Sudden unexpected death in epilepsy (SUDEP) is the most frequent cause of premature death in epileptic patients. Most SUDEP events occur at night and frequently go unnoticed; the exact pathophysiological mechanisms of this phenomenon therefore remain undetermined. Nevertheless, most cases of SUDEP are attributed to an infrequent yet extremely severe complication of epileptic seizures.
DevelopmentWe conducted a systematic literature search on PubMed. Our review article summarises scientific evidence on the classification, pathophysiological mechanisms, risk factors, biomarkers, and prevention of SUDEP. Likewise, we propose new lines of research and critically analyse findings that are relevant to clinical practice.
ConclusionsCurrent knowledge suggests that SUDEP is a heterogeneous phenomenon caused by multiple factors. In most cases, however, SUDEP is thought to be due to postictal cardiorespiratory failure triggered by generalised tonic-clonic seizures and ultimately leading to cardiac arrest. The underlying pathophysiological mechanism involves multiple factors, ranging from genetic predisposition to environmental factors. Risk of SUDEP is higher in young adults with uncontrolled generalised tonic-clonic seizures. However, patients apparently at lower risk may also experience SUDEP. Current research focuses on identifying genetic and neuroimaging biomarkers that may help determine which patients are at high risk for SUDEP. Antiepileptic treatment is the only preventive measure proven effective to date. Night-time monitoring together with early resuscitation may reduce the risk of SUDEP.
La muerte súbita (e inesperada) en epilepsia (MSE) es la causa más frecuente de muerte prematura en este grupo de pacientes. La gran mayoría de los casos de MSE ocurren durante la noche, y por lo tanto no son observados, por lo que el mecanismo patológico exacto no ha sido aclarado aún. A pesar de ello la mayoría de los casos de muerte no explicable en pacientes con crisis epilépticas pueden atribuirse a una complicación poco frecuente pero muy grave de las mismas.
DesarrolloSe realiza un proceso de revisión de la literatura basada en una búsqueda sistemática y completa de publicaciones indexadas en PubMed. En esta revisión se resumen los principales desarrollos en clasificación, mecanismos fisiopatológicos, factores de riesgo, biomarcadores y prevención de MSE y se discuten nuevas áreas de investigación, valorándose de forma crítica una serie de conclusiones relevantes para la práctica clínica.
ConclusionesSegún el estado actual de conocimiento la MSE es probablemente un fenómeno heterogéneo con diferentes causas. Sin embargo, en la mayoría de los casos la causa más probable es una insuficiencia cardiorrespiratoria postictal, desencadenada por crisis generalizadas tónico-clónicas y que finalmente conduce a un paro cardíaco. La fisiopatología subyacente engloba probablemente múltiples factores, incluyendo tanto una predisposición genética como factores ambientales. Jóvenes adultos con crisis tónico-clónicas generalizadas no controladas están en mayor riesgo de fallecer por MSE. No obstante, también pacientes con un riesgo aparentemente bajo pueden sufrir una MSE. Actualmente se encuentran en investigación biomarcadores genéticos y de neuroimagen que podrían mejorar la identificación de pacientes de alto riesgo. La única medida preventiva demostrada hasta ahora es una terapia eficaz con antiepilépticos. Posiblemente una vigilancia del paciente durante la noche, así como una reanimación prematura podrían reducir la MSE.
Epilepsy is one of the most frequent neurological diseases, affecting between 35 million and 70 million people worldwide, with a prevalence of 0.5% to 1%.1 Two-thirds of patients achieve good seizure control with proper antiepileptic drug (AED) treatment, and the majority are able to lead a normal life.2 However, young adults with epilepsy are 24 times more likely to die suddenly than individuals without epilepsy.3 These deaths largely correspond to sudden unexpected death in epilepsy (SUDEP), which accounts for 20% of premature deaths in this patient group.4 No data are available on SUDEP incidence in Spain; however, based on USA data, we calculate that there are between 250 and 450 cases per year.5 In the light of these figures, and given the exchange of information between patients, families, and support groups over social media, both patients and medical professionals in Spain recognise the need for clinical and experimental research on SUDEP. The Spanish Society of Neurology recently launched a project on its web page inviting physicians to register cases of SUDEP, with a view to establishing global prevention strategies for patients with epilepsy (Spanish national SUDEP registry, available at http://www.sen.es/investigacion/id/renmuertesubita). The present study reviews the latest and most important information on SUDEP, with emphasis on the epidemiology, pathophysiology, risk factors, and prevention of the phenomenon.
