Vagus nerve stimulation (VNS) is used as a complementary therapy to pharmacological treatment in patients with refractory epilepsy. This study aims to evaluate the efficacy of VNS in reducing seizure frequency, severity, and duration; reducing the number of antiepileptic drugs administered; and improving patients’ quality of life.
Material and methodsWe analysed the clinical progression of 70 patients with refractory epilepsy treated with VNS at Hospital Universitario de Alicante and Hospital Clínico de Valencia. Data were collected before and after the procedure. The difference in seizure frequency pre- and post-VNS was classified using the McHugh scale. Data were also collected on seizure duration and severity, the number of drugs administered, and quality of life.
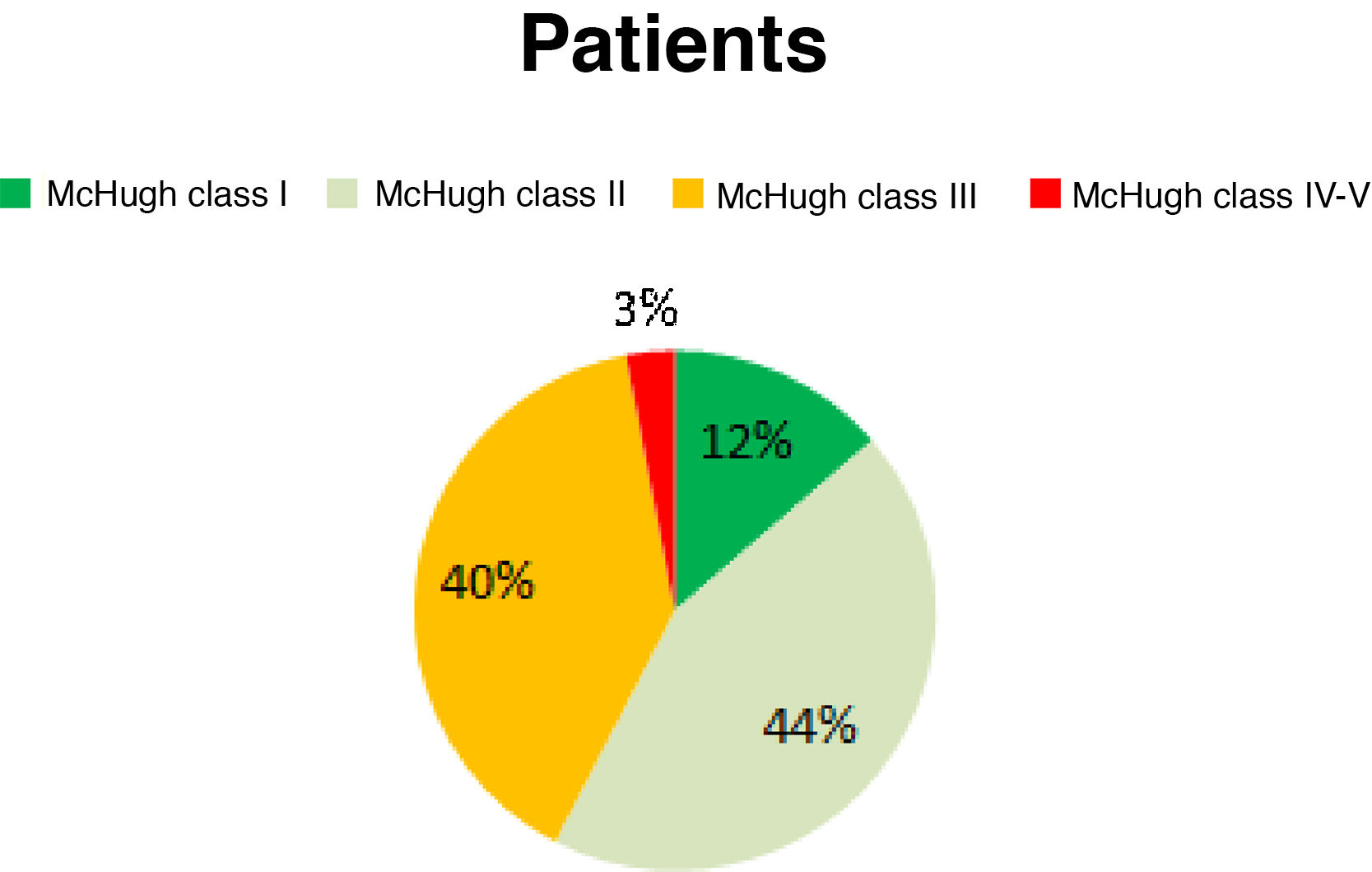
ResultsAccording to the McHugh classification, 12.86% of the patients were Class I, 44.29% were Class II, 40% were Class III, and the remaining 2.86% of patients were Class IV-V. A ≥ 50% reduction in seizure frequency was observed in 57.15% of patients. Improvements were observed in seizure duration in 88% of patients and in seizure severity in 68%; the number of drugs administered was reduced in 66% of patients, and 93% reported better quality of life.
ConclusionsVNS is effective for reducing seizure frequency, duration, and severity and the number of antiepileptic drugs administered. It also enables an improvement in patients’ quality of life.
La estimulación del nervio vago (ENV) se ha mostrado como una terapia complementaria al tratamiento farmacológico en pacientes con epilepsia refractaria. Nuestro objetivo es evaluar la eficacia de la ENV en relación con la disminución del número, intensidad y duración de las crisis, con la reducción del número de fármacos antiepilépticos y con la mejoría de la calidad de vida.
