Cervical artery dissection (CAD) is responsible for up to 20% of all ischaemic strokes in patients younger than 45. The benefits of acute-phase reperfusion therapy in these patients have yet to be confirmed.
MethodsWe conducted a retrospective review of patients with CAD admitted to a comprehensive stroke centre between 2010 and 2015. We recorded baseline clinical characteristics, treatments, functional outcomes, and mortality.
ResultsWe identified 35 cases of CAD (23 carotid/12 vertebral); mean age was 43.5±9.5 years and 67.7% were men. Ten patients (32.3%) had a history of trauma. The most frequent risk factors were arterial hypertension (29%) and smoking (35.5%). The most common clinical presentation was ischaemic stroke (29 patients, 93.5%). The median baseline National Institute of Health Stroke Scale score was 6 (range, 0-41). The most frequently used diagnostic method was CT angiography (74.2%), followed by MRI (64.5%) and digital subtraction angiography (45.6%). Seven patients (22.6%) were treated with intravenous fibrinolysis and 11 (35.5%) with endovascular treatment plus intravenous fibrinolysis; at 3 months, functional independence (modified Rankin Scale scores 0-2) was achieved by 57.1% and 63.6% of these cases, respectively. One patient died (3.2%).
ConclusionsIn our sample, the most common form of presentation of CAD was ischaemic stroke. Reperfusion therapy seems to be a safe and effective option for these patients, and outcomes resemble those of other patients with ischaemic stroke. Larger comparative studies are necessary to better assess response to reperfusion therapy in acute ischaemic stroke.
Las disecciones de arteriales cervicales (DAC) provocan hasta el 20% de los ictus isquémicos en menores de 45 años. El beneficio de los tratamientos de reperfusión en fase aguda no está plenamente clarificado.
MétodosRevisión retrospectiva de pacientes con DAC ingresados en un centro terciario de ictus desde 2010 hasta 2015. Recogemos las características basales, clínicas, los tratamientos, el pronóstico funcional y la mortalidad.
ResultadosSe registraron 35 DAC (23 carotídeas/12 vertebrales). La edad media fue de 43,5±9,5 años y el 67,7% fueron varones. En 10 casos (32,3%) hubo antecedente de un traumatismo. Los factores de riesgo más frecuentes fueron la hipertensión arterial (29%) y el tabaquismo (35,5%). La presentación clínica más frecuente fue el infarto cerebral en 29 pacientes (93,5%). La mediana de puntuación National Institute of Health Stroke Scale basal fue de 6 (0-41). El método diagnóstico más empleado fue la angio-TC (74,2%), seguido de resonancia magnética (64,5%) y arteriografía cerebral (45,6%). Siete pacientes (22,6%) fueron tratados con fibrinólisis intravenosa y 11 (35,5%) con tratamiento endovascular (TEV)±fibrinólisis intravenosa. A los 3 meses, la independencia funcional (escala de Rankin 0-2) fue del 57,1% y del 63,6%, respectivamente. Falleció un paciente (3,2%).
ConclusionesLa forma de presentación más frecuente de la DAC fue el infarto cerebral. Estos casos pueden beneficiarse de terapias de reperfusión, con un pronóstico similar al resto de enfermos con ictus isquémicos. Se requieren registros más extensos para conocer mejor la respuesta a los tratamientos de reperfusión en fase aguda en este grupo de pacientes.
