Traumatic brain injury (TBI) is one of the leading causes of death and disability globally. We present a study describing epidemiological changes in severe TBI and the impact these changes have had on management and analysing alternatives that may improve outcomes in this new population.
Materials and methodsWe performed a retrospective, descriptive, cross-sectional analysis of patients presenting severe TBI at our hospital in the period of 1992-1996 and 2009-2013. We analysed demographic data, including age, sex, mortality, aetiology, anticoagulation, treatment, and functional outcome.
ResultsWe reviewed data from 220 patients. In the second cohort, there were 40% fewer patients, mean age was 12 years older, patients were more frequently receiving anticoagulation therapy, and the percentage of interventions was halved. Aetiology varied, with traffic accidents being the main cause in the first group, and accidental falls and being hit by cars in the second group. There were no intergroup differences for mortality or functional outcomes.
ConclusionThe age of patients admitted due to severe TBI has increased. As a result of this, the main cause of severe TBI in our population is accidental falls in elderly, anticoagulated patients. Despite the low-energy nature of trauma, patients in the second cohort presented a poorer baseline status, and were less frequently eligible for surgery, with no improvement in mortality or functional outcomes.
El TCE es una de las principales causas de muerte y discapacidad a nivel mundial. Presentamos este estudio con el objetivo de detallar el cambio epidemiológico de la población que sufre TCE severo, su influencia en el tipo de tratamiento ofrecido y analizar alternativas que mejoren los resultados ante el nuevo tipo de población que afrontamos.
Material y métodosSe ha realizado un análisis descriptivo, transversal y retrospectivo de los pacientes que sufrieron TCE severo en nuestro hospital en los periodos 1992-1996 y 2009-2013. Se analizaron datos demográficos como edad, sexo, mortalidad, etiología, anticoagulación, tratamiento realizado y resultados funcionales.
ResultadosSe revisaron 220 pacientes. En la segunda cohorte el número de pacientes con TCE severo disminuyó un 40%, eran de media 12 años mayores, más frecuentemente anticoagulados y las intervenciones se redujeron a la mitad. Varió la etiología, predominando en el primer grupo los accidentes de tráfico y en el segundo las caídas casuales y los atropellos. No hubo diferencias en la mortalidad de ambos grupos, y sí en su situación funcional.
ConclusiónEn este estudio encontramos un envejecimiento de la población que ingresa por TCE severo. Ello hace que, en la actualidad, la principal causa de TCE severo en nuestra población sean las caídas casuales en pacientes anticoagulados mayores. A pesar de ser traumatismos de poca energía, los pacientes presentan peores condiciones basales y son menos candidatos a cirugía, sin que mejoren la mortalidad ni la situación funcional.
Traumatic brain injury (TBI) is one of the leading causes of death and disability worldwide. According to the World Health Organization, it will become the main cause of death and disability in 2020.1
Rapid population ageing in developed countries is rendering many concepts and management strategies obsolete. The population is now older, with higher rates of multimorbidity, which should lead to changes in management approaches.
The purpose of this study is to analyse changes in the demographics and treatment of patients with severe TBI at our hospital over the past 2 decades.
Material and methodsWe conducted a descriptive, cross-sectional, retrospective study of all patients older than 18 years admitted to our hospital's intensive medicine department (IMD) due to severe TBI, defined as a post-resuscitation Glasgow Coma Scale (GCS) score ≤ 8.2 We analysed 2 different cohorts: patients admitted between January 1992 and December 1996, and patients admitted between January 2009 and December 2013. We did not exclude patients with pupillary abnormalities or those with a GCS score of 3.
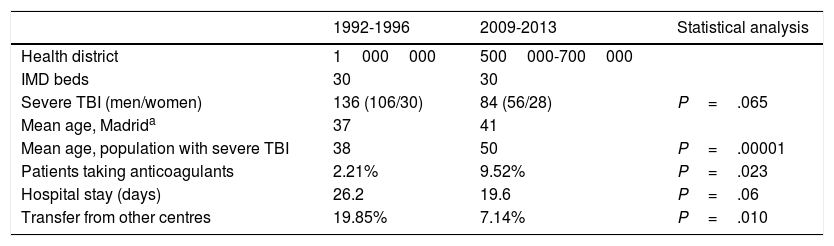
Hospital Universitario La Paz, a tertiary-level hospital, is one of Madrid's reference centres for patients with multiple trauma; our hospital has a team of intensivists and neurosurgeons on site 365 days a year. Our centre serves a population that has ranged from 500000 to 1000000 people over time (Table 1). Our IMD is a multidisciplinary unit with 30 beds. Between the first and the second periods studied, 2 new hospitals with neurosurgery departments were established in healthcare districts originally covered by our centre.