Material y métodosSe analizó la evolución de 70 pacientes con epilepsia refractaria, trata dos mediante ENV en el Hospital General Universitario de Alicante y en el Hospital Clínico de Valencia. Se recogieron variables pre- y postoperatorias. La diferencia en la frecuencia tras la estimulación vagal se clasificó mediante la escala de McHugh. También se recogieron los cambios en la duración e intensidad de las crisis y la disminución de la medicación junto con la modificación de la calidad de vida.
ResultadosEl 12,86% de los pacientes se clasificaron como McHugh I, el 44,29% como II, el 40% como III y el 2,86% como IV-V. Un 57,15% de los pacientes presentaron una reducción superior al 50% en la frecuencia de las crisis. Un 88% de los pacientes presentaron una mejoría en la duración de las crisis, en el 68% disminuyó la intensidad, un 66% toman menos fármacos y en el 93% mejoró la calidad de vida.
ConclusionesLa ENV ha mostrado disminuir la frecuencia de las crisis, así como la duración, la intensidad y el consumo de fármacos, ofreciendo además una mejoría en la calidad de vida de nuestros pacientes.
Refractory epilepsy is defined as the persistence of unprovoked epileptic seizures at a frequency that interferes with daily living activities, social and family life, employment, and education, leading to personal dissatisfaction, despite full adherence to treatment with 2 antiepileptic drugs (AED) at the maximum tolerated dose for a minimum of 2 years.1–3
Refractory epilepsy poses major social and healthcare problems, as up to one-third of all patients with epilepsy do not respond to AEDs. Of these, 25%-40% are not eligible for resective surgery.1,4 Penry and Dean4 were the first to implant a vagus nerve stimulation (VNS) device in a human patient in 1988. In 1997, the United States Food and Drug Administration approved VNS as adjunctive therapy for refractory epilepsy. Since then, VNS has emerged as a safe complementary treatment for patients with epilepsy who present insufficient response to pharmacological treatment and are not eligible for resective, ablative, or disconnection surgery.5,6
VNS consists of retrograde stimulation of the vagus nerve through a generator (a device resembling a pacemaker) implanted into a subcutaneous pocket and connected to an electrode wrapped around the cervical portion of the nerve. The generator supplies intermittent stimulation whose intensity, frequency, and duration can be defined according to the parameters set on the telemetric wand. Furthermore, patients can switch the device on using a small magnet when they anticipate an imminent seizure.7
Many questions surround this treatment. Several articles with small samples have evaluated the efficacy of VNS, reporting heterogeneous results.8,9 While all researchers agree that VNS is safe (the most frequent adverse reactions are heart rate alterations, hoarseness, voice changes, neck pain, dysphonia, and cough),10,11 the efficacy of this treatment is controversial.
In a meta-analysis conducted in 2013 by Chambers and Bowen,12 VNS was found to reduce seizure frequency by half in up to 25% of patients. However, recent studies report greater efficacy. For instance, a meta-analysis by Connor et al.13 shows a decrease of over 50% in seizure frequency in up to 50.9% of patients.
No consensus has been reached on which epileptic syndromes are associated with the highest success rates; future studies should address this issue.
There is also disagreement regarding whether age at implantation of the device has an impact on the efficacy of treatment. Some authors postulate that efficacy is significantly better in children younger than 12 years,14,15 whereas others have found no age-related differences.16,17
According to some studies, VNS reduces not only seizure frequency but also seizure duration and intensity, as well as medication use.18–20 However, few studies have addressed these effects, with most studies focusing only on seizure frequency.
Although most experimental and clinical studies conducted in recent decades focus exclusively on seizure control, increasing emphasis is being placed on the effects of VNS on quality of life; this parameter should be analysed in future studies into VNS.
In the light of these considerations, it is important to analyse the effects of VNS not only on seizure frequency but also on seizure intensity and duration, AED use, and quality of life.
ObjectivesThis study aimed to evaluate the efficacy of VNS in reducing seizure frequency, intensity, duration, and AED use, and improving quality of life in our patients.
As a secondary objective, we evaluated the efficacy of VNS by seizure type, aetiology, and age, and determined the technique’s safety considering the main adverse reactions (heart rate alterations, hoarseness, infections, neck pain, dysphonia, and cough).
Material and methodsWe conducted a non-randomised retrospective clinical study of 70 patients treated with VNS at the neurosurgery departments of Hospital General Universitario de Alicante and Hospital Clínico Universitario de Valencia, in Spain.
We retrospectively reviewed the clinical histories of patients undergoing VNS implantation, gathering data from the 3 months prior to the intervention and one year after onset of VNS therapy.
A Cyberonics vagus nerve stimulator (model 103) was implanted in all cases. The device was set to deliver discharges at an intensity of 0.5 μA, frequency of 30 Hz, pulse width of 500 ms, on-time of 30 s, off-time of 10 minutes, 24 hours a day.
Clinical variables were recorded by patients and/or their relatives in a seizure diary.
ParametersClinical and demographic variablesWe gathered data on sex, age, date at implantation of the device, seizure type, and seizure aetiology.
Seizure frequencyWe evaluated seizure frequency (mean number of seizures per week) by seizure type, seizure aetiology, and age (≤ 12 years vs > 12 years). To quantify reductions in seizure frequency, we used the McHugh classification. This classification uses the patient’s or his/her relatives’ responses to questions on seizure frequency at the time of survey completion (one year after the intervention) and before device implantation, comparing the number of seizures before and after the intervention. The McHugh classification has greater inter-rater reliability (as measured with the kappa coefficient) than other scales currently available.21 McHugh class I indicates a decrease of at least 80% in seizure frequency, class II indicates a 50%-79% decrease, class III indicates decreases of less than 50%, class IV indicates that improvements were only observed with use of the magnet, and class V indicates no improvement. Seizure outcomes were classified according to the Engel classification.
