Drugs impact brain reward circuits, causing dependence and addiction, in a condition currently described as substance use disorders. Mechanisms of synaptic plasticity in these circuits are crucial in the development of addictive behaviour, and endocannabinoids, particularly anandamide and 2-arachidonyl-glycerol, participate in normal neuroplasticity. Substance use disorders are known to be associated with disruption of endocannabinoid-mediated synaptic plasticity, among other phenomena. Endocannabinoids mediate neuroplasticity in the short and the long term. In the short term, we may stress “inhibitory” phenomena, such as depolarisation-induced suppression of inhibition and depolarisation-induced suppression of excitation, and such “disinhibitory” phenomena as long-lasting disinhibition of neuronal activity, particularly in the striatum, and suppression of hippocampal GABA release. Drugs of abuse can also disrupt normal endocannabinoid-mediated long-term potentiation and long-term depression. Endocannabinoids are also involved in the development of drug-induced hypofrontality and sensitisation. In summary, substance abuse causes a disruption in the synaptic plasticity of the brain circuits involved in addiction, with the alteration of normal endocannabinoid activity playing a prominent role. This facilitates abnormal changes in the brain and the development of the addictive behaviours that characterise substance use disorders.
Las drogas impactan en los circuitos de recompensa cerebrales y originan dependencia y adicción, lo que se define actualmente como trastornos por consumo de drogas. Los mecanismos de plasticidad sináptica en dichos circuitos son cruciales en el desarrollo de la conducta adictiva, y los endocannabinoides, entre los que destacan la anandamida y el 2-araquidonil-glicerol, participan en la normal neuroplasticidad. Se sabe que los trastornos por consumo de drogas se asocian, entre otros fenómenos, a disrupción de la plasticidad sináptica mediada por endocannabinoides. Estas moléculas median neuroplasticidad de corta duración y perdurable. Respecto a la de corta duración, destacan fenómenos de carácter «inhibidor», como la supresión de la inhibición inducida por despolarización y la supresión de la excitación inducida por despolarización; y otros «desinhibidores», como la desinhibición de la actividad neuronal, sobre todo en el núcleo estriado, y la supresión de la liberación GABA en el hipocampo. Por otra parte, las drogas pueden alterar la normal potenciación perdurable y la depresión perdurable mediadas por endocannabinoides. Los endocannabinoides también influyen en el desarrollo de hipofrontalismo y sensibilización causados por las drogas. En fin, el abuso de drogas origina una disrupción en la plasticidad sináptica de circuitos cerebrales involucrados en la adicción y en ello juega un destacado papel la alteración de la normal actividad endocannabinoide. Ello facilita los cambios anómalos cerebrales y el desarrollo de conductas adictivas que caracterizan a los trastornos por consumo de drogas.
While it is clear that drugs can be addictive, “addictive” is a difficult word to define. Several terms have been used to define the psychobiological effect caused by drugs of abuse; these include addiction, dependence, abuse (Diagnostic and Statistical Manual of Mental Disorders-IV [DSM-IV]), and harmful use (International Classification of Diseases, 10th revision [ICD-10]).1 Addiction is defined as a chronic and recurrent disease of the brain, characterised by searching for and compulsively consuming drugs, despite their harmful consequences.2 Dependence would be a broader term than addiction, encompassing mild (for example, dependence on caffeine) to compulsive needs (an established addiction), according to 6 criteria.1 Abuse (DSM-IV terminology), or harmful use (ICD-10 terminology), is based on a list of somatic and psychological effects of drug use. Today, the DSM-V3 no longer uses these terms; instead, it includes “substance use disorders” (SUD), defined according to 11 criteria (Table 1) and ranging from mild (presence of fewer than 3 criteria) to severe (more than 6 criteria).