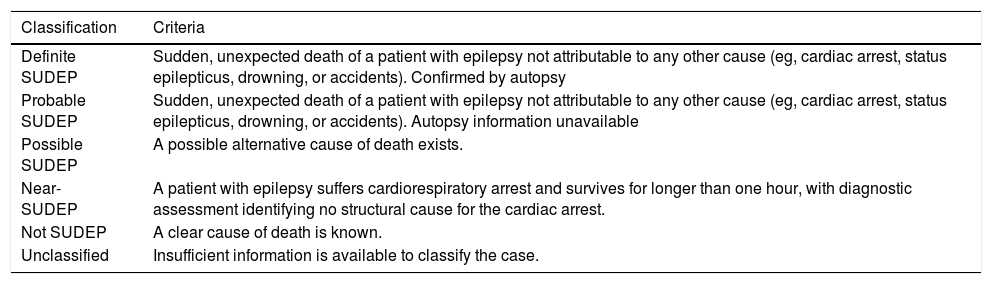
Definition of sudden unexpected death in epilepsySUDEP refers to the sudden, unexpected death of patients with epilepsy not attributable to other causes (eg, cardiac arrest, status epilepticus, drowning, or accidents confirmed by autopsy).6 However, studies on SUDEP incidence often use different definitions, probably influencing their findings.7 This led to the proposal of a more differentiated classification of SUDEP, aiming to improve diagnostic accuracy and to facilitate comparisons between studies. This classification distinguishes between definite, probable, possible, and near-SUDEP, and unclassified cases (Table 1).8 SUDEP is classified as definite if all criteria of SUDEP are met, including a postmortem examination. The definition of possible SUDEP allows for a compatible cause to which death cannot be attributed with absolute certainty. Near-SUDEP covers cases in which patients with epilepsy suffer cardiorespiratory arrest and survive for longer than one hour, with diagnostic assessment identifying no structural cause for the cardiac arrest.
Proposal for the classification of SUDEP (2012).
| Classification | Criteria |
|---|---|
| Definite SUDEP | Sudden, unexpected death of a patient with epilepsy not attributable to any other cause (eg, cardiac arrest, status epilepticus, drowning, or accidents). Confirmed by autopsy |
| Probable SUDEP | Sudden, unexpected death of a patient with epilepsy not attributable to any other cause (eg, cardiac arrest, status epilepticus, drowning, or accidents). Autopsy information unavailable |
| Possible SUDEP | A possible alternative cause of death exists. |
| Near-SUDEP | A patient with epilepsy suffers cardiorespiratory arrest and survives for longer than one hour, with diagnostic assessment identifying no structural cause for the cardiac arrest. |
| Not SUDEP | A clear cause of death is known. |
| Unclassified | Insufficient information is available to classify the case. |
SUDEP: sudden unexplained death in epilepsy.