Seizure durationPatients were classified into 3 groups, according to whether seizure duration was reduced by 0%-33% (small decrease), 33%-66% (moderate decrease), or 66%-100% (large decrease). Seizure duration was reported by the patient’s relatives or companions, and was defined as the time (minutes and seconds) from seizure onset to partial or complete recovery of awareness and/or resolution of involuntary movements.
Seizure intensityPatients and/or their relatives were asked to rate the intensity of seizures as high, moderate, or low before and after vagus nerve stimulator implantation. We identified improvements in seizure intensity by comparing pre- and post-intervention ratings.
Antiepileptic drug useWe calculated the percentage of reduction in the number of AEDs used after VNS therapy. Patients were classified into 3 groups, according to whether AED use was reduced by 0%-33% (small decrease), 33%-66% (moderate decrease), or 66%-100% (large decrease).
Adverse events after surgeryData were gathered on heart rate alterations (palpitations, heart rate alterations detected by primary care physicians and/or emergency department physicians), hoarseness, voice changes, neck pain, infection, dysphonia, and cough. These variables were gathered during consultations, based on data provided by patients and/or their relatives upon direct questioning.
Quality of lifeQuality of life was measured before and after the intervention with a modified version of the Quality of Life in Epilepsy (QOLIE-10) scale (Table 1). The questionnaire was administered by telephone. This scale has not been validated in Spanish.
Patients were classified according to QOLIE-10 score: group I (19-25 points), group II (12-18 points), or group III (5-11 points); higher scores indicate poorer quality of life.
Statistical analysisStatistical analysis was performed with the Epidat 3.5 software (Xunta de Galicia) and Excel (Microsoft). Data were analysed using the Fisher exact test (frequency study) and the McNemar test (quality of life).
ResultsPatientsOf the 70 patients included in the study, 53% were men and 47% were women. The mean age of our sample was 30 years (range, 8-69). Mean age at implantation was 23 years (range, 4-58). In our sample, 21 patients were younger than 12 years, and the remaining 49 patients were older than 12.