Criteria for the diagnosis of substance use disorders.
| 1. Taking the substance in larger amounts or for longer than you’re meant to. |
| 2. Wanting to cut down or stop using the substance but not managing to. |
| 3. Spending a lot of time getting, using, or recovering from use of the substance. |
| 4. Cravings and urges to use the substance. |
| 5. Not managing to do what you should at work, home, or school because of substance use. |
| 6. Continuing to use, even when it causes problems in relationships. |
| 7. Giving up important social, occupational, or recreational activities because of substance use. |
| 8. Using substances again and again, even when it puts you in danger. |
| 9. Continuing to use, even when you know you have a physical or psychological problem that could have been caused or made worse by the substance. |
| 10. Needing more of the substance to get the effect you want (tolerance). |
| 11. Development of withdrawal symptoms, which can be relieved by taking more of the substance. |
These criteria are based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-V).3
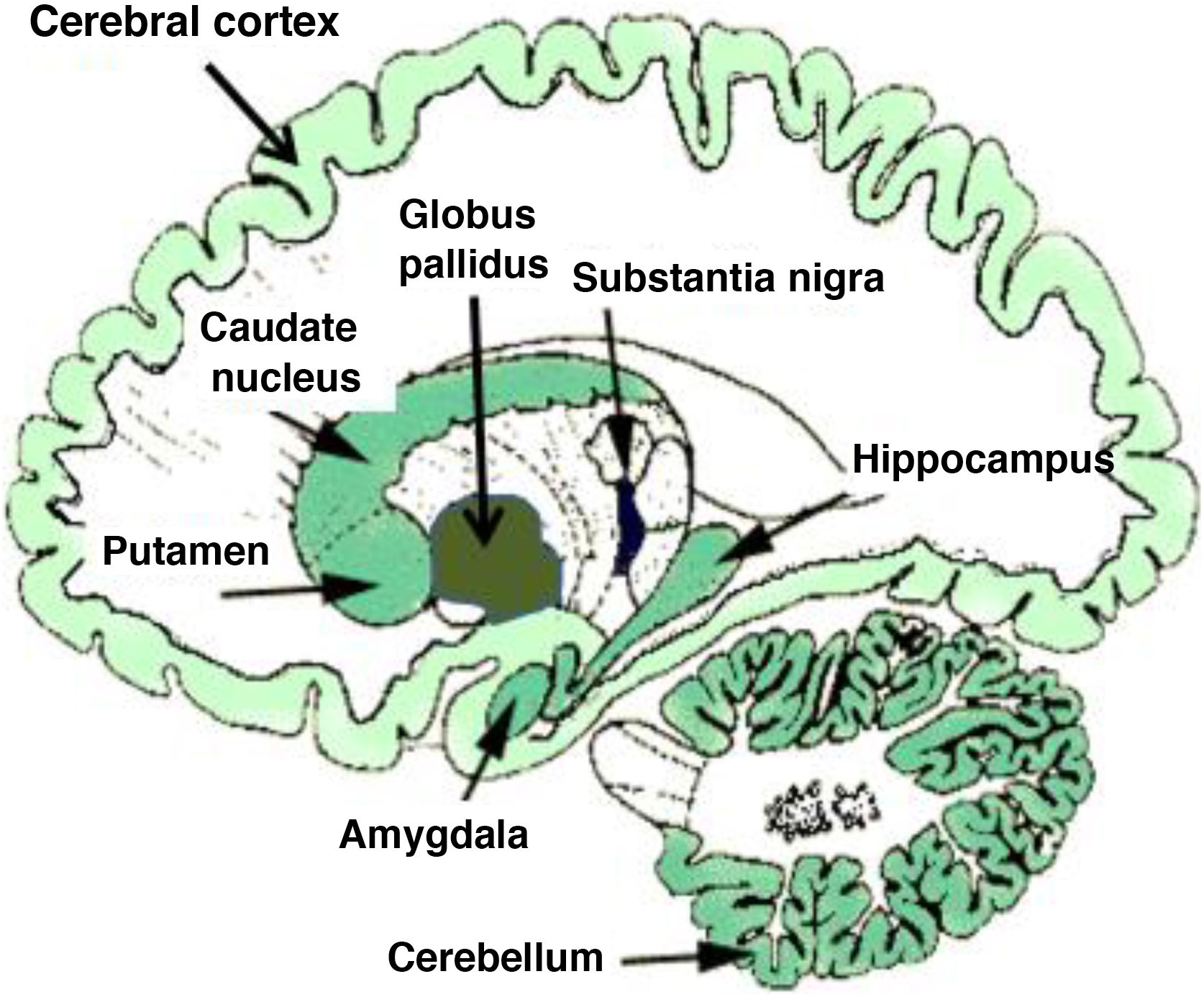
From a neurobiological viewpoint, drugs or addictive substances have an impact on the brain’s reward circuits, originating in the ventral tegmental area (VTA), as well as on limbic and memory-related regions including the amygdala and the hippocampus. Brain reinforcement mechanisms and synaptic plasticity in these circuits are essential in the development of addiction and addictive behaviour.2,4–6 Basic neuroplasticity events occur in mesolimbic reward circuits, which include the nucleus accumbens and ventral striate, the prefrontal cortex, and the VTA in the midbrain, as mentioned previously. These limbic structures consolidate the abnormal behaviour, associating numerous internal and external events with drug-related reward (conditioning). These conditioned effects are critical in the development of addiction, both in the active consumption phase and in the abstinence phase.7 In terms of neurobiological effects, the neurotransmitters dopamine, GABA, and glutamate are key factors in mesolimbic neuroplasticity; the increasing relevance of such other neuromessengers as endocannabinoids is also worth mentioning. Endocannabinoids are known to cause retrograde modulation of synaptic signals, especially in glutamatergic and GABAergic synapses, and play a crucial role in synaptic neuroplasticity phenomena. SUDs are associated with a disruption of endocannabinoid-mediated plasticity. Fig. 1 shows the distribution of endocannabinoids in the human brain.