Source: Nashef et al.8
According to the population studies reviewed, incidence ranges from 0.09 to 2.3 cases per 1000 patient-years.9 However, SUDEP is more frequent in patients with refractory epilepsy, with an incidence of 2.2 to 10 cases per 1000 patient-years.10 In paediatric patients, there are only 0 to 0.2 cases of SUDEP per 1000 patient-years.10 Cumulative risk for a patient with refractory epilepsy beginning in childhood amounts to nearly 15%.5,11 Although the phenomenon is relatively rare compared to other neurological disorders, in the USA it constitutes the second-largest cause of years of potential life lost among neurological diseases (after stroke), as mainly young adults are affected.10 The majority of cases occur before the age of 40, hence the significance and impact of SUDEP both for patients and for health systems.5
Causes of sudden unexpected death in epilepsyThe pathogenesis of SUDEP is not fully understood and is currently an active area of research. This lack of information is mainly explained by the fact that most patients are not under observation at the time of death.12 Furthermore, the nature of the phenomenon means that no structural causes are identified in post mortem examinations8; investigating the underlying pathophysiology therefore represents a challenge. Given that the majority of observed cases of SUDEP occur immediately after generalised seizures, death is probably due to an associated complication in many cases.12,13
Some studies suggest that the main underlying cause is cardiac dysfunction, such as arrhythmia associated with epileptic seizures.14 Other authors associate SUDEP with respiratory dysfunction, such as seizure-induced apnoea.15 An interaction between both factors may also be responsible. Given the frequency of cardiorespiratory problems both during and between epileptic seizures, SUDEP may be caused by various factors, rather than having a uniform cause.16 In this article we shall place particular emphasis on cardiac and pulmonary dysfunction associated with epileptic seizures.
Cardiac dysfunction associated with epileptic seizuresVarious types of cardiac dysfunction have been described during epileptic seizures.17 According to various studies, 60% to 70% of seizures in patients with focal epilepsy feature ictal tachycardia, or an increase in heart rate (HR) to more than 120bpm during the seizure.18 The severity of ictal tachycardia depends on seizure type and duration and the spread of epileptic activity through the brain.19,20 Bradycardia (HR<60bpm) and asystole (> 4s) associated with epileptic seizures are far less frequent (affecting 7% and 0.2%-0.4% of patients, respectively).21–23 According to a recent meta-analysis, bradyarrhythmia occurring immediately after seizures may be dangerous, whereas ictal bradyarrhythmia appears to be more benign.24
Epileptic patients often display disorders of cardiac repolarisation, such as long QT syndrome, during seizures.20 These disorders can promote ventricular tachycardia, a known risk factor for sudden cardiac death.25 One recent study found that abnormal ventricular conduction patterns are 3 times more frequent in cases of SUDEP than in epilepsy controls.26 However, ventricular tachycardia associated with seizures is a rare phenomenon. To date, only 3 cases have been reported in the literature, of which one was classified as SUDEP and 2 as near-SUDEP.24
Besides cardiac arrhythmia, generalised seizures can be associated with cardiac mechanical dysfunction, such as takotsubo cardiomyopathy.27 This condition is generally triggered by an emotional stimulus and resembles acute coronary syndrome in terms of symptoms.28 It is characterised by abnormal movement of the left ventricle; coronary artery occlusion is not observed, unlike in cases of acute coronary syndrome. Of 74 published cases of patients with takotsubo cardiomyopathy, 2 died during or shortly after an epileptic seizure.29
Unlike for heart rate, little data is available on the association between seizures and the modulation of systemic arterial blood pressure.30 One recent study reports higher interictal diastolic blood pressure in patients who subsequently died of SUDEP than in controls with refractory epilepsy; this may be indicative of autonomic dysfunction.31 Another study systematically measured peri-ictal changes in blood pressure for the first time, in a sample of 37 patients, finding a 30% increase during focal seizures.32 The extent of the increase and the profile depend on seizure type (focal aware or focal impaired awareness seizures). Less frequently, blood pressure may drop during seizures. Unfortunately, movement artefacts compromised blood pressure recordings during secondarily generalised seizures; however, in the early postictal phase, the researchers observed a curious dissociation between HR and arterial blood pressure: HR increased by 75%, persisting at that level throughout the postictal phase, whereas blood pressure increased by only 15%, decreasing after 5minutes to below preictal levels. A drop in blood pressure may support the hypothesis of cerebral shutdown, according to which the central inhibition triggered by epileptic seizures may lead to cardiorespiratory insufficiency, potentially resulting in death.30
