Tuberous sclerosis complex (TSC) is one of the most frequent neurocutaneous disorders. Cortical tubers are the most common pathological changes in TSC and they are directly related to the disease's main clinical manifestations: seizures, mental retardation, and autistic behaviour.
ObjectiveThe aim of this study is to establish a correlation between tuber size and the severity of clinical features in TSC.
Material and methodsWe performed a retrospective study of the clinical and imaging findings from 45 TSC patients (22 females and 23 males) and compared the clinical features with the location, size, and number of the cortical tubers in each patient.
ResultsFour patients had voluminous tubers located in 1 or both cerebral hemispheres. All of these patients had intractable seizures and severe mental retardation; 3 of these cases also presented with autistic behaviour, despite tubers having been resected in all 4 patients. Thirteen patients had tubers of large-to-average size, and all patients in this group showed intractable seizures and mental retardation. Nine patients who had experienced infantile spasms during the first year of life presented autistic behaviour. Multiple tubers of small to average size were found in 28 patients. In general, this group had seizures that responded well to antiepileptic drugs and a low prevalence of autism. In 3 patients who all presented good seizure control and normal intelligence, single cortical/subcortical tubers were located in the frontal or occipital lobes. Of the total of 45 patients, 13 had cerebellar as well as cerebral tubers; these were generally present in cases with more severe clinical features.
ConclusionsAlthough large tubers are less common than small to medium-sized ones, they are much more likely to be accompanied by severe clinical symptoms (seizures, mental retardation and autistic behaviour), even when the smaller tubers are quite numerous.
El complejo esclerosis tuberosa (CET) es uno de los trastornos neurocutáneos más frecuentes. Las tuberosidades corticales son las alteraciones patológicas más frecuentes y están relacionadas directamente con las principales expresiones clínicas, crisis epilépticas, retraso mental y comportamiento autista. El motivo de este trabajo es mostrar la importancia de los diferentes tipos de tuberosidades en la expresión clínica de los pacientes.
ObjetivoLa finalidad de este trabajo es relacionar el tamaño de las tuberosidades con la severidad de las alteraciones clínicas.
Material y métodosSe estudiaron retrospectivamente los hallazgos clínicos y neurorradioló-gicos de 45 pacientes infantiles (22 mujeres y 23 varones) con CET y comparamos los hallazgos clínicos con la localización, el tamaño y el número de las tuberosidades corticales en cada paciente.
ResultadosCuatro pacientes tenían tuberosidades muy voluminosas en los hemisferios cere-brales. Todas mostraban crisis epilépticas muy rebeldes y retraso mental profundo con comportamiento autista en 3 de ellos, pese a que se extirparon las tuberosidades en los 4 casos. Trece pacientes tenían tuberosidades de tamaño promedio-grande. Todos tenían crisis epilépticas muy rebeldes y retraso mental. Nueve pacientes habían tenido espasmos infantiles durante el primer año de vida y presentaban comportamiento autista. Veintiocho pacientes mostraban muchas tuberosidades de tamaño promedio-pequeño. La mayoría de ellos tenían crisis con buena respuesta al tratamiento farmacológico y poca prevalencia del autismo. Tres pacientes mostraban tuberosidad córtico-subcortical única en un polo frontal u occipital, todos ellos con crisis controladas con medicación y cociente intelectual normal. Trece pacientes de los 45 tenían tuberosidades cerebelosas, siempre asociadas a algún tipo de tuberosidad hemisférica y generalmente presentes en casos con mayor expresividad clínica.
ConclusionesLas tuberosidades de gran tamaño, aunque sean poco numerosas, tienen mucha mayor relación con la presencia de sintomatología clínica severa—crisis epilépticas, retraso mental y comportamiento autista—que las tuberosidades de pequeño-mediano tamaño, aunque sean muy numerosas.