Comparison of demographic data between periods.
| 1992-1996 | 2009-2013 | Statistical analysis | |
|---|---|---|---|
| Health district | 1000000 | 500000-700000 | |
| IMD beds | 30 | 30 | |
| Severe TBI (men/women) | 136 (106/30) | 84 (56/28) | P=.065 |
| Mean age, Madrida | 37 | 41 | |
| Mean age, population with severe TBI | 38 | 50 | P=.00001 |
| Patients taking anticoagulants | 2.21% | 9.52% | P=.023 |
| Hospital stay (days) | 26.2 | 19.6 | P=.06 |
| Transfer from other centres | 19.85% | 7.14% | P=.010 |
IMD: intensive medicine department; TBI: traumatic brain injury.
We gathered data on the following epidemiological variables: age, sex, mechanism of injury (categories are subsequently subdivided into high-energy or low-energy trauma), and transfer from another hospital. We also gathered the following clinical data: post-resuscitation GCS score (once the patient was stable and before neurosurgery), baseline injury severity according to the Traumatic Coma Data Bank CT classification, duration of hospital stay, long-term anticoagulation treatment, comorbidities, pupillary reactivity at admission, duration of stay at the IMD (in days), mortality at the IMD (early, < 72 hours, or late), functional status at 6 months according to the Glasgow Outcome Scale (GOS; classified as good vs poor), emergency neurosurgery and type of intervention (craniotomy vs craniectomy), intracranial pressure (ICP) monitoring, and use of an external ventricular drain.
Statistical analysisWe used the Fisher exact test and the chi-square test to compare categorical variables, and the t test to analyse continuous variables with homogeneous variances. We also conducted multivariate logistic regression analyses of mortality and GOS classification. Statistical analysis was conducted using version 12 of the Stata software (StataCorp, 2011; College Station, TX, USA).
ResultsA total of 220 patients with severe TBI were admitted to our unit during the periods studied: 136 between 1992 and 1996 and 84 between 2009 and 2013. Table 1 summarises the demographic variables of the 2 groups. In the second period, mean age was 12 years older (P=.00001), and more patients were women (22% vs 33%; not significant). The number of transfers from other hospitals was significantly lower in the second period (7.14% vs 19.85% in the first period; P=.010). More patients from the second period received anticoagulation therapy (9.52% vs 2.21%; P=.023). No significant differences were observed in length of stay at the IMD (26.2 days in the first period vs 19.6 days in the second; P=.06).