Table 2 summarises the aetiologies of epilepsy in our patients. Table 3 presents the types of seizures reported.
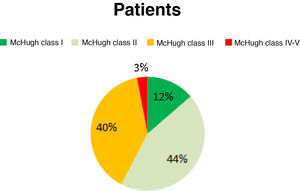
Before the intervention, our patients presented a mean seizure frequency of 15 seizures/week. After the intervention, 12.86% of patients were classified as McHugh class I, 44.29% as class II, 40% as class III, and 2.86% as classes IV or V (Fig. 1). Therefore, 57.15% of patients presented a reduction in seizure frequency of over 50% (classes I and II). The mean reduction in seizure frequency after a year of VNS therapy was 53%. None of our patients remained completely seizure-free after VNS implantation.
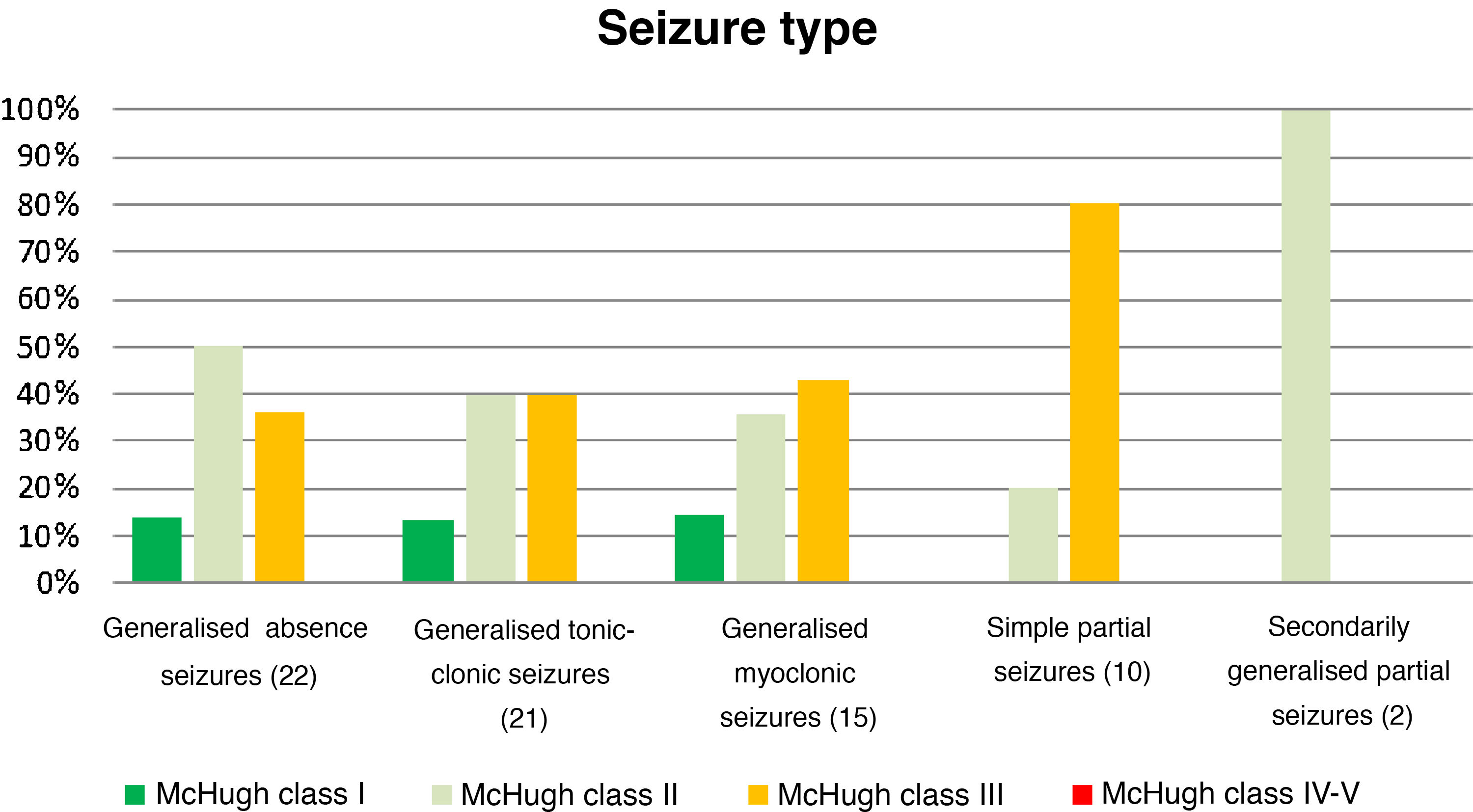
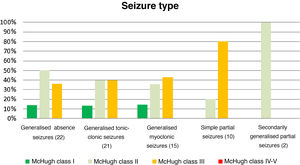
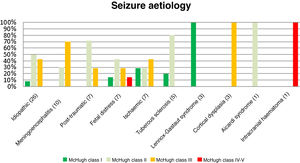
Table 4 and Fig. 2 analyse the decrease in seizure frequency as a function of seizure type (P = .32). Patients with generalised tonic-clonic seizures benefited the most from VNS.
Decreases in seizure frequency by seizure type (P > .05).
| Seizure type (n) | McHugh I (%) | McHugh II (%) | McHugh III (%) | McHugh IV-V (%) |
|---|---|---|---|---|
| Generalised absence seizures (22) | 13.64 | 50 | 36.36 | 0 |
| Generalised tonic-clonic seizures (21) | 19.05 | 47.62 | 28.57 | 4.76 |
| Generalised myoclonic seizures (15) | 13.33 | 40 | 40 | 0 |
| Simple partial seizures (10) | 0 | 20 | 80 | 0 |
| SGPS (2) | 0 | 100 | 0 | 0 |
SGPS: secondarily generalised partial seizures.
Given the high number of variables analysed and the small size of our sample, we compared decreases in seizure frequency by establishing 2 broad groups: patients presenting decreases ≥ 50% and patients presenting decreases < 50% (Table 5). Approximately 63% of patients with generalised seizures presented reductions of over 50% in seizure frequency, as compared to only 20% of those with partial seizures (P = .01).
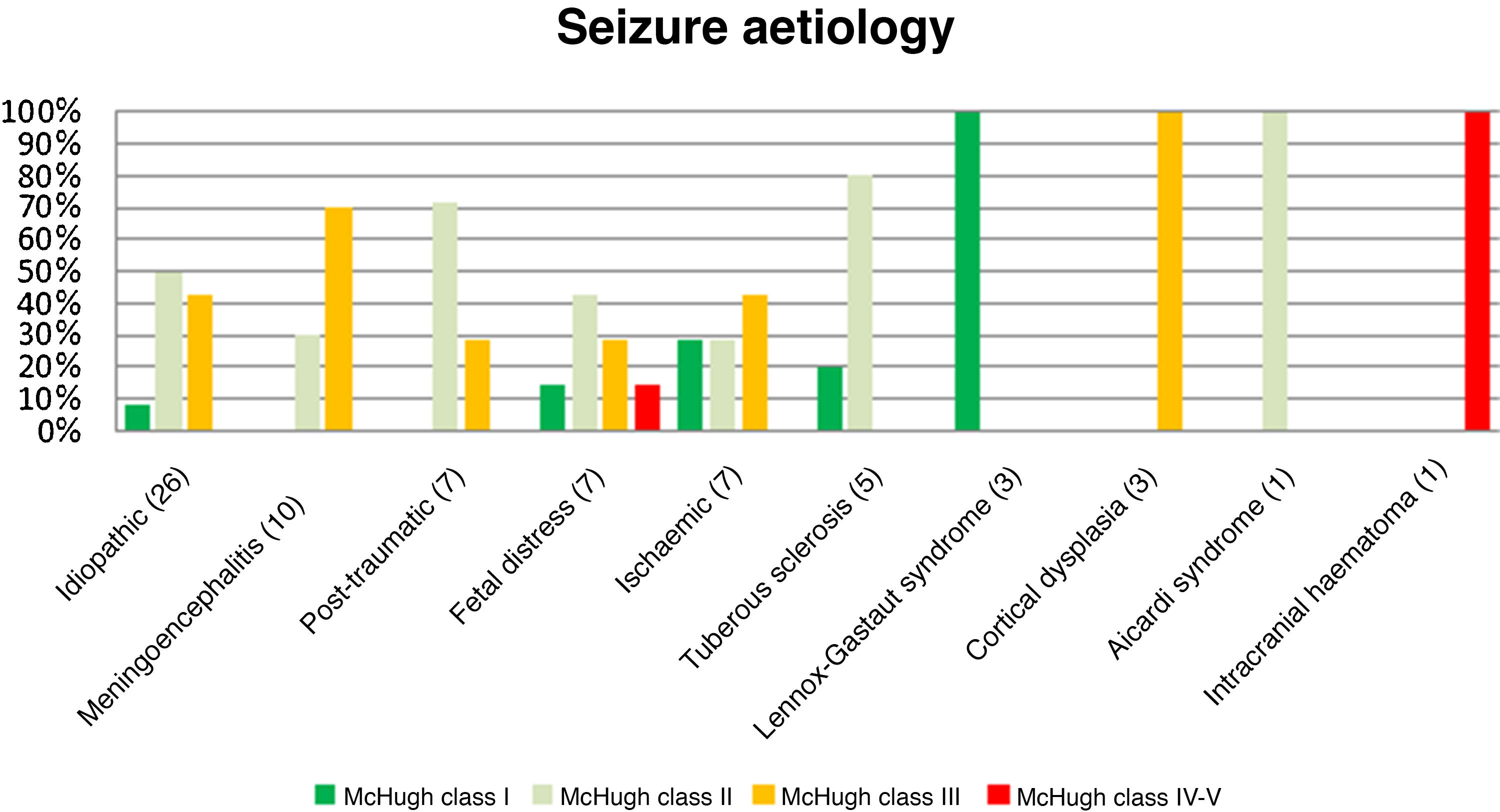
Fig. 3 presents the decreases in seizure frequency in patients with different seizure types (P > .05). Patients with Lennox-Gastaut syndrome, post-traumatic epilepsy, and tuberous sclerosis responded particularly well to VNS therapy.