Tuberous sclerosis complex (TSC) (OMIM 605284 and 191092) is a multi-systemic autosomal dominant disorder caused by mutations in the tumour-suppressing genes hamartin (TSC1) and tuberin (TSC2), located on chromosomes 9q34 and 16p13, respectively. TSC is generally associated with drug-resistant epilepsy and delayed mental development in children. The disorder is directly related to the presence of cortical tubers, the histological entities most representative of TSC. Histology studies of TSC brain lesions began in 1901 with Pelazzi.1 On the macroscopic level, tubers appear as lesions located on the cerebral surface in 82% to 100% of all patients with TSC, and they participate directly in triggering crises.2 Tubers are prominent in cerebral folds and they are firm, soft, and paler in colour than the nearby unaffected tissue. They vary considerably in number, size, localisation, and appearance in MRI studies when such studies are completed.3,4 On the histological level, cortical tubers are hypomyelinated hamartomas characterised by abnormal cortical lamination and the presence of giant balloon cells. Cellularity is abnormal, with a reduced number of neurons and an increased number of giant cells, balloon cells, dysplastic neurons, and large astrocytes.5–8
Tubers have been identified in fetuses at 20 weeks, and they remain present for life. Sequential MR studies of patients with TSC show that tubers change as patients age.4
Approximately 95% of patients with TSC present cerebral tubers.9 Epilepsy, developmental delay, and autism are the main concerns of patients with TSC, and they may be lifelong problems. Epileptic seizures often appear in the first year of life, and a high percentage of all TSC cases present infantile spasms. Other types of seizures, such as focal and myoclonic seizures, appear somewhat later, between years 1 and 2. They may also present in older children.
Epileptogenesis in cases of TSC is caused by anatomical or functional characteristics of the cerebral cortex surrounding cortical tubers, rather than by the tubers themselves.10 In recent years, histochemical studies of oxidative mitochondrial enzymes have revealed focal cortical dysplasia and dispersed or grouped neurons that are more active than others. These neurons have been located in epileptic foci in frozen sections of tubers recently excised from patients with TSC.11,12 The direct relationship between mental development, epilepsy, and the characteristics of cortical tubers, especially their number and localisation, seems obvious.13–15 Likewise, cerebral tubers appear to be a potential direct cause of epileptic seizures and developmental delay.16,17
Several studies in the literature suggest that there may be an association between the findings provided by brain MRI studies and the number and localisation of tubers, cognitive function, presence of seizures (especially infantile spasms), and age at onset of seizures in TSC patients.13,18–22 Nevertheless, few studies link the severity of neurological alterations to tuber size, and even fewer link alterations to specific tuber localisations.23 Likewise, not many studies have explored the relationship between tuber activity, epilepsy, and cognitive function,24 or between condition severity and the time elapsed from seizure onset to beginning treatment.25,26
Based on this retrospective review of 45 patients with TSC who underwent MR studies throughout childhood in addition to long-term follow-up (to adolescence and adulthood in some cases), we conclude that tuber size is the most important of all the alterations in the disease that are associated with severe sequelae. Cases of TSC that were studied before MRI techniques were available, and which were published because of their scientific interest,27,28 were not included unless they were studied with MRI at a later date.
Material and methodsBetween 1996 and 2004, 45 patients with TSC (22 females and 23 males) were studied in the paediatric neurology department at Hospital Universitario La Paz, Madrid, Spain. A few patients also underwent molecular genetics studies to determine whether the transmitter gene was TSC1 or TSC2, but since there were so few cases, we did not include these results when framing this study. Table 1 displays a list of patients and their conditions. Table 2 lists the tests performed on those patients. Almost all MRI studies in each patient were performed with a 1.5-Tesla machine. As 25 patients had previous MR studies that had been completed in other hospitals according to protocols that were not as systematic as our own, and some had been taken with a 1.0-Tesla machine, we repeated those studies.
Patients with TSC under monitoring (1996–2004).
| Total: 45 (23 M; 22 F) |
| Ages: 2 months to 12 years |
| Follow-up: up to 20 years in 5 cases |
| Main reason for consultation |
| Epileptic seizures: 45 cases |
| Other reasons |
| Psychomotor delay |
| Autistic behaviour |
| Attention deficit/hyperactivity |
| Skin lesions |
| Kidney lesions |
Electroencephalographic studies were very important for determining presence or absence of hypsarrhythmia, a finding that may help indicate an appropriate treatment in very early stages. They were also useful for identifying one or more epileptogenic foci able to provide data about seizure localisation and surgical possibilities, as well as indicating whether one or more tubers would have to be resected.