Respiratory dysfunction associated with epileptic seizuresEpileptic seizures are also frequently associated with disorders of respiratory function, such as decreased oxygen saturation (hypoxaemia).33 As the majority of epilepsy units do not constantly monitor respiratory rate or oxygen saturation, few data are available on this subject. A recent study recorded 304 seizures in 56 patients, detecting hypoxaemia (oxygen saturation < 90%) in one-third of cases, with a mean duration of 70 seconds. Fewer than 4% of seizures provoked severe hypoxaemia (saturation < 70%). The frequency, duration, and degree of hypoxaemia depended on seizure type and duration.15 Hypoxaemia is particularly frequent in generalised seizures, as well as in focal impaired awareness seizures. Central respiratory disorders were observed in 50% of episodes. Another study reports an increase of over 50mm Hg in end-tidal CO2 in 30% of focal seizures.34 In addition to the respiratory disorders described above, generalised seizures can also lead to neurogenic pulmonary oedema.35
The results of the studies mentioned suggest that respiratory dysfunction associated with epileptic seizures can be severe enough to cause SUDEP. On the other hand, they also raise the question of why seizures do not more frequently lead to medical complications, given the respiratory disorders frequently associated with them.36
ProgressionAs the great majority of cases of SUDEP occur in patients who are not under observation, the limited available evidence on the pathophysiology of the condition is based on information from eyewitnesses or reports of isolated cases recorded during video-EEG monitoring.13 The Mortality in Epilepsy Monitoring Unit Study (MORTEMUS), a large-scale, multicentre, retrospective study into the incidence and mechanisms of SUDEP and near-SUDEP at epilepsy units, has been very important in this regard.12 During the study period, video-EEG was used to record 16 cases of SUDEP and 9 cases of near-SUDEP at 147 epilepsy units. In addition, ECG data were also available for 9 cases of SUDEP and 3 cases of near-SUDEP. Despite the large number of pathogenic mechanisms potentially involved in SUDEP, the study demonstrated that the sequence of events was largely similar between individuals: all cases of SUDEP and 7 of the 9 cases of near-SUDEP occurred after a generalised tonic-clonic seizure, with cardiorespiratory failure occurring in the first 3minutes after the episode. Cardiorespiratory failure took the form of bradycardia or asystole alternating with periods of accelerated HR, and bradypnoea or apnoea alternating with periods of increased work of breathing. EEG recordings showed postictal generalised EEG suppression (PGES), with amplitude reduced to below 10μV.37 Finally, all cases of SUDEP ended in terminal apnoea and asystole after 3 to 15minutes. From a clinical perspective, it is also striking that in 14 of the 16 cases of SUDEP, the event took place at night and patients were found prone.12 In 7 of the 9 cases of near-SUDEP, cardiopulmonary resuscitation was performed within the first 3minutes after the seizure ended. In contrast, cardiopulmonary resuscitation was not attempted until 10minutes after the seizure in 8 of 12 cases of SUDEP.
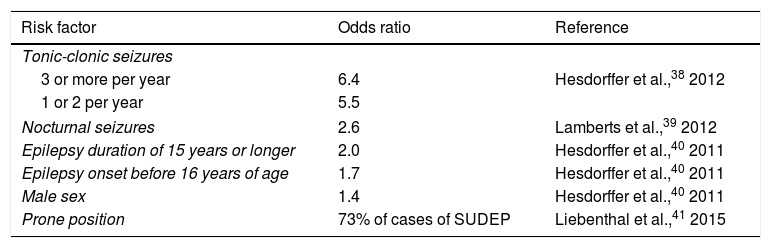
Risk factorsWhat patient groups are at greatest risk of SUDEP? Recognition of SUDEP risk factors may result in early implementation of measures to protect patients. Poor seizure control is the main risk factor identified to date, with SUDEP prevalence being greater in cases of refractory epilepsy than in patients with better seizure control. Patients presenting more than 2 generalised tonic-clonic seizures per year are at particular risk of SUDEP.38 Patients with nocturnal seizures are also at greater risk (Table 2).39 Early onset of epilepsy (before the age of 16) and long disease duration (≥ 15 years) are both associated with increased risk of SUDEP.40 Sex also has an influence, with men being more vulnerable than women.40 Curiously, body position also appears to influence the risk of SUDEP: in one study, 73% of patients were found in a prone position, similarly to the circumstances observed in sudden infant death syndrome.41 The authors propose a postictal respiratory dysfunction due to the prone position as a possible explanation for this observation.41 According to recent studies, the use of multiple AEDs is not an independent risk factor; rather, it is a marker of refractory epilepsy.38 On the contrary, poor treatment adherence is probably associated with greater SUDEP risk, with some studies reporting blood AED levels at zero or below the therapeutic range in 35% to 55% of cases.42,43 An extensive case-control study performed in the United Kingdom found no specific treatment or polytherapy to be associated with increased risk.13 A recent study provides a checklist summarising all known SUDEP risk factors to assist in the clinical identification of patients at increased risk.44
SUDEP risk factors identified in a meta-analysis.
