre- and post-treatment assessments; this, combined with the small sample size, represents a limitation for extrapolating the results.
It should be noted that all studies analysed in this review were performed independently, despite some being conducted by the same authors.
DiscussionThe aim of this study is to present a systematic review of the literature on the rehabilitation of SC in patients with TBI. The study has several objectives: to establish the amount of work published to date on this topic; to evaluate the methodological quality of the research; and to assess the level of evidence of the interventions used.
An initial overview shows that little research has been done on this subject. We only identified 10 studies meeting the inclusion criteria. Of these, 6 were RCTs,33,37–41 one was a PCT,42 and 3 were case studies.43–45
The methodological quality of RCTs33,37–41 and the PCT42 ranged from good to excellent. The case studies43–45 were not evaluated with the PEDro scale and thus were considered to have poor methodological quality. According to the system proposed by Sackett et al.,36 the levels of evidence were level 1a for the RCTs,33,37–41 level 2 for the PCT,42 and level 5 for the case studies.43–45 However, it should be noted that the methodological quality of the articles was evaluated with the PEDro scale only, and other factors potentially affecting quality (eg, sample size) were not taken into account. Therefore, the levels of evidence established require clarification.
Nine studies33,37–41,43–45 report positive or partially positive results after treatment. This suggests that it is possible to improve SC in patients with TBI through the application of specific techniques by properly trained professionals. The PCT42 was the only study that did not find significant differences between the experimental and control groups.
A more detailed evaluation of the analysis performed and the characteristics of participating patients raises the following questions: is the effect of treatment affected by the stage of progression of TBI? What is the optimal duration and intensity of treatment? Should treatments be applied in isolation or in combination?
Early onset of rehabilitation treatment has classically been considered a positive prognostic factor for patient recovery.51–53 León-Carrión and Machuca54 report that spontaneous recovery ceases 8 months post-trauma; from that time, practically no cognitive improvement is observed without rehabilitation treatment. Similarly, other studies have shown that specialised treatments may lead to significant improvements in the functions targeted, even when treatment is applied years after trauma.55 The authors of the latter study posit that specialised interventions affect brain plasticity and cause changes even after spontaneous recovery has ceased. However, other studies describe a plateau phase in recovery,56,57 or even a tendency toward functional worsening over time,58 independently of treatment.
In the light of the observations discussed above, any systematic review must analyse the role of progression time. In 9 of the 10 studies analysed in this review, interventions were applied to patients with progression times ranging from 2.5 to 24 years; despite the inclusion of chronic patients, all studies (except for the PCT by McDonald et al.42) report positive changes after treatment. These results support the hypothesis, also proposed by Machuca et al.,59 that specific treatment performed by properly trained professionals can lead to the recovery of cognitive functions (and therefore SC), even in chronic patients. Another noteworthy observation is the lack of studies analysing the benefits of SC rehabilitation in patients with subacute and post-acute TBI. The positive results described in chronic patients should be replicated in patients with shorter progression times. In the light of this hypothesis, there is a clear need to implement SC rehabilitation programmes early after trauma as part of any holistic neurorehabilitation treatment. This represents a new field of research, as no study to date has addressed SC rehabilitation in patients with subacute and post-acute TBI.
The literature includes several studies reporting a direct relationship between treatment intensity and cognitive improvement.60–65 According to León-Carrión et al.,61 patients with TBI must receive more than 300 hours’ intensive rehabilitation therapy in order to obtain favourable outcomes. Positive associations are also reported between post-treatment improvements and treatment duration. Sandhaug et al.66 observed that longer stays at rehabilitation units were associated with better functional status at discharge. This improvement with longer treatment durations is even observed in chronic patients. It is also reported that patients with more severe TBI require greater treatment intensity and duration, in order to obtain better outcomes. This is exemplified in the study by Ashley et al.,67 which included patients with TBI of over one year’s progression: patients with mild-to-moderate disability (Disability Rating Scale68) showed improvements after 90 days of rehabilitation, whereas those with severe disability needed at least 180 days of treatment to achieve similar outcomes.
The results discussed in the previous paragraph may be interpreted differently: patients with greater initial severity will spend the most time receiving high-intensity rehabilitation. As these patients will have a poorer cognitive status at treatment onset, they will also be those who show the greatest changes after prolonged, intensive treatment. Hart et al.69 analyse the differences in treatment duration and intensity between the healthcare systems of 2 countries with similar characteristics (Denmark and the United States of America). Contrary to expectations, no differences were observed between Danish patients (who received more rehabilitation during the first year post-trauma) and American patients (who received less treatment) after the groups were adjusted to account for initial TBI severity.