The MRI study protocol included coronal, axial, and sagittal images and used spin-echo T1-weighted, spin-echo T2-weighted, and contrast-enriched T1-weighted images. We also used three-dimensional MR techniques and fluid-attenuated inversion recovery sequences and cortical reconstruction in cases in which cortical lesions had to be defined precisely.
The main focus of this study is the number, form or type, size, and localisation of tubers and their relationship with epileptic seizures (especially infantile spasms), mental development, frequency and significance of cerebellar tubers, and also the link between mental capacity and the characteristics of different types of tubers. We are aware of the obstacles to classifying tubers by size (Table 3), and this is why there are no such descriptions in the literature. On the other hand, many studies point to the importance of the number of tubers13–15 with respect to the way seizures present, psychomotor impairment, and autism. We have not found any earlier publications that would serve as models. We therefore devised one of our own that is somewhat artificial, but which seems to be a better match for the clinical severity of the syndrome than the more widespread method of counting the number of tubers. There is no consensus on whether if the latter method is valid. We classified tubers as voluminous if they extended throughout an entire lobe; medium-large if they measured more than 3cm in diameter but did not occupy an entire lobe; and medium-small if they measured less than 3cm. We did not measure cerebellar tubers, but they were usually type 2 or medium-large.
ResultsThe results for tuber type are displayed in Table 4. Except for the 3 patients with single cerebral tubers corresponding to the medium-small group (1 located in the frontal lobe and 2 in occipital lobes), all cases presented multiple tubers of different sizes that were distributed fairly evenly across both hemispheres. Contrast uptake was good in all or part of each tuber. Cerebellar tubers were also observed in 13 patients. Rather than being isolated, these tubers were always associated with tubers in the cerebral hemispheres. Abnormalities in each group according to the classification by the 3 sizes of tubers are shown in Table 5.
Neurological alterations found in patients from each group.
| 1. The 4 patients with voluminous tubers presented: |
| Different types of drug-resistant seizures |
| Infantile spasms |
| Profound intellectual disability: all 4 cases |
| Autistic behaviour: 3 of the 4 cases |
| Tubers were resected in all cases, resulting in: |
| Effective epilepsy control |
| No change in intellectual level |
| 2. The 13 patients with medium-large tubers presented: |