The incidence of cervical artery dissection (CAD) in the general population is approximately 2.6 to 3 cases per 100000 person-years; while it represents a small percentage of all ischaemic strokes, it is the most frequent cause of stroke in young adults.1–4
Current guidelines recommend antithrombotic treatment during the acute phase of CAD, especially in patients with acute stroke or transient ischaemic attack (TIA).5 However, the latest published studies report that anticoagulation does not seem to provide any benefit over antiplatelet therapy with aspirin.6
Although several studies support the safety of intravenous fibrinolysis in the acute phase of stroke secondary to CAD,7,8 physicians are still reluctant to treat these patients with intravenous fibrinolysis due to the risk of such complications as intramural haematoma extension, subarachnoid haemorrhage in cases of intracranial dissection, haemorrhagic transformation, or dissecting aneurysm-associated complications, among others.9 Furthermore, although CAD is not considered a reason to rule out thrombolytic treatment, several studies have found no significant difference in the prognosis of patients with CADs treated with thrombolysis and those receiving conservative treatment.10,11
Endovascular treatment (EVT) is a new alternative for acute management of these patients; some centres report good results with this treatment.12–14 The recent implementation of endovascular techniques in stroke reference centres has also facilitated early diagnosis of CAD and has demonstrated that endovascular management of these patients is feasible and safe.15
Our aim is to study the acute-phase treatment and clinical progression of patients with ischaemic stroke secondary to CAD admitted to a comprehensive stroke centre in the past 6 years.
Patients and methodsWe conducted a retrospective study including all patients diagnosed with CADs and examined by the neurology department at a comprehensive stroke centre between 2010 and 2015. Cases were drawn from the stroke unit database and the hospital's IT system. We collected all cases of CAD (carotid or vertebral arteries, spontaneous or traumatic), regardless of the symptoms and treatment received. We excluded those cases with no brain ischaemia (stroke or TIA) secondary to CAD from the final analysis.
We included data on the following risk factors previous to stroke: arterial hypertension, diabetes mellitus, dyslipidaemia, smoking, and heart disease. Local symptoms associated with dissection included headache, neck pain, and Horner syndrome.
Stroke severity was assessed with the National Institutes of Health Stroke Scale (NIHSS).16 The modified Rankin Scale (mRS)17 was used to assess functional status at discharge and at 3 months (a score of 0-2 is considered to indicate functional independence, 3-5 dependence, and 6 represents death).
Radiological diagnosis of dissection was performed using computed tomography (CT), CT angiography, magnetic resonance imaging (MRI), MRI angiography, carotid Doppler ultrasound, and digital angiography.
Angiography findings after the endovascular procedure were assessed using the Thrombolysis in Cerebral Infarction (TICI) scale18; a TICI score≥2b was considered satisfactory.
Quantitative variables are expressed as mean±standard deviation (SD), with the exception of NIHSS and mRS scores, which are expressed as median and range. Qualitative variables are shown as the absolute number and the percentage of individuals meeting a specific condition in each category. To perform the comparative analysis of quantitative variables with a normal distribution, we used the t-test or one-way ANOVA, with the Scheffe test as post hoc test. The Kruskal–Wallis test was used for the analysis of quantitative variables not following a normal distribution. Qualitative variables were compared using the chi-square test and where needed, the Fisher exact test. Statistical analysis was performed with SPSS version 15.0 for Windows.
ResultsWe collected a total of 35 cases, 31 with ischaemic stroke. Four had presented isolated Horner syndrome and were therefore excluded from the final analysis. Nineteen patients presented internal carotid dissection and 12 vertebral artery dissection. Dissection affected the left side in 61.3% of cases and was bilateral in one case (3.2%).