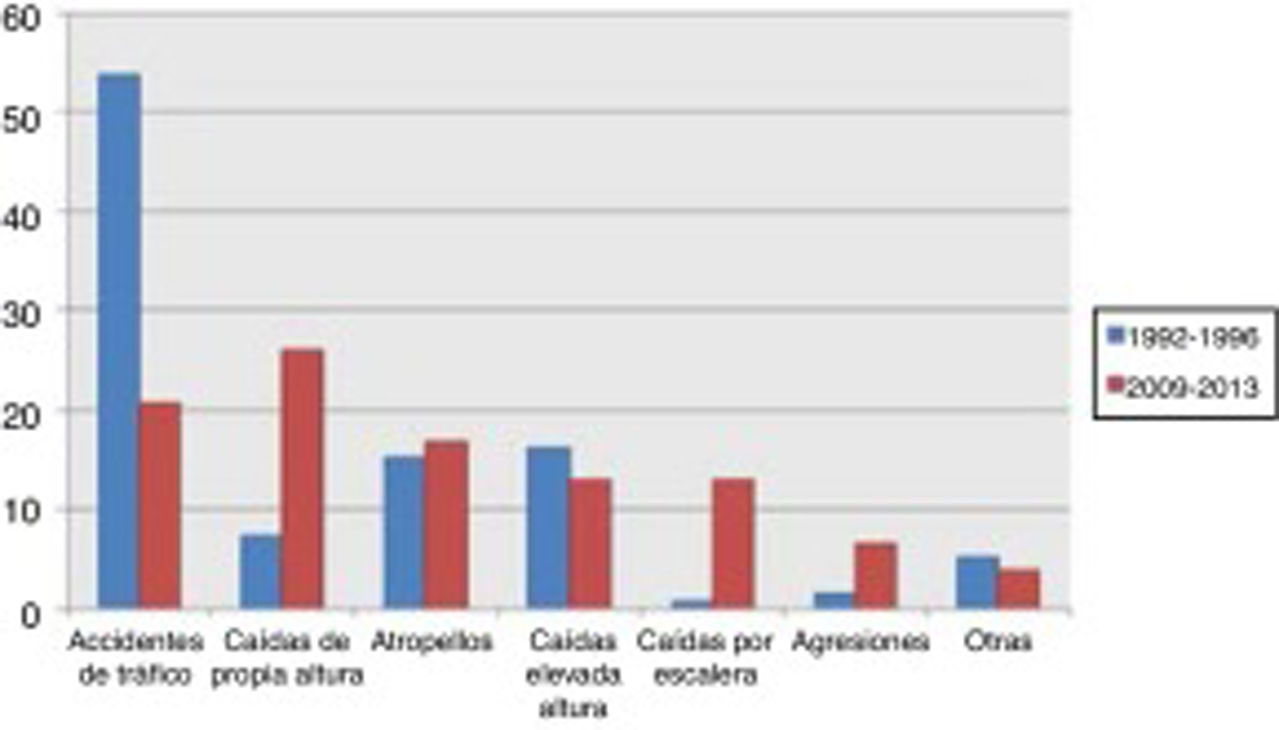
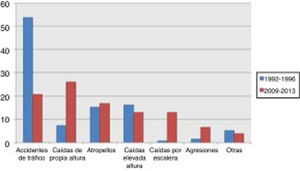
Traffic accidents decreased from 52.9% of cases in the first period to 17.9% in the second period, and therefore ceased to be the leading cause of severe TBI in our population. Falls from the same level constituted the main cause of severe TBI in the 2009-2013 period, with a four-fold increase in frequency (from 8% to 36.9%). No significant changes were observed in other mechanisms of injury, including pedestrian accidents, despite the latter being one of the main causes of severe TBI (Fig. 1).
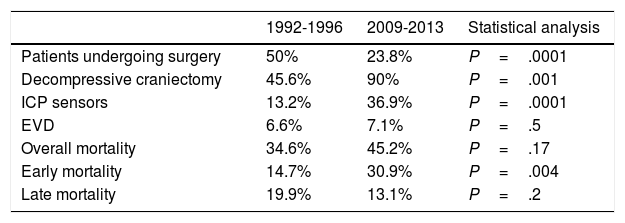
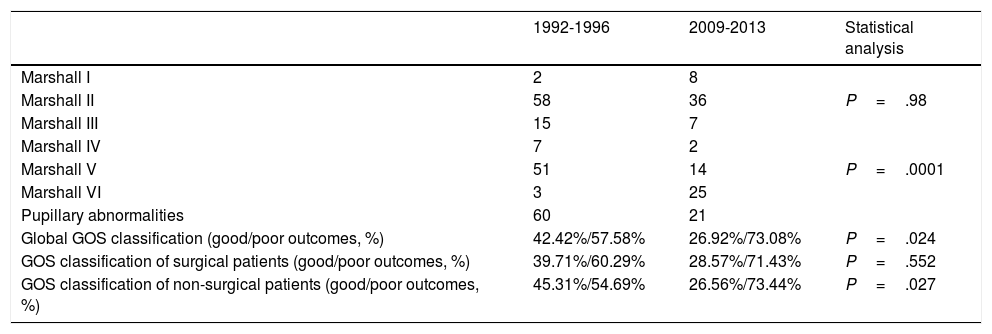
Regarding surgical management, the percentage of patients undergoing surgery decreased by half. Fifty percent of patients underwent surgery between 1992 and 1996, compared to 23.8% between 2009 and 2013 (P=.0001) (Table 2). The surgical technique used has changed over time: while 90% of surgeries in the second period consisted of decompressive craniectomy, only 45.6% of surgical patients in the first period underwent this procedure (P=.001). Insertion of ICP sensors increased from 13.2% to 36.9% (P=.0001). No significant changes were observed in the use of external ventricular drains (Table 2) or in the radiological distribution of lesions according to the Marshall classification, except for categories V and VI (lesions surgically evacuated and not surgically evacuated) (Table 3). Likewise, no significant changes were found in the percentage of patients with pupillary abnormalities (Table 3).