Table 6 presents the decrease in seizure frequency by age (≤ 12 years or > 12 years). Sixty-six percent of patients younger than 12 years presented decreases of over 50% in seizure frequency after one year of VNS, as compared to only 53% of patients older than 12 (P = .21).
According to the Engel classification, 15.7% of patients (11 patients) were in class I, 45.7% (32) in class II, 35.7% (25) in class III, and only 2.9% (2) in class IV.
Seizure durationTreatment reduced seizure duration in 88% of patients. The decreases were small in 19% of these patients, moderate in 46%, and marked in 35%.
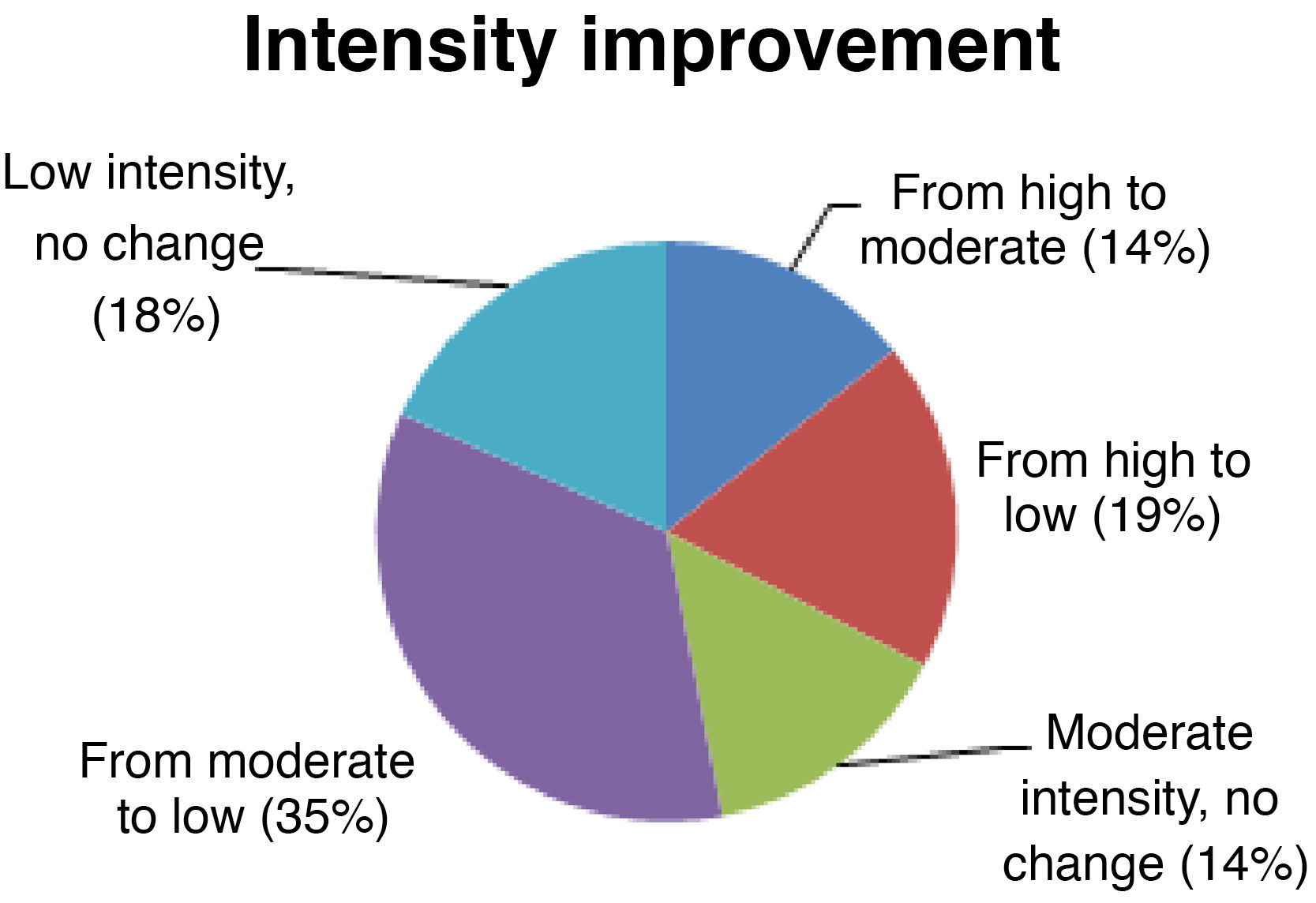
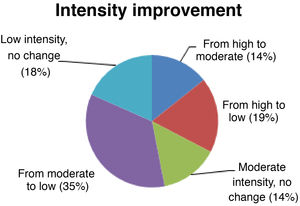
Seizure intensitySeizure intensity decreased from high to moderate in 14% of patients, from high to low in 19%, and from moderate to low in 34% (Fig. 4). The remaining patients did not report any changes in seizure intensity (14% of patients continue to have moderate-intensity seizures and 18% continue to have low-intensity seizures). All patients with high-intensity seizures showed improvements with VNS therapy.