Distribution of endocannabinoids in the human brain. Darker tone indicates higher density of endocannabinoid receptors.
The first endocannabinoid to be discovered was anandamide (N-arachidonoylethanolamine, AEA), followed by 2-arachidonoylglycerol (2-AG) 2 years later.8,9 Other cannabinoid molecules have subsequently been identified, including 2-arachidonoylglycerol ether, N-arachidonoyl dopamine, virodhamine, N-homo-gamma-linolenoylethanolamine, and N-docosatetra-7,10,13,16-enoylethanolamine.10–13 Because endocannabinoids are lipidic compounds, synaptic vesicular storage is impossible due to their high liposolubility. Therefore, they are synthesised on demand by membrane precursors. After acting, endocannabinoids are rapidly degraded by reuptake (both by neurons and by glial cells) and subsequent hydrolysis.14,15
Endocannabinoids act on specific cannabinoid receptors, especially types 1 and 2 (CB1 and CB2). The first cannabinoid receptor described in the brain, in 1988,16 was CB1; it was molecularly cloned in humans,17 and identified as a member of the superfamily of receptors of G-protein-coupled neurotransmitters. A second cannabinoid receptor, CB2, also belonging to the family of G-protein-coupled membrane receptors, was identified at the peripheral level.18 CB2 receptors share an overall homology of 44% with CB1 receptors (68% in transmembrane regions); they are abundantly expressed in lymphocytes, which suggests that this subtype of receptor mediates the immunomodulatory action of cannabinoids. Although the CB2 receptor has been identified in glial cells in the brain,19 CB1 is the main cerebral cannabinoid receptor in neurons. In the mammal brain, CB1 proteins are preferentially expressed in neuronal populations closely related to addiction and reward systems. A high density of CB1 receptors is found in the neurons of the nucleus accumbens, dorsal striatum, and cerebellum, which also explains the effects of acute stimulation (ataxia, dysmetria, and hypokinesia); the receptor is also found in the neurons of the hippocampus and amygdala. The remaining areas, such as the neocortex, superior colliculus, and habenula, show a moderate presence of these receptors. As previously mentioned, drugs of abuse alter both short- and long-term endocannabinoids-mediated synaptic neuroplasticity. Table 2 lists the SUD-related synaptic plasticity phenomena involving endocannabinoids.