Analysis of patients undergoing surgery and mortality in the intensive medicine department.
| 1992-1996 | 2009-2013 | Statistical analysis | |
|---|---|---|---|
| Patients undergoing surgery | 50% | 23.8% | P=.0001 |
| Decompressive craniectomy | 45.6% | 90% | P=.001 |
| ICP sensors | 13.2% | 36.9% | P=.0001 |
| EVD | 6.6% | 7.1% | P=.5 |
| Overall mortality | 34.6% | 45.2% | P=.17 |
| Early mortality | 14.7% | 30.9% | P=.004 |
| Late mortality | 19.9% | 13.1% | P=.2 |
EVD: external ventricular drain; ICP: intracranial pressure.
Marshall classification after patient stabilisation, pupillary abnormalities at admission, and Glasgow Outcome Scale scores in our sample, by period.
| 1992-1996 | 2009-2013 | Statistical analysis | |
|---|---|---|---|
| Marshall I | 2 | 8 | |
| Marshall II | 58 | 36 | P=.98 |
| Marshall III | 15 | 7 | |
| Marshall IV | 7 | 2 | |
| Marshall V | 51 | 14 | P=.0001 |
| Marshall VI | 3 | 25 | |
| Pupillary abnormalities | 60 | 21 | |
| Global GOS classification (good/poor outcomes, %) | 42.42%/57.58% | 26.92%/73.08% | P=.024 |
| GOS classification of surgical patients (good/poor outcomes, %) | 39.71%/60.29% | 28.57%/71.43% | P=.552 |
| GOS classification of non-surgical patients (good/poor outcomes, %) | 45.31%/54.69% | 26.56%/73.44% | P=.027 |
GOS: Glasgow Outcome Scale (good outcome: 1-2 points; poor outcome: 3-5 points). GOS: 1=good recovery; 2=moderate disability but patient is independent for daily living activities; 3=severe disability, patient is dependent for daily living activities; 4=neurovegetative state; 5=death.
Overall mortality increased from 34.6% in the first period to 45.2% in the second period; this change was not statistically significant. Early mortality increased from 14.7% to 30.9% (P=.004). Late mortality, in contrast, decreased from 19.9% to 13.1% (P=.2) (Table 2).
Regarding functional status, 42.4% of patients in the first period presented good outcomes, compared to 26.9% in the second period (P=.2). No significant differences in GOS classification were observed between patients undergoing surgery in the 2 periods, although we did observe significant differences between patients not undergoing surgery (Table 3).
A multivariate logistic regression analysis of mortality found that age older than 45 years increases the risk of mortality, with an OR of 2.31 (95% CI, 1.15-4.61), regardless of lesion distribution (Marshall classification), presence of pupillary abnormalities, or cause of trauma. The OR of mortality for presence of pupillary abnormalities was 3.19 (95% CI, 1.45-7.06). Regarding GOS classification, age older than 45 years (OR: 0.12; 95% CI, 0.05-0.25), surgery (OR: 0.12; 95% CI, 0.03-0.97), and presence of pupillary abnormalities (OR: 0.33; 95% CI, 0.13-0.84) were found to independently reduce the likelihood of achieving good functional outcomes; these differences were statistically significant.