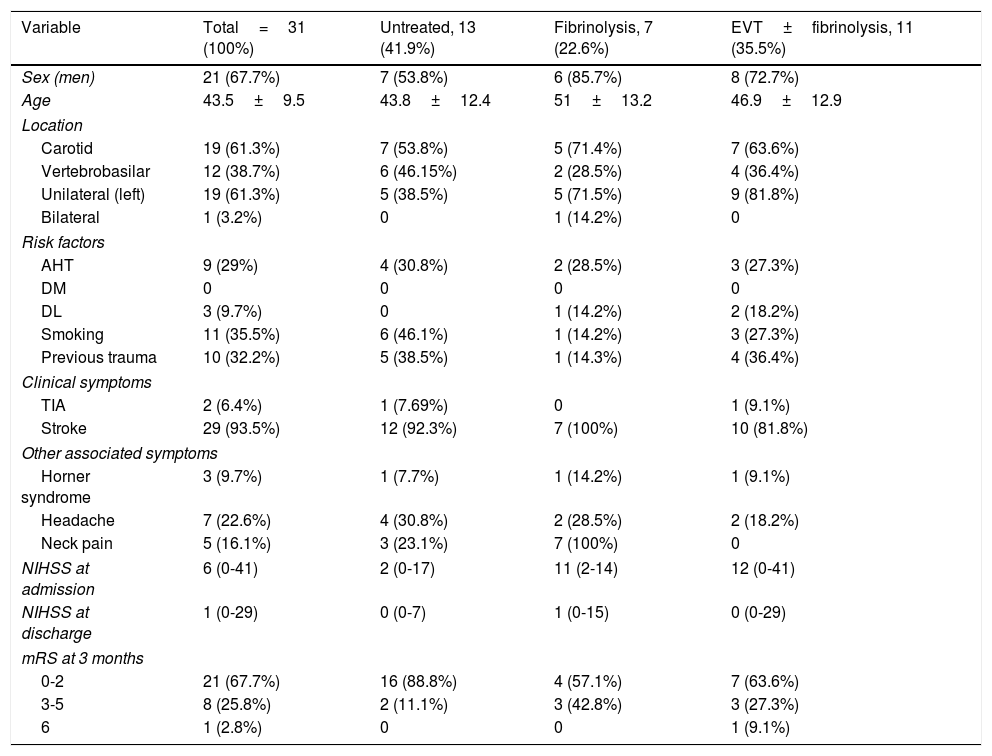
Demographic and clinical characteristics, the treatment used and patients’ progression are listed in Table 1. Mean age was 43.5±9.5 years; 21 patients (67.7%) were men. Ten patients (32.2%) had history of trauma; trauma was major (bicycle or car accident) in some cases and minor in others. We observed no causal relationship, but a time relationship was established (cervical manipulation, roller coaster ride, intense cross-fit training with weights, and sustained neck flexion during a flight). Our series includes a case of CAD secondary to tumour compression due to a cervical vertebral bone metastasis. Other possible aetiologies included underlying Cushing syndrome in one patient, but no connective tissue disease was identified in any other patient.
Demographic characteristics, type of presentation, and progression.
| Variable | Total=31 (100%) | Untreated, 13 (41.9%) | Fibrinolysis, 7 (22.6%) | EVT±fibrinolysis, 11 (35.5%) |
|---|---|---|---|---|
| Sex (men) | 21 (67.7%) | 7 (53.8%) | 6 (85.7%) | 8 (72.7%) |
| Age | 43.5±9.5 | 43.8±12.4 | 51±13.2 | 46.9±12.9 |
| Location | ||||
| Carotid | 19 (61.3%) | 7 (53.8%) | 5 (71.4%) | 7 (63.6%) |
| Vertebrobasilar | 12 (38.7%) | 6 (46.15%) | 2 (28.5%) | 4 (36.4%) |
| Unilateral (left) | 19 (61.3%) | 5 (38.5%) | 5 (71.5%) | 9 (81.8%) |
| Bilateral | 1 (3.2%) | 0 | 1 (14.2%) | 0 |
| Risk factors | ||||
| AHT | 9 (29%) | 4 (30.8%) | 2 (28.5%) | 3 (27.3%) |
| DM | 0 | 0 | 0 | 0 |
| DL | 3 (9.7%) | 0 | 1 (14.2%) | 2 (18.2%) |
| Smoking | 11 (35.5%) | 6 (46.1%) | 1 (14.2%) | 3 (27.3%) |
| Previous trauma | 10 (32.2%) | 5 (38.5%) | 1 (14.3%) | 4 (36.4%) |
| Clinical symptoms | ||||
| TIA | 2 (6.4%) | 1 (7.69%) | 0 | 1 (9.1%) |
| Stroke | 29 (93.5%) | 12 (92.3%) | 7 (100%) | 10 (81.8%) |
| Other associated symptoms | ||||
| Horner syndrome | 3 (9.7%) | 1 (7.7%) | 1 (14.2%) | 1 (9.1%) |
| Headache | 7 (22.6%) | 4 (30.8%) | 2 (28.5%) | 2 (18.2%) |
| Neck pain | 5 (16.1%) | 3 (23.1%) | 7 (100%) | 0 |
| NIHSS at admission | 6 (0-41) | 2 (0-17) | 11 (2-14) | 12 (0-41) |
| NIHSS at discharge | 1 (0-29) | 0 (0-7) | 1 (0-15) | 0 (0-29) |
| mRS at 3 months | ||||
| 0-2 | 21 (67.7%) | 16 (88.8%) | 4 (57.1%) | 7 (63.6%) |
| 3-5 | 8 (25.8%) | 2 (11.1%) | 3 (42.8%) | 3 (27.3%) |
| 6 | 1 (2.8%) | 0 | 0 | 1 (9.1%) |