The articles reviewed here report disparate results, and no firm conclusions can be drawn. Treatment duration ranged from 2hours (4 experimental conditions of 30minutes’ duration each in a single session),45 for the least intense intervention, to 25hours (1 weekly session of 2.5hours over 10 weeks).41 The changes observed seem to present no clear correlation with treatment intensity. However, it should be noted that the only study not clearly demonstrating positive results used a very low-intensity treatment (a total of 3hours).42
Regarding the benefits of monotherapy or combined therapy, current clinical practice guidelines recommend integrated, holistic neuropsychological rehabilitation addressing cognitive and functional deficits in patients with moderate or severe TBI.64 Our results on this issue are unclear. They do not support a clear relationship between treatment effectiveness and the number of techniques used. We may only indicate that the study using the greatest number of techniques37 obtained clearly positive outcomes, whereas the 3 studies using monotherapy43–45 obtained partially positive results in 2 cases and positive results in just one. Incidentally, the 3 studies using monotherapy are the 3 case studies reviewed.
The reviews published on the rehabilitation of SC in patients with TBI19,70–72 do not evaluate the methodological quality or level of evidence of the interventions applied. These reviews aimed to describe the available studies and to reflect on the mainly positive results presented in our “Summary of results” section. However, one review does question the benefits of treatment due to the methodological characteristics of the studies.
Similarly, and as commented above, we should be very cautious when interpreting results about the level of evidence of the different studies. Many of them used small patient samples, resulting in reduced statistical power for hypothesis testing. With small samples, statistically significant results can only be detected for very large effect sizes. However, clinical experience shows that effect sizes for this type of intervention tend to be small. Therefore, the fact that the studies reviewed include small samples but mainly report positive outcomes may suggest the possibility of false positives in the results, with the actual level of evidence being lower than the proposed level. In addition to this, the fact that some studies analyse numerous outcome measures, or have multiple arms, considerably increases the need to be cautious in our interpretation of the results reported.
In summary, these studies’ level of evidence may have been exaggerated. One possible solution would be the use of some methodology accounting for these variables when establishing the level of evidence. We should therefore temper our optimism about the effects and levels of evidence reported. Similarly, these considerations should be taken into account when interpreting the assertions made above. In accordance with the issues addressed above, it would be appropriate to indicate that the studies analysed present correct methodological quality and acceptable levels of evidence.
However, a significant limitation of this review is the small number of studies addressing SC rehabilitation in patients with TBI. Given this situation, we are unable to offer a clear response to the issues raised throughout the discussion. Future studies should aim to establish guidelines for the application of rehabilitation therapies for SC in patients with TBI. However, we must also stress that this study mainly aimed to establish the “doses” used (intensity, frequency, duration) and patient characteristics, rather than focusing on what Whyte et al.73 describe as the “active ingredients” of the treatment: the techniques used (content) and the process by which they are applied.
The main international guidelines on systematic reviews (eg, the PRISMA guidelines46) recommend that they be registered on public databases (eg, PROSPERO74) in order to ensure the transparency and replicability of the study performed. Similarly, data should be extracted by 2 independent researchers. Our study meets neither of these criteria; this limitation should be taken into account in future reviews.
Finally, we should mention the limitations inherent to evidence-based practice in neurorehabilitation. Evidence gives general guidance on how to treat patients; therefore, while treatment of patients should follow the available evidence, therapists are responsible for deciding how to treat patients as individuals.
ConclusionsThis systematic review demonstrates that few studies have addressed SC rehabilitation in patients with TBI, although the experimental studies analysed present correct methodological quality and an acceptable level of evidence, which suggests that the treatments administered are largely effective. Another issue that should be highlighted is the fact that the majority of rehabilitation strategies used improve SC following TBI, despite the inclusion of chronic patients in these studies.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank Olga Araujo (Centre de Documentació Santi Beso Arnalot, Institut Guttmann) for her assistance in the literature search. We are also grateful to the reviewers for their comments, which undoubtedly improved the quality of the manuscript.
Please cite this article as: Rodríguez-Rajo P, Leno Colorado D, Enseñat-Cantallops A, García-Molina A. Rehabilitación de la cognición social en el traumatismo craneoencefálico: una revisión sistemática. Neurología. 2022;37:767–780.