DiscussionOur study shows significant differences in the population attended at our hospital due to severe TBI over the last 2 decades. Mean age was 12 years older in the second period. Accidental falls are currently the most frequent cause of severe TBI. Traffic accidents (a frequent cause in the young population) have decreased, whereas other causes of TBI more commonly observed in elderly individuals, such as stairway falls, have increased. Pedestrian accidents were observed in both groups, with no significant differences. We also observed changes in the management of these patients: in the second period, the frequency of emergency surgeries decreased by half; this may be explained by the higher proportion of elderly, multimorbid patients, who more frequently receive anticoagulants, resulting in poorer surgical prognosis. Our experience with the first cohort, with a high percentage of patients presenting sequelae despite the young mean age, may have led us not to indicate surgery for patients in the second group, who had a higher mean age, as older individuals are more likely to present poorer functional recovery. This, together with a change in the pattern of TBI, may have played a role in the change in surgical indication.
The natural history of TBI has changed considerably over time due to a number of reasons. In Spain, the annual incidence of TBI is estimated at 200 new cases per 100000 population. Of these patients, 70% present good recovery, 9% die before reaching hospital, 6% die during hospitalisation, and 15% present functional disability.3–6 Severe TBI (GCS score ≤ 8) accounts for 10% of all cases.7
As life expectancy has continued to increase, age-related problems have become increasingly common. The group of individuals older than 60 years, and particularly that of individuals older than 80, is growing faster than any other age group.8 Population ageing is more frequent in developed countries, such as Spain, and is also observed in our population (Table 1). The incidence of TBI in the elderly population is estimated to have doubled over the past 20 years.8
Severe TBI is greatly influenced by the epidemiological characteristics of each population. In a review of TBI in China, Wu et al.9 presented a series with a mean age of 36 years where the main cause of TBI was traffic accidents, as in our first cohort. A French series,10 in contrast, shows a mean age of 44 years, which is similar to our second cohort. That series also displayed a change in the aetiology of TBI, similar to that observed in our study. In our cohort, the incidence of traffic accidents as the cause of severe TBI decreased considerably from the first period to the second. This is explained by cultural changes and the development of driver education programmes, leading to a decrease in the incidence of traffic accidents, which have traditionally been found to be more common among younger men (Fig. 2). At present, the most common cause of severe TBI is accidental falls.