| Different types of epileptic seizures: 13 cases |
| Infantile spasms in the first year of life: 9 cases |
| Intellectual disability: all 13 cases |
| Autistic behaviour: 9 cases |
| 3. The 28 patients with medium-small tubers presented: |
| More than 12 tubers located across both hemispheres, in 25 patients |
| Seizures of any type in 24 patients (96%) |
| Infantile spasms in the first year of life in 10 patients (40%) |
| Seizure control in 18 patients (73%) |
| Normal intellectual level (IQ>85) in 7 (27%) |
| Borderline intellectual level (IQ=70–85) in 12 (46%) |
| Lower intellectual level (IQ<70) in 9 (31%) |
| Autistic behaviour in 4 (15%) |
Seizures were highly resistant to all types of antiepileptic drugs employed. The 4 patients with voluminous tubers displayed profound intellectual disability and uncontrolled epilepsy; they underwent operations between the ages of 3 and 17 years. At the age of 17, the first patient underwent resection of an enormous tuber that had increased in size since its initial diagnosis in early childhood. By the patient's adolescent years, it had extended throughout the entire right frontal lobe (Fig. 1); by this time, the patient's seizures had resolved, but his intellectual level and autistic behaviour remained unchanged. Another patient with 2 large tubers experienced very frequent partial seizures, intellectual disability with an IQ of less than 50, and no autistic behaviour. One tuber extended throughout the entire right temporal lobe, and the other was localised in the left parasagittal frontoparietal zone (Fig. 2). When the patient was 8, the temporal lobe tuber was resected, which resulted in a definitive decrease in seizure frequency of more than 90%, again with no changes in his intellectual level. A third patient with refractory seizures and profound intellectual disability underwent surgery to remove a voluminous tuber with very diffuse edges, located in the left parieto-occipital zone, at the age of 3. The operation did not resolve seizures or improve either his intellectual level or autistic behaviour. The fourth patient, a girl, had experienced infantile spasms since her earliest months of life, after which she continued to present different types of seizures, especially focal seizures, with complex symptoms. In addition, she displayed profound intellectual disability, autistic behaviour, and marked restlessness. The MRI study showed tubers in both hemispheres; 3 were voluminous, and they included one that extended throughout the right frontal and temporal lobes, and another in the left occipital lobe. When the patient was 12 years old, the tuber in the right hemisphere was removed. Seizures remained completely controlled during 2 years, after which they reappeared and were