Synaptic plasticity phenomena associated with substance use and involving the participation of endocannabinoids.
| Short-term plasticity |
|---|
| Depolarisation-induced suppression of inhibition |
| Depolarisation-induced suppression of excitation |
| Long-lasting disinhibition of neuronal activity |
| Suppression of GABAergic release in the hippocampus |
| Long term plasticity |
|---|
| Long term depression |
| Long term potentiation |
| Hypofrontality |
| Sensitisation |
Endocannabinoids mediate short-term plasticity. Short-term plasticity phenomena may be either inhibitory or disinhibitory. Among inhibitory endocannabinoid-mediated phenomena, particularly important processes are depolarisation-induced suppression of inhibition (DSI) and depolarisation-induced suppression of excitation (DSE); these phenomena reduce the synaptic release of glutamate, GABA, or glycine.20–22 Both processes are based on the fact that release of endocannabinoids after synaptic activity inhibits the subsequent presynaptic calcium influx and the release of the (excitatory or inhibitory) neurotransmitter, as has been shown in the cerebellum,21 hippocampus,20 or brainstem nuclei.22 Endocannabinoids induce DSI and DSE through CB1 receptors: CB1 antagonists and agonists block and stimulate those synaptic phenomena, respectively.20,23 DSI phenomena are associated with dependence; for example, caffeine provokes a considerable alteration of DSI mediated by GABA.24
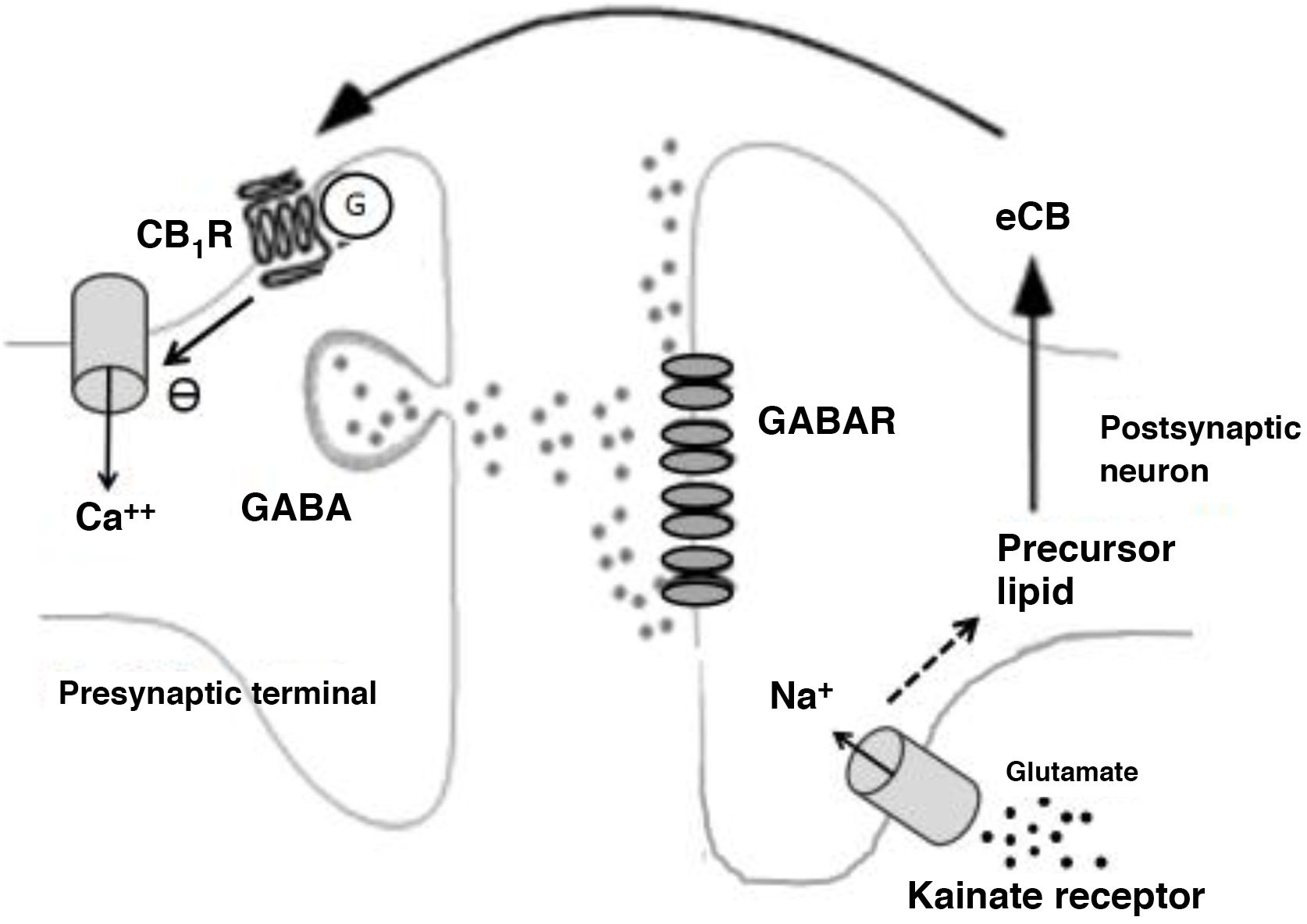
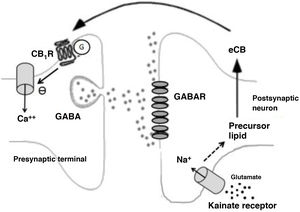
Among disinhibitory endocannabinoid-mediated phenomena, we should highlight the disinhibition of neuronal activity, especially endocannabinoid-mediated disinhibition in the striatum (long-lasting disinhibition; DLL), and the suppression of GABA release in the hippocampus.26 Both processes are mediated by CB receptors.25 In the case of the suppression of GABA release in the hippocampus, glutamate release activates kainate receptors, which in turn stimulate the production of endocannabinoids that suppress synaptic GABA release (Fig. 2).26