| Risk factor | Odds ratio | Reference |
|---|---|---|
| Tonic-clonic seizures | ||
| 3 or more per year | 6.4 | Hesdorffer et al.,38 2012 |
| 1 or 2 per year | 5.5 | |
| Nocturnal seizures | 2.6 | Lamberts et al.,39 2012 |
| Epilepsy duration of 15 years or longer | 2.0 | Hesdorffer et al.,40 2011 |
| Epilepsy onset before 16 years of age | 1.7 | Hesdorffer et al.,40 2011 |
| Male sex | 1.4 | Hesdorffer et al.,40 2011 |
| Prone position | 73% of cases of SUDEP | Liebenthal et al.,41 2015 |
SUDEP: sudden unexplained death in epilepsy.
While SUDEP is a rare phenomenon, it is the leading cause of premature death in epileptic patients. The underlying pathophysiology is not fully understood. According to current evidence, cardiac and respiratory dysfunction occurring in the early postictal phase (probably caused by generalised tonic-clonic seizures) eventually lead to cardiorespiratory arrest. Epidemiological studies indicate that young men with frequent generalised tonic-clonic seizures are at particularly high risk of SUDEP. Identifying additional markers of SUDEP would assist in better informing patients and their families; a number of recent studies have focused on searching for genetic, EEG, and neuroimaging biomarkers. These are described below.
Genetic biomarkersThe identification of genetic biomarkers may play a key role in assessing the risk of SUDEP. As is the case for such diseases as dementia and Parkinson’s disease, the genetic factors involved in SUDEP are also an active area of research and debate.45,46 The ion channel genes expressed in the brain and heart are important candidates. For instance, evidence from animal models suggests that mutations of a gene frequently related to classic long QT syndrome can cause not only potentially fatal arrhythmias, but also epilepsy, due to abnormal ion channel expression in the brain.47,48 An example is the SCN1A gene, expressed mainly in the brain; mutations in this gene cause Dravet syndrome and are associated with a significantly higher risk of SUDEP.49 Animal studies have also demonstrated that SCN1A mutations can result in fatal cardiac dysfunction.50 Various animal and human studies have identified at least 9 genes associated with “neurocardiopathies” that are potentially related to SUDEP; these may be candidate genes.45 However, the significance of these genes in predicting SUDEP risk is yet to be determined. Surprisingly, overlap between the genes identified in various series is moderate, with each study identifying new candidate genes.51,52 Frequent methodological issues are small sample sizes, the inclusion of cases of possible rather than definite SUDEP, the lack of controls, and patient heterogeneity.
Significantly, the only study with a reasonable sample size (a cohort of 68 cases of definite SUDEP) obtained a negative result for the gene of interest.53 The study found that mutations in the PHOX2B gene, a promising candidate according to pathophysiological data, were not a common risk factor for SUDEP. Based on this evidence, we may conclude that no single gene or group of genes is likely to be related with SUDEP. The genetic causes of the phenomenon are probably complex and may constitute a combination of single nucleotide polymorphisms (variations in individual base pairs within a gene) and copy number variations in different genes (deviations in the number of copies of a specific segment of a gene), including SCN1A, KCNA1, RYR3, and HTR2C, which are involved in neurocardiac and respiratory control pathways.54 Another study compared 18 cases of SUDEP with 97 patients with epilepsy and 1479 healthy controls, finding a