AHT: arterial hypertension; DL: dyslipidaemia; DM: diabetes mellitus; EVT: endovascular treatment; TIA: transient ischaemic attack; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale.
Age is expressed as mean±standard deviation.
NIHSS score is expressed as median (P25–P75).
The most frequent vascular risk factors were arterial hypertension (29%) and smoking (35.5%).
The most frequent clinical manifestation was cerebral infarction (29 cases), whereas 2 patients presented TIA. Median NIHSS score at admission was 6 (P25-P75, 0-41).
Other local symptoms associated with CAD included headache in 7 patients (22.6%), neck pain in 5 (16.1%), and Horner syndrome in 3 (9.7%).
A CT angiography of the supra-aortic trunks and brain was performed in 74.2% of the patients, a duplex ultrasound in 61.3%, and a brain MRI/MRI angiography in 64.5%. All patients undergoing reperfusion therapy were previously assessed with CT angiography before receiving treatment.
An embolism distal to the dissection was observed in 12 patients (38.7%), with intracranial large-vessel occlusion. No haemodynamic lesions were detected.
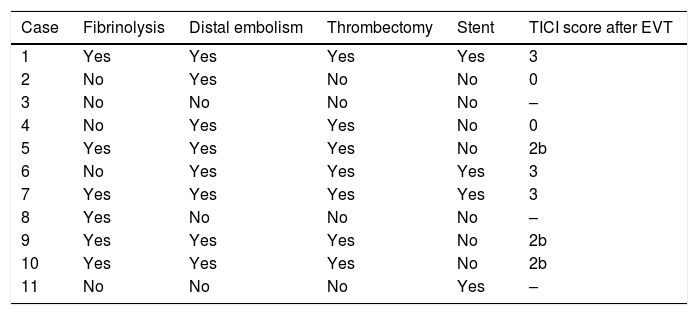
Reperfusion therapy during the acute phase was performed in 18 patients: 7 received intravenous fibrinolysis, and 11 underwent thrombectomy following a diagnostic arteriography (6 had received intravenous fibrinolysis). Distal embolism was observed in 8 patients, with 7 undergoing thrombectomy. The thrombus could not be extracted in one patient, as catheterising the true lumen was technically impossible. Successful recanalisation was achieved in 75% of patients (Table 2). Haemorrhagic transformation did not occur in any patient.
Characteristics of patients who underwent endovascular treatment.
| Case | Fibrinolysis | Distal embolism | Thrombectomy | Stent | TICI score after EVT |
|---|---|---|---|---|---|
| 1 | Yes | Yes | Yes | Yes | 3 |
| 2 | No | Yes | No | No | 0 |
| 3 | No | No | No | No | – |
| 4 | No | Yes | Yes | No | 0 |
| 5 | Yes | Yes | Yes | No | 2b |
| 6 | No | Yes | Yes | Yes | 3 |
| 7 | Yes | Yes | Yes | Yes | 3 |
| 8 | Yes | No | No | No | – |
| 9 | Yes | Yes | Yes | No | 2b |
| 10 | Yes | Yes | Yes | No | 2b |
| 11 | No | No | No | Yes | – |
EVT: endovascular treatment; TICI: Thrombolysis in Cerebral Infarction.