Biochemical phenomena occurring during endocannabinoid-mediated suppression of GABA release in the hippocampus. Glutamate release activates postsynaptic kainate receptors, which induce sodium influx and production of endocannabinoids. Endocannabinoids are released into the synaptic space and act on G protein–coupled CB1 receptors on terminals that release GABA. This induces a decrease in presynaptic calcium influx and a decrease in GABA release.
Ca++: calcium; CB1R: CB1 receptor; eCB: endocannabinoid; G: G protein; GABAR: GABA receptor; Na+: sodium.
Short-term changes seem to be mediated mainly by 2-AG.27 Short-term synaptic changes last only minutes, but modify synaptic transmission and may underlie acute hedonic and motor changes caused by drugs. For example, alcohol blocks DLL phenomena induced by endocannabinoids in the dorsal striatum, which is associated with abnormal motor response.28 Alcohol alters the delicate balance of excitation and inhibition in the striatum, mediated by endocannabinoids.28 Drugs alter DSI and DSE phenomena in several regions associated with addiction, such as the VTA, amygdala, striatum, hippocampus, and neocortex.29–32
Endocannabinoids and long-term neuroplasticityEndocannabinoids also participate in long-term plasticity phenomena, which are longer-lasting and more stable in the brain engram; these phenomena include long-term potentiation (LTP) and long-term depression (LTD). LTP and LTD are important in memory and learning consolidation; drugs modify the normal neurophysiology of these processes, promoting the consolidation of addiction.