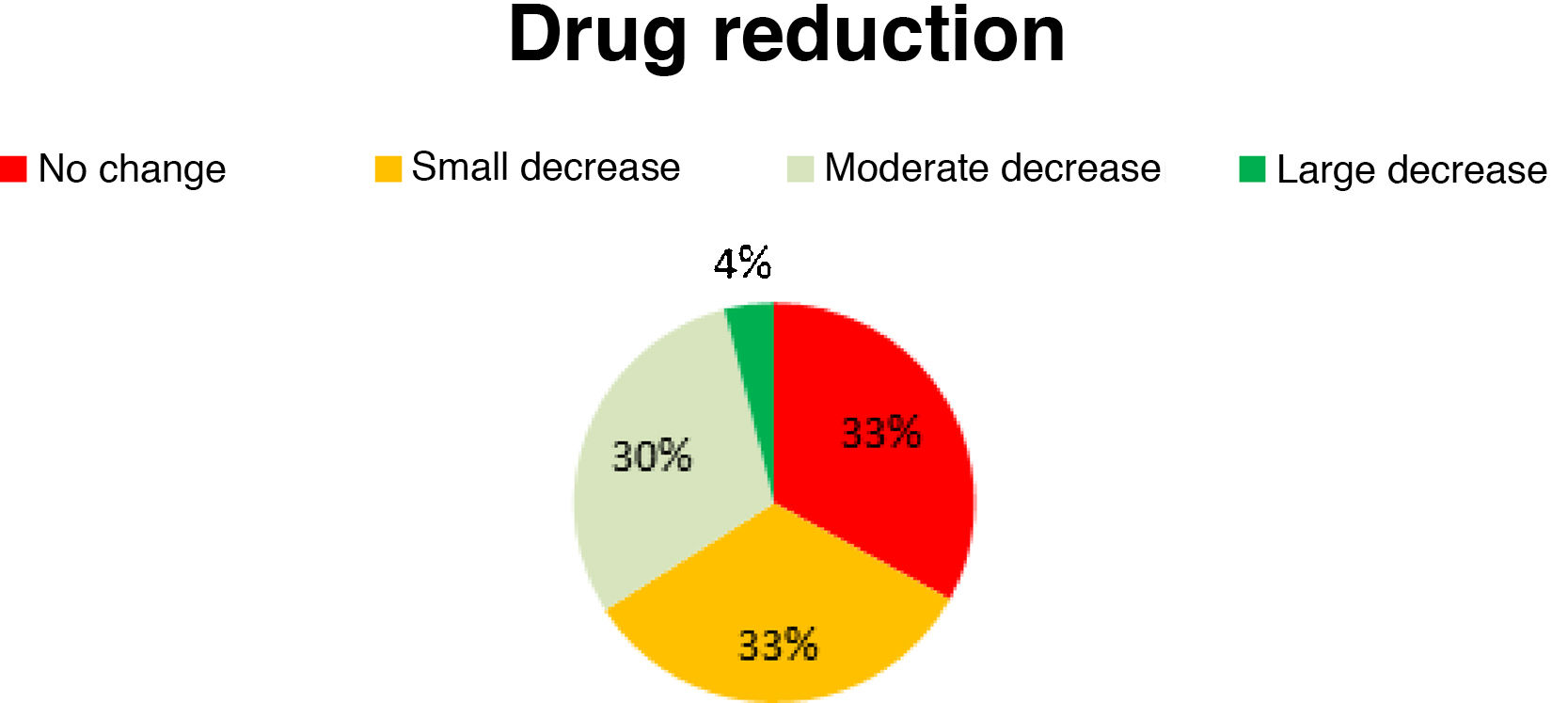
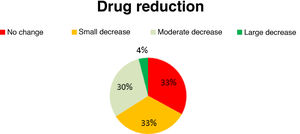
Antiepileptic drug useAED use decreased slightly in 33% of patients, moderately in 30%, and considerably in 4%; the remaining 33% presented no changes in AED use (Fig. 5). No new AEDs were introduced after implantation of the vagal nerve stimulator.
Adverse reactionsTwenty-one percent of patients reported adverse reactions: dysphonia (4.55%), cough (10.61%), cough and dysphonia (4.55%), and cervical dysaesthesia (3%). None of our patients presented heart rate alterations, hoarseness, or infections at the implantation site.