Stent-assisted angioplasty was performed in 4 patients (2 on the carotid artery and 2 on the vertebral artery): during the procedure to access the distal thrombus in 3, and to treat recurrent TIAs in a patient without distal embolism.
Stroke severity score at admission was higher in patients receiving treatment (median NIHSS of 2 [P25-P75, 0-17] in untreated patients, 11 [2-14] in the intravenous fibrinolysis group, and 12 [0-41] in the EVT±intravenous fibrinolysis group). At discharge, these scores reached 0 (0-7), 1 (0-15), and 0 (0-29), respectively.
At 3 months, 67.7% of patients were functionally independent, with no statistically significant differences in the analysis by treatment subgroup.
One patient died after a new embolism of the basilar artery 24hours after a vertebral artery dissection. Although endovascular treatment achieved a good angiographic outcome, this patient presented an extensive established infarction of the brainstem and died.
DiscussionCADs constitute a frequent cause of stroke. Symptoms can be mild and transient, which make it difficult to estimate the actual incidence of this condition. However, more severe cases represent a therapeutic challenge in the acute phase.
Our results support the use of intravenous fibrinolysis in stroke secondary to arterial dissection, given the absence of haemorrhagic complications and the good functional outcomes of these patients (57.1% at 3 months), similar to those reported in strokes of other aetiologies.19 These results are consistent with those obtained in previous studies, which have reported that intravenous fibrinolysis does not present more adverse effects in patients with stroke secondary to arterial dissection; therefore, arterial dissection should not be a reason for exclusion from treatment.20–22
The role of EVT in the acute phase in this type of case remains to be established. Patients with CAD may present vascular alterations and a tendency to develop pseudoaneurysm and may have more fragile vessel walls. Furthermore, dissections can represent an additional challenge for interventional procedures (risk of dissection progression, formation of pseudoaneurysms, vessel rupture...). Despite the baseline severity of patients undergoing EVT, we observed high rates of angiographic recanalisation (75% of cases with distal embolism) and functional independence at 3 months (63.6%). This is in line with the rates observed in other published series (Ohta et al.13 and Fields et al.14), which also suggest that treatment is safe and effective in these cases.
In our series, stents were placed across the dissection in 4 patients, who subsequently showed favourable progression and no complications or further embolic phenomena, as observed in other published cases.23
Six of the patients undergoing EVT had received intravenous fibrinolytic treatment, and no haemorrhagic complications occurred during the procedure. Limitations to our study include the fact that no statistically significant differences were found, probably because the groups at the end of each arm are too small. However, we should highlight the fact that despite presenting the highest neurological severity at admission, patients treated with reperfusion therapy in the acute phase showed a favourable prognosis in a high percentage of cases (with as many as 63.6% showing functional independence at 3 months). Furthermore, this is a retrospective study, with the resultant statistical limitations for drawing conclusions. More extensive prospective studies are needed to analyse in depth the benefits of acute-phase reperfusion therapies for patients with CAD.
Our results suggest that fibrinolysis and EVT in stroke secondary to CAD are safe, effective, and lead to a higher probability of functional recovery.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Crespo Araico LA, Vera Lechuga R, Cruz-Culebras A, Matute Lozano C, de Felipe Mimbrera A, Agüero Rabes P, et al. Tratamientos de reperfusión en el infarto cerebral agudo por disección de arterias cervicales. Neurología. 2019;34:153–158.
This work was presented in poster format at the 62nd Annual Meeting of the Spanish Society of Neurology, the 13th Annual Meeting of the Madrid Association of Neurology, and the second edition of the European Stroke Organisation Conference.