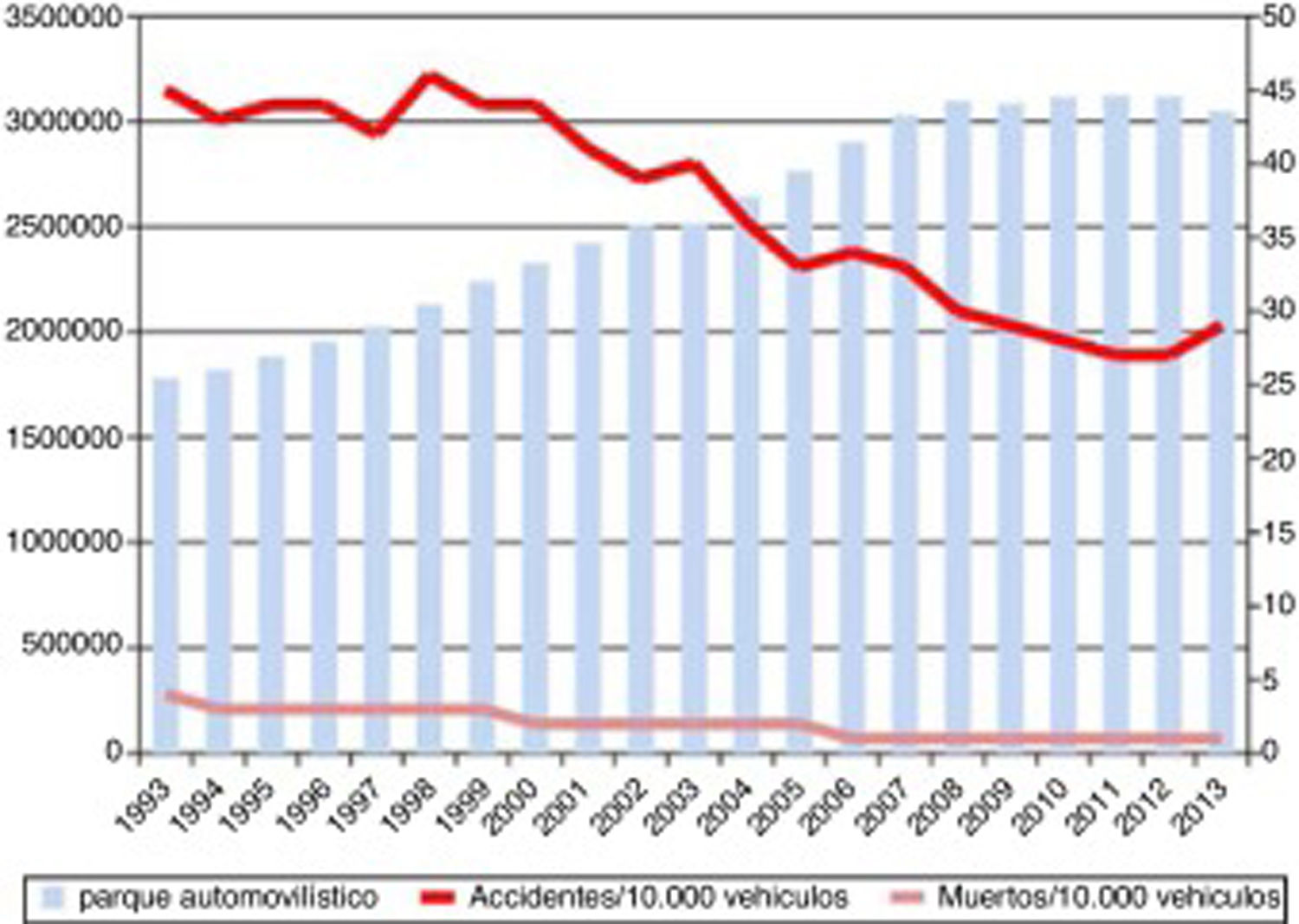
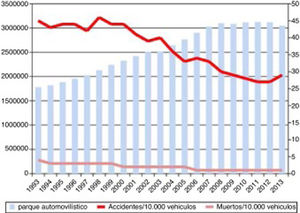
Progression of lethality (number of deaths/number of victims×100) and number of traffic accidents from 1993 to 2013, and number of motor vehicles during the same period.
Source: Spanish Ministry of Home Affairs, General Directorate of Traffic: Anuario Estadístico de Accidentes 2013, p. 56.
The number of surgeries, especially solid organ surgeries, in patients with severe TBI has decreased in recent decades, but this has not had a negative impact on outcomes.11–14 In our study, the number of surgeries decreased dramatically, perhaps because the second cohort comprised older patients, with poorer baseline status and more frequently receiving anticoagulants (Table 1). More patients from the first cohort underwent surgery, due to the belief that good baseline status and younger age may improve prognosis; however, this was not the case, which may have influenced the therapeutic decisions made during the second period. The prognosis of severe TBI remains very poor.3–6
We also observed changes in the type of intervention, with most surgical patients from the second cohort undergoing decompressive craniectomy. Rebleeding is more likely to occur in elderly patients receiving anticoagulation therapy. Furthermore, ICP monitoring is easier when the bone flap is not put back in place. In daily clinical practice, subdural haematomas, which are particularly frequent among the elderly, are more common than epidural haematomas, which have classically been associated with younger age. The bone flap is usually put back in place during the intervention in patients with epidural haematomas; this is not the case for subdural haematoma (Fig. 3).
We also observed an increase in the use of ICP sensors in our population. ICP monitoring may have prevented surgery in patients who traditionally underwent these procedures. The Brain Trauma Foundation's guidelines for the management of severe TBI15,16 recommend ICP monitoring, although controversy has also arisen after the publication of the results of the BEST TRIP randomised controlled trial.17 The percentage of patients undergoing neuromonitoring may be lower than expected; this may be explained by the fact that patients are frequently indicated for surgery following emergency CT without first undergoing neuromonitoring or assessment at the IMD.