completely refractory to treatment. Two months later, she developed status epilepticus and died during seizure. The 4 patients in this group also presented cerebellar tubers. An anatomical histology study of the excised tissue samples from these 4 cases revealed very similar changes, characterised by ill-defined borders between white matter and grey matter, loss of cortical lamination, decrease in the number of normal neurons, increase in the number of giant astrocytes, and presence of giant cells. The 13 patients with medium-large tubers presented severe intellectual disability (IQ <50); 9 had experienced infantile spasms throughout the first year of life, and all 9 developed autistic behaviour. There were no noteworthy differences in symptoms between patients who presented one or multiple large tubers, or with regard to tuber location or the patient's sex. Three of these patients also presented either 1 or 2 tubers in the cerebellum.
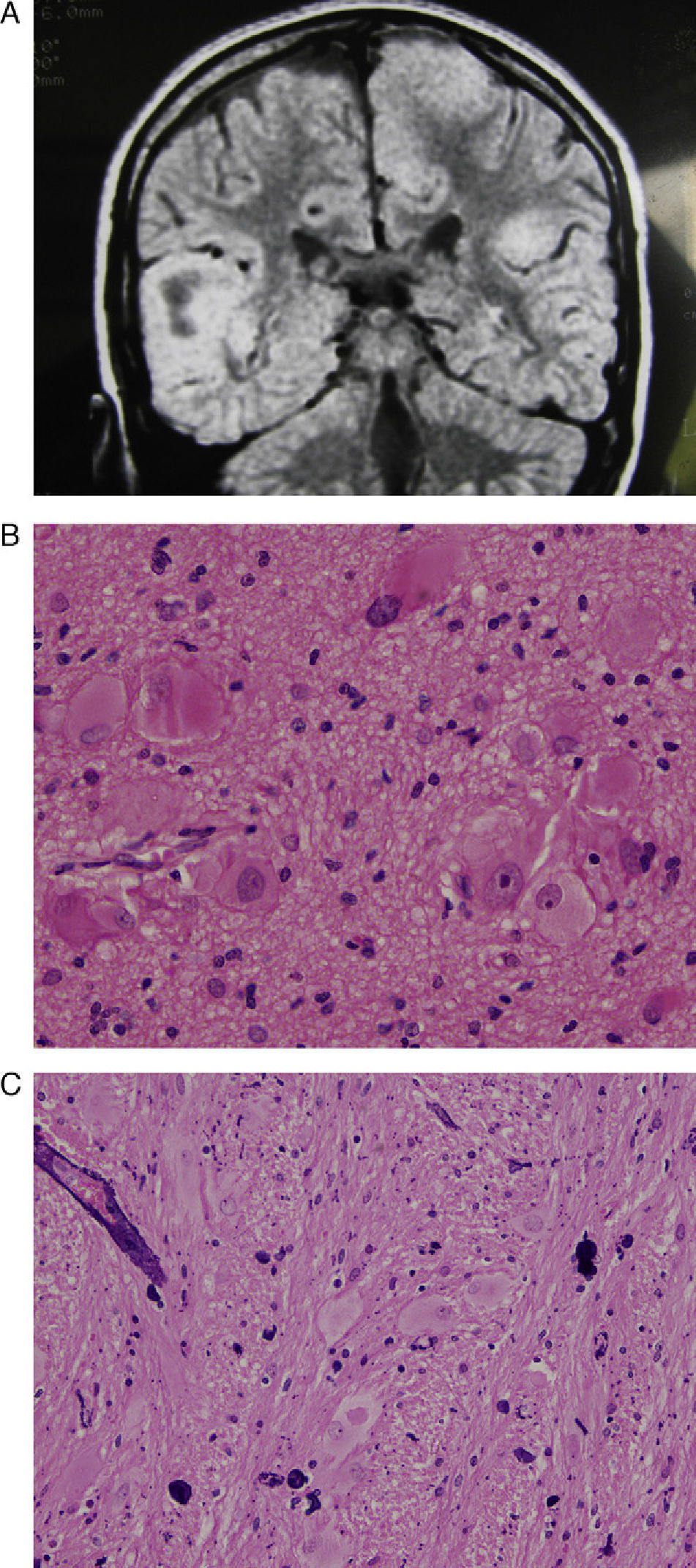
(A) Axial T2-weighted MR image showing a voluminous tuber occupying the entire right frontal lobe and extending to the medial zone of the left frontal lobe. (B) Same case. Postoperative MR image shows the large void in the anterior part of the right hemisphere. (C) Haematoxylin and eosin stain of a histological slice of the resected tuber, showing diverse types of dysmorphic and enlarged cells, especially balloon cells.
(A) Coronal T2-weighted MR image with the principal finding of a voluminous tuber occupying the entire right temporal lobe, another very large tuber in the paramedian zone of the left hemisphere, and tuberous areas in the peripheral parts of both cerebellar hemispheres. (B) Histological slice from the temporal tuber. Haematoxylin–eosin stain. Abundant giant and deformed cells of different types, especially balloon cells. (C) Another histological slice with an H&E stain showing numerous blood vessels with perivascular calcifications.
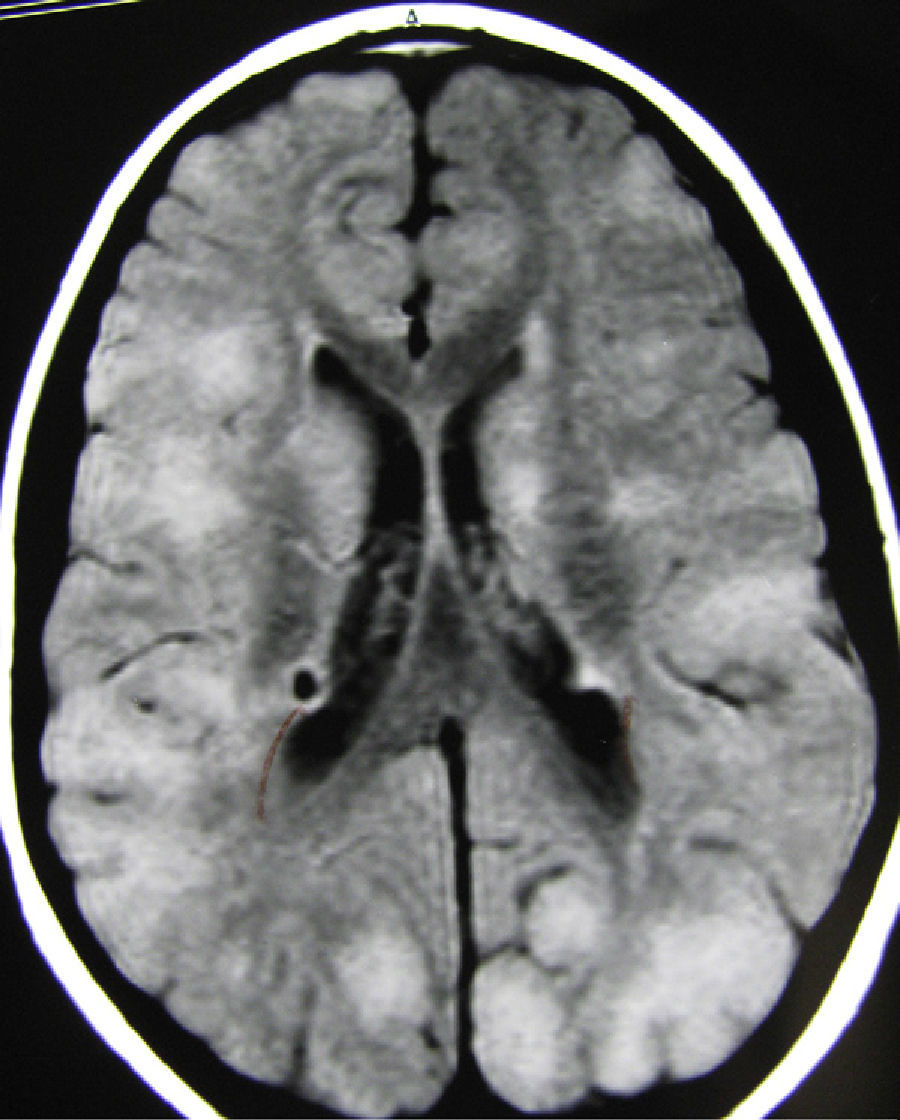
In the group of patients with medium-small tubers, tuber localisations were quite variable, with some patients showing marked differences between the two hemispheres (Fig. 3). Clinical abnormalities in patients in this tuber size group are listed in Table 5, along with results for the other 2 tuber size groups. Most of the patients with a low intellectual capacity and the 4 with autistic behaviour also had either uncontrolled epilepsy or a history of infantile spasms from a very early age which had later evolved into other types of seizures that could not be controlled completely. Six of these patients also had cerebellar tubers, but these tubers did not seem to affect seizure frequency or the patient's intellectual development.