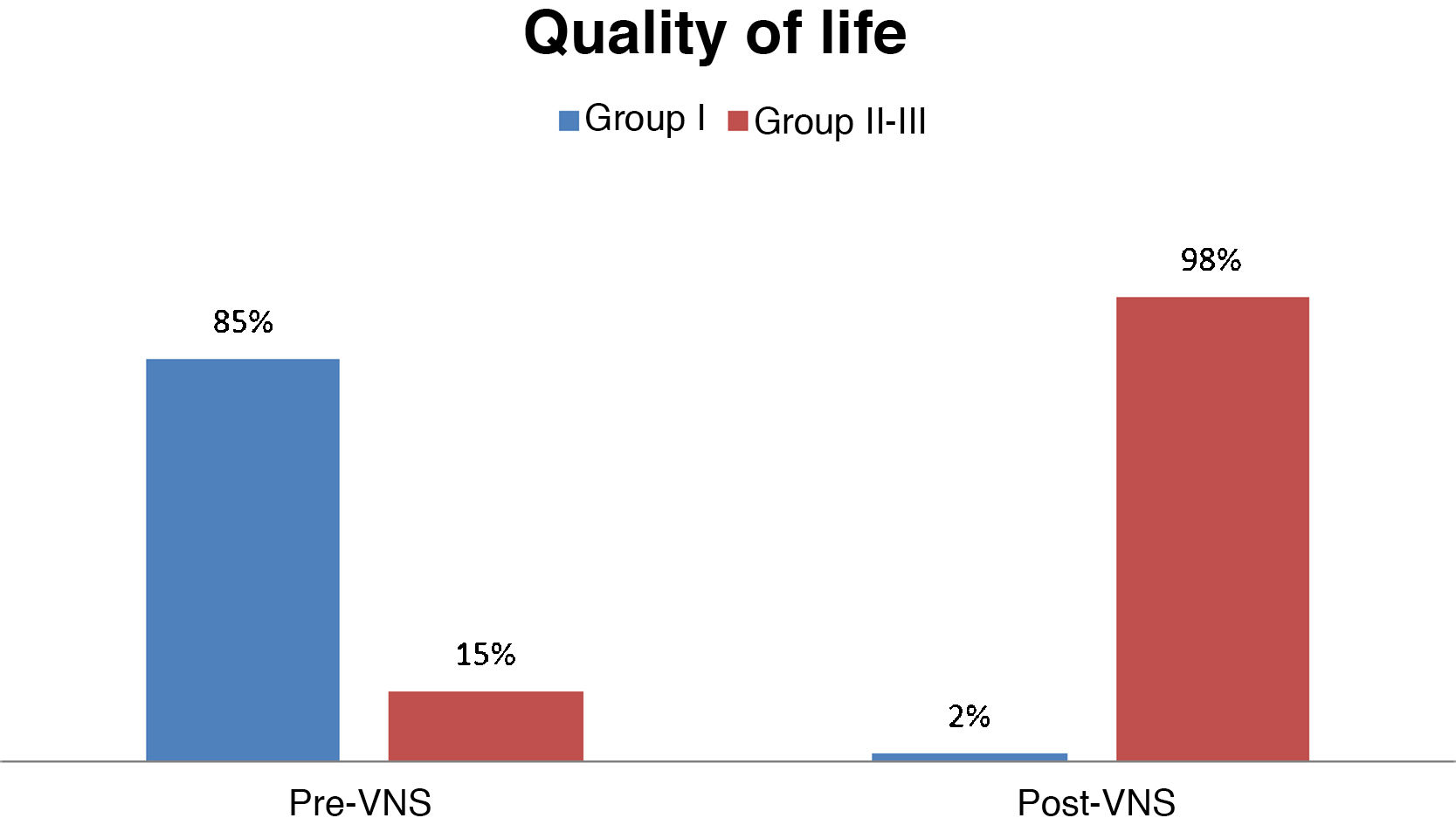
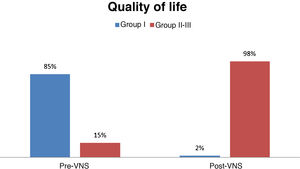
Quality of lifeBefore VNS, 85% of patients were in group I (poorer quality of life), 15% were in group II; and none of the patients were in group III (better quality of life). After VNS, only 2% of patients were in group I, whereas 98% were in groups II and III (59% in group II and 39% in group III); this change was statistically significant (P < .001) (Fig. 6).
Our sample showed a mean (SD) decrease of 8.45 points (7.2) in QOLIE-10 scale scores.
DiscussionThe available evidence suggests that VNS is a good alternative to pharmacological treatment in patients with refractory epilepsy. However, although many studies have evaluated the efficacy of the technique in reducing seizure frequency, reporting heterogeneous results, few have analysed its impact on seizure intensity and duration, AED use, and quality of life. This is largely due to the difficulty of gathering these data in retrospective studies.22–24
A high percentage of our patients presented a meaningful decrease in the total number of seizures (> 50% decrease in 57% of patients). In a meta-analysis by Connor et al.,13 50.9% of patients presented a decrease of over 50% in seizure frequency. Meng et al.25 classified 94 patients with the McHugh classification, finding that 35.1% were in class I, 28.7% in class II, 21.3% in class III, and 14% in class IV-V (63.8% of patients presented decreases of over 50% in seizure frequency). Wasade et al.26 reported similar results. In their study of 152 patients treated with VNS, seizure frequency decreased by over 50% in 68% of participants.
In the meta-analysis published in 2013 by Chambers and Bowen,12 only a quarter of patients achieved a decrease of over 50% in seizure frequency. However, this may have been due to the fact that most of the studies included in the meta-analysis gathered data at 3 and 6 months after implantation of the stimulator. Efficacy has been found to increase significantly with time (due to chronic thalamic deactivation)25; therefore, these results may be explained by the short duration of treatment.