Decompressive craniectomy is increasingly recommended.18–21 The available evidence on the use of the procedure for TBI shows ambiguous short-term results, and the technique has not consistently been shown to reduce morbidity and mortality or to improve quality of life in the short term.22–25 One of the most recent studies into the topic is the RESCUEicp study, which confirmed a decrease in the mortality associated with decompressive craniectomy as a secondary treatment for refractory intracranial hypertension, but also shows an increase in the rate of dependence, which confirms recent perceptions.26
The decrease in the number of neurosurgical interventions for severe TBI has previously been reported. Flynn-O’Brien et al.27 analysed 22974 patients with severe TBI from Washington State between 1995 and 2002, and found that the percentage of those undergoing surgical procedures decreased from 36% to 7%, despite the incidence of TBI remaining stable.
In recent years, great advances have been made in the management of patients with multiple trauma and TBI. A meta-analysis of the progression of TBI over more than 150 years, including over 140000 patients found a 50% decrease in mortality rates.28 Between 1930 and 1970, mortality remained stable, which the authors attributed to the increasingly widespread use of motor vehicles and the number of traffic accidents during that period. The great improvement observed between 1970 and 1990 was associated with medical advances (medical management, routine CT for patients with multiple trauma, etc). The fact that mortality rates did not continue to decrease after 1990 was attributed to population ageing, which results in poorer outcomes of TBI. This underscores the need to develop new approaches to the management of these patients and to focus on strategies that may improve outcomes, such as optimising coagulation (anticoagulation therapy is increasingly common, which may increase the risk of death).
The overall rate of mortality due to TBI is not easy to establish due to methodological differences between studies. Our mortality rates are similar to those reported in other studies, some of which were also conducted in Spain.29–33 Mortality within 72hours of TBI was much higher in our second cohort, probably as a result of improvements in prehospital emergency services, preventing patient deaths at the site of the accident, leading to higher in-hospital mortality rates due to these patients’ extremely poor clinical status. Mortality later than 72hours after the accident decreased considerably in the second period, despite the fact that far fewer patients from the second cohort underwent surgery. These findings are consistent with those of studies with larger samples.27,28
Although trauma in elderly individuals is usually low-energy, older age is associated with poorer functional prognosis and greater mortality.8,34–36 Some researchers report mortality rates of up to 80% among patients with severe TBI older than 70 years,37 with very poor functional outcomes and no improvements in these rates over the years. The poorer outcomes (GOS classification) observed in the second cohort also reflect the effect of population ageing. As shown by the multivariate analyses, older age, presence of pupillary abnormalities, and surgery were associated with significant increases in the likelihood of disability. Patel et al.38 observed a nearly exponential increase: while patients with severe TBI between the ages of 65 and 70 years presented mortality rates of 47%-56%, those older than 75 years presented a mortality rate of 78.5%.
Due to the retrospective design of our study, we can suggest hypotheses to explain our findings but cannot establish causal relationships, some data may also be missing due to retrospective data collection. Other limitations include the small size of our sample, the fact that the sample was drawn from a single centre, and the wide range of scales used to assess TBI in the included studies.
Comparison between studies is extremely difficult. TBI is greatly influenced by the epidemiological characteristics of the population under study. For instance, in Spain the use of motorcycle helmets became compulsory in 1992, whereas in China this was not the case until 2004. Furthermore, different studies use different assessment scales, and many gathered data from large registries using the ICD-9 classification, which is known to have coding errors.
ConclusionThe epidemiology of TBI has changed over time. Generally, patients with severe TBI tend to be older, particularly in developed countries. Although falls from the same level are the main cause of severe TBI at present, the high incidence of comorbidities, the widespread use of anticoagulants in this population, and the high percentage of dependent patients have led to a considerable decrease in the indication of surgery for severe TBI in our centre. The population with severe TBI has changed, which has also changed the management of the condition, with the need to introduce new approaches and strategies focused on achieving better outcomes, such as optimising coagulation and providing neurorehabilitation therapy.
FundingThis study received no funding of any kind.
Conflicts of interestThe authors of this study have no conflicts of interest to declare.
Please cite this article as: Giner J, Mesa Galán L, Yus Teruel S, Guallar Espallargas MC, Pérez López C, Isla Guerrero A, et al. El traumatismo craneoencefálico severo en el nuevo milenio. Nueva población y nuevo manejo. Neurología. 2022;37:383–389.