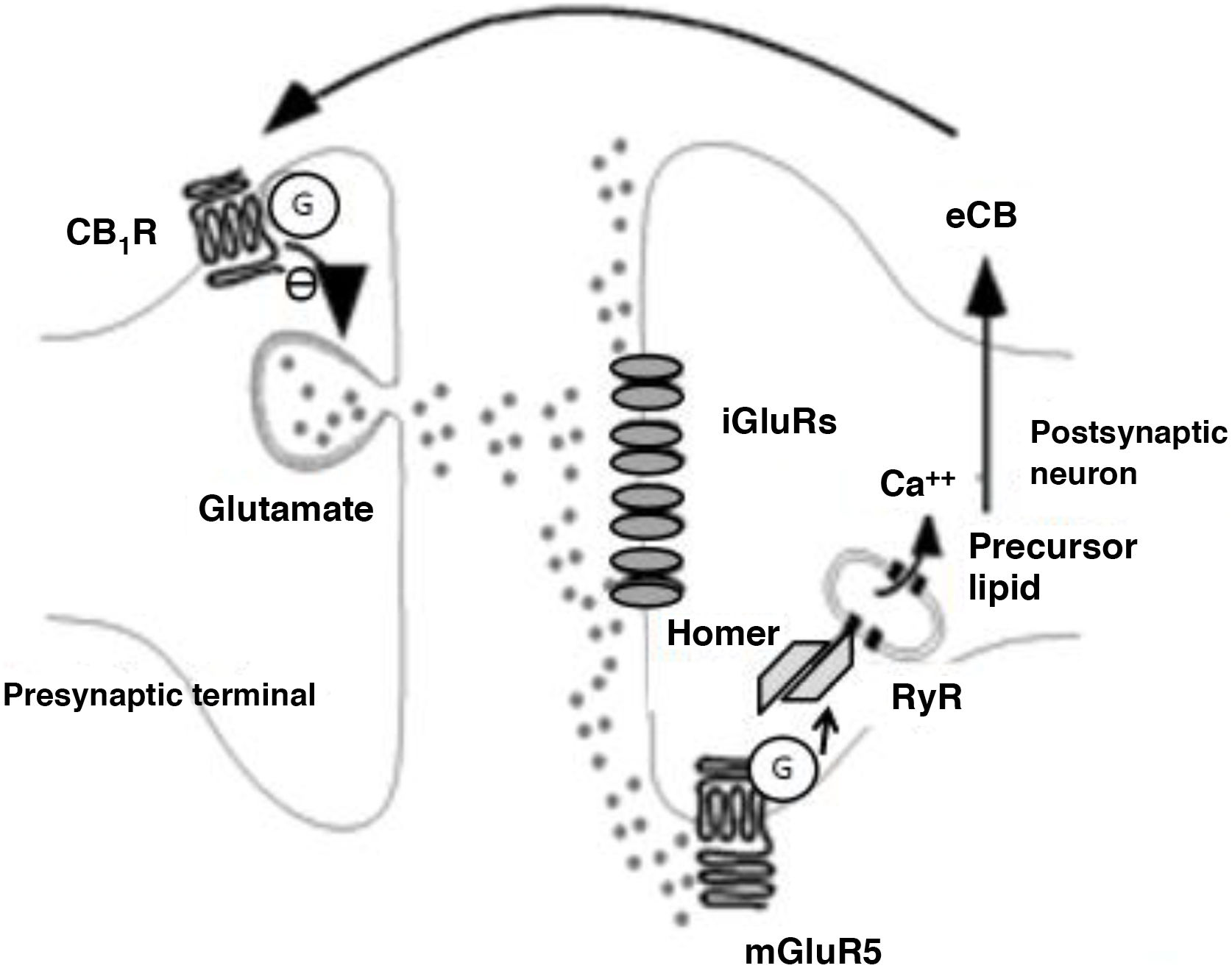
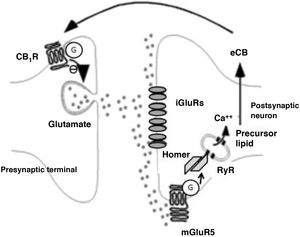
Endocannabinoids mainly participate in LTD phenomena. Endocannabinoid-mediated LTD occurs after a transient increase in glutamate levels, triggering increased postsynaptic endocannabinoid production, which in turn causes a long-term decrease in glutamate release, as is shown in Fig. 3.33 This phenomenon has been detected in several structures associated with addiction, such as the nucleus accumbens, dorsal striatum, prefrontal cortex, amygdala, hippocampus, and VTA. The main LTD-mediating endocannabinoids is AEA,34 except in the hippocampus, where it seems to be 2-AG.35 Such drugs as δ9-THC and cocaine block endocannabinoid-induced LTD in the nucleus accumbens, at least in animal models.36,37 Alcohol also blocks endocannabinoid-induced LTD phenomena, in this case in the dorsal striatum.28 Amphetamines block LTD in the amygdala through their action on endocannabinoids.38
Biochemical phenomena occurring during endocannabinoid-mediated long-term depression. Glutamate release activates postsynaptic ionotropic receptors and type 5 metabotropic glutamate receptors. The latter induce the production of endocannabinoids through Homer proteins and ryanodine receptors in calcium-storing vesicles and the endoplasmic reticulum. Endocannabinoids are released into the synaptic space and act on G protein–coupled CB1 receptors on terminals that release glutamate. This induces a decrease in presynaptic glutamate release.