All patients with an IQ above 70 had experienced infantile spasms after the age of 6 months. Infantile spasms appeared before the age of 6 months in those patients with voluminous tubers.
These spasms were treated mainly with adrenocorticotropic hormone and vigabatrin; patients were also closely monitored, not only to follow up on any seizures, but also to watch for the severe adverse effects that those 2 substances may provoke. To this end, when spasms became controlled but focal and generalised epileptic seizures persisted, doctors prescribed vigabatrin treatment for short periods only, since it often causes severe damage to the visual field. It was substituted with levetiracetam, which, like vigabatrin, had a favourable effect on seizures. Surgical treatment was only carried out in the 4 patients described above who had extremely large tubers. No cases were treated with a ketogenic diet or with vagus nerve stimulation.
DiscussionDiagnostic criteria for TSC have been available since 1998.29
Magnetic resonance images for the 2 tuber types (Pellazzi 1 and Pellazzi 2) were described in 1992 by Braffman et al.30 and researchers recently observed an association between type 2 cortical tubers and mutations in the TSC2 gene.31 Gallagher et al.3 identified 3 types of tubers based on signal intensity in the subcortical white matter component. He also identified 3 patient groups according to the dominant type of tuber. Highlighting the importance of MRI findings, Gama et al.32 indicated the (infrequent) presence of abnormalities such as mesial temporal sclerosis (MTS), and concluded that this type of lesion would have been caused by febrile convulsions in the first year of life. The presence of both findings, with tubers being primary and MTS being secondary, plus epileptogenic zones in patients with TSC, does not necessarily contraindicate surgical treatment in selected patients.33 The number, size, localisation, and unilateral or bilateral presence of tubers, the patient's age at onset of seizures, presence or absence of infantile spasms,21 response of seizures to antiepileptic drugs, and time elapsed between first seizure and beginning treatment are important factors that affect the degree of cerebral impairment.14 Some authors19 have suggested that the presence of more than 7 tubers is associated with a high risk of presenting infantile spasms and intellectual disability; other researchers14 state that 10 or more tubers must be present to cause epileptic seizures and intellectual disability. Nevertheless, a genetic, radiology, and clinical study in a large series34 was unable to confirm a link between the number of tubers in the hemispheres and intellectual disability in affected patients. Our own series cannot confirm this link either.