Differences in the decrease in seizure frequency between patients with different seizure types were not statistically significant, probably due to the small size of our sample with regard to the number of variables under study. However, we did observe significant differences between patients with partial seizures and those with generalised seizures: while 63% of patients with generalised seizures presented a decrease of over 50% in seizure frequency, only 20% of patients with partial seizures achieved that outcome. Few studies have compared the efficacy of VNS in patients with these 2 types of seizures, using very small samples.
No significant differences were observed between patients younger than and older than 12 years in the decrease in seizure frequency. These findings are consistent with the results of a retrospective study by Elliott et al.,16 including 141 paediatric patients, in which VNS showed similar efficacy and safety in children younger than and older than 12 years. Murphy et al.17 also did not find significant differences.
However, in a study of 70 patients, Lagae et al.14 found that young age at VNS implantation predicts good prognosis. However, the authors only found statistically significant differences in the percentage of patients achieving seizure freedom between children younger than and older than 5 years (3 out of 4 patients achieving seizure freedom were younger than 5 years), but did not observe a higher global response rate between age groups.
Despite the lack of statistical significance, the trend observed in our study seems to support the hypothesis that device implantation at younger ages is associated with better treatment response. This hypothesis should be analysed in future studies.
Regarding seizure duration, up to 88% of our patients presented clear decreases. Of these, 85% presented moderate to high decreases.
We also observed significant decreases in seizure intensity (up to 68% of patients). Given that 18% of our patients already presented low-intensity seizures before VNS, only 14% did not benefit in this respect. Furthermore, seizure intensity decreased from high/moderate to low in 54% of patients; our results are therefore satisfactory.
In our sample, 33% of patients did not use fewer AEDs after onset of VNS therapy. The remaining patients (67%) presented a significant decrease in the number of AEDs used after implantation of the vagus nerve stimulator.
As mentioned previously, few studies have analysed the effect of VNS on seizure duration and intensity and AED use. One of the few studies addressing this topic is a retrospective study of 347 children, conducted by Orosz et al.,18 who observed decreased seizure duration in 42.9% of patients, decreased seizure intensity in 34%-42%, and decreased AED use in 50.4%. Future studies should analyse these parameters and confirm these results, as they have a major impact on patient quality of life.
The safety of the technique is also of great importance. In our study, only 21% of patients presented adverse reactions to VNS, which were mild in all cases (dysphonia, cough, cervical dysaesthesia). This finding is consistent with those reported in the literature. In a study of 68 patients undergoing VNS, 25.8% presented adverse reactions, most of which were mild (a single patient presented left vocal cord paralysis; none of the patients presented alterations in cardiac function).27
All these parameters have a major impact on quality of life. Quality of life increased significantly in our series. A large percentage of patients changed from class I before VNS to class II or III after treatment, with nearly 40% in class III (good quality of life). Quality of life is one of the most important variables in assessing the efficacy of VNS, as it reflects the impact of the remaining parameters.
Ryvlin et al.28 used the QOLIE-89 scale to compare improvements in quality of life between patients treated with VNS plus pharmacological treatment and patients receiving pharmacological treatment only. The authors observed mean decreases (SD) of 5.5 points (7.2) in the group receiving combination therapy and 1.2 points (7.9) in the group receiving pharmacological treatment only, concluding that combination therapy with VNS and pharmacological treatment significantly improves quality of life as compared to pharmacological treatment alone.
Our study has a number of limitations. Firstly, this study is retrospective; therefore, stimulation parameters were not standardised (although the study does reflect the parameters commonly set in clinical practice). The retrospective design did not allow us to include a control group; however, this would have been unethical, given the duration of the study. We were therefore unable to determine the influence of the placebo effect. Secondly, our sample was small for some comparisons, particularly for assessing the efficacy of VNS by seizure aetiology. Furthermore, we evaluated quality of life using a modified version of the QOLIE-10 scale, which included fewer questions. A more comprehensive analysis of quality of life would require the use of other scales, such as the QOLIE-10, QOLIE-39, or QOLIE-89 scales. Quality of life data were gathered after a year of VNS therapy, using a cross-sectional approach. We were therefore unable to analyse time-dependent fluctuations during that period, which very likely would have occurred. Lastly, clinical variables were recorded by patients and/or their relatives using a seizure diary. This data collection method may have resulted in an overestimation of the response to VNS, since some seizures may have gone undetected (particularly after the decreases in seizure intensity and duration), especially myoclonic and absence seizures.
ConclusionsVNS effectively decreases seizure frequency, duration, and intensity, and AED use. It is also a safe technique, presenting very few, mild adverse reactions. None of our patients presented severe or life-threatening complications or were forced to terminate treatment due to adverse reactions to VNS.
VNS was found to be more efficacious for the treatment of generalised seizures than for partial seizures; future studies should confirm these results.
Like other researchers, we observed no significant differences in treatment efficacy between paediatric patients, on the one hand, and adolescents and adults, on the other; however, this should be confirmed by multivariate analyses of larger samples.
VNS implantation is an efficacious surgical technique for the treatment of refractory epilepsy, achieving significant improvements in these patients’ quality of life.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Martorell-Llobregat C, González-López P, Luna E, Asensio-Asensio M, Jadraque-Rodríguez R, García-March G, et al. Papel de la estimulación del nervio vago en el tratamiento de la epilepsia refractaria. Resultados clínicos e impacto en la calidad de vida. Neurología. 2022;37:450–458.