Ca++: calcium; CB1R: CB1 receptor; eCB: endocannabinoid; G: G protein; iGluR: ionotropic glutamate receptors; mGluR5: type 5 metabotropic glutamate receptors; RyR: ryanodine receptor.
Another important plasticity phenomenon caused by drugs is hypofrontality: in the prefrontal cortex, interaction between dopamine (through D2 receptors), endocannabinoids, and several drugs of abuse may provoke prefrontal dopaminergic hypoactivity.39 The importance of this process in the development of addiction is increasingly recognised, and it constitutes the basis of hypofrontality.40 There is mounting evidence suggesting that frontal areas, and especially the prefrontal area, prepare the subject to adequately respond to acute stress and show resilience to future stress. Endocannabinoids are key mediators in frontal activity, and endocannabinoid alterations due to drug abuse significantly modify the subject’s response and resilience to stress.41
Another significant plasticity phenomenon in the development of addiction is sensitisation to addiction, although the role of endocannabinoids in these cases is not so relevant.42 Acute administration of several drugs increases dopamine release in the main areas of the mesolimbic circuit, ie, the VTA and nucleus accumbens. This dopamine release is reinforced by the chronic consumption of the drug, in a process called dopaminergic sensitisation.2,43,44 This represents a crucial aspect differentiating addictive drugs from natural reinforcers (food, water, and sex), which do not present dopaminergic sensitisation.2 Sensitisation is related to LTP, and several drugs including psychostimulants, opiates, ethanol, and nicotine are known to induce the LTP phenomenon in the VTA.45–47 However, from a biochemical perspective, sensitisation is mainly mediated by an increase in glutamatergic transmission, NMDA and D1 dopamine receptor upregulation, and hyperactivity of the cAMP/protein kinase A pathway.2 Therefore, endocannabinoids do not play a relevant role; they are only known to play a clear role in sensitisation to opiates.48 In fact, endocannabinoids act on CB1 receptors located on dopaminergic neurons in the VTA, and mediate sensitisation to opiates. Sensitisation to cocaine has also recently been associated with CB1 receptors and, possibly, with endocannabinoids.49,50
Lastly, endocannabinoids and CB1 receptors are important in incentive sensitisation phenomena, which is the potentiation over time of the reinforcing role of spatial cues associated with the consumption of the drug; this type of conditioning is also essential in the development of drug addiction. For example, endocannabinoids facilitate behavioural association to spatial cues caused by cocaine,51 nicotine,52 and alcohol.53
ConclusionsDrug abuse disrupts the synaptic plasticity of cerebral circuits involved in the development of the addiction and plays an important role in the alteration of the normal endocannabinoid activity. Endocannabinoid alteration facilitates anomalous changes in the brain and the development of the addictive behaviours that characterise SUD. Without doubt, good understanding of these phenomena will facilitate the development of treatments based on the modulation of endocannabinoids in the brain, with the aim of minimising the devastating effects of SUD.
Conflicts of interestThe authors have no conflicts of interest to declare.
We are grateful for the scientific support of Dr Fernando Rodríguez de Fonseca (IMABIS, Hospital Regional Universitario de Málaga). This study was supported by the Addictive Disorder Network (RETICS subprogramme, RD16/0001/0017, Institute of Health Carlos III).
Please cite this article as: Fernández-Espejo E, Núñez-Domínguez L. La plasticidad sináptica mediada por endocannabinoides y «trastornos por consumo de drogas». Neurología. 2022;37:459–465.