Presence of epileptic seizures is considered an important factor that affects intellectual development in patients with TSC; in some series, seizures were present in 99% of the patients with intellectual disability.34
Cortical tuber studies using MR spectroscopy (MRS) have detected a decrease in the NAA/Cr ratio and an increase in myo-inositol/Cr, with no differences in the Cho/Cr ratio,35 between patients and normal controls. Nevertheless, we believe that the diagnostic value of this technique is not comparable to that of other neuroimaging approaches, such as measuring tuber size. It is true, however, that we did not compare the 2 parameters since only a few of our cases had been studied using MRS.
Clinical data reported by studies of patients with TSC show that the syndrome is associated with intellectual disability.36,37 Almost all patients with TSC, even those with no epileptic seizures, experience major cognitive and behavioural problems.36 Based on results in our series, tuber size rather than tuber number plays a leading role as a cause of epileptic seizures, especially the infantile spasms that provoke intellectual disability. Some authors, however, feel that tubers and seizures are neither necessary nor sufficient to explain the clinical manifestations of TSC, and stress that there must be another molecular mechanism at the root of the syndrome.38 One such mechanism could be related to the cause of mutations in the TSC2 gene,34 but this connection will require further study if it is to be assessed more objectively.
Pharmacological treatment is still considered the treatment of choice, and vigabatrin and levetiracetam are the most common drugs employed, even for seizures that are not infantile spasms. The list of possible treatments grows longer as the patient ages.
Vagus nerve stimulation has also been used in patients with TSC and seizures refractory to all treatment approaches, but results have been poor.39
Treatment with mTOR inhibitors (the family containing sirolimus/rapamycin), which has been described as very effective for treating subependymal giant-cell astrocytomas,40,41 is not yet considered a treatment alternative for cortical tubers.
Recent literature on the subject suggests that surgical resection of tubers that cause drug-resistant epilepsy is beneficial in patients selected based on semiology, ictal and inter-ictal EEG, tuber size and location, and findings from video-EEG images.42 Traditionally, doctors have believed that surgery for lesions including the rolandic and perirolandic cortex in patients with TSC involves a greater risk of motor and sensory sequelae in the contralateral side of the body, but several authors have not confirmed this tendency.43 The decreased level of a risk that should apparently be present could be due to reorganisation of cortical function, or to tubers not having a specific function.43 Electrocorticography studies have shown that epileptogenesis in cortical tubers is caused more by the disturbance to or abnormal development of the neighbouring cerebral cortex than by the tuber itself.44 Identifying epileptogenic areas may be difficult in some cases, but diagnostic techniques tend to improve over time45 and provide non-invasive methods of reaching a preoperative diagnosis of the tuber and the surrounding epileptogenic areas. Some imaging techniques offer more data than SPECT or PET, as has been shown by combining functional MRI and EEG.46
Given the size difference among tubers, resection may be performed by excising the tuber only, or an entire lobe, or even contiguous areas of the brain. The latter approach was used to treat one of our patients who experienced no new seizures after the operation. Nevertheless, studies of surgical cases that have been monitored for several years show that surgical treatment is more effective at the beginning of the postoperative stage, and seizure control decreases with the passing of time.46
Autistic behaviour is associated with intellectual disability, which is profound in most cases of TSC. This occurred in 15 (33.3%) of the 45 patients in our series, broken down as 64.7% of the patients with voluminous hemispheric tubers and 15.4% of the patients with more numerous, smaller tubers. The percentages of patients with autistic behaviour described in different TSC series range from 17%47 to 68%.48,49 The relationship between epileptic seizures and autistic behaviour has been highlighted by most series, and we also observed it here. Attention deficit disorder associated with autistic behaviour is a very frequent finding in this disease.48
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Pascual-Castroviejo I, Hernández-Moneo J, Pascual-Pascual S, Viaño J, Gutiérrez-Molina M, Velazquez-Fragua R, et al. Importancia del tamaño de las tuberosidades en las complicaciones del complejo esclerosis tuberosa. Neurología. 2013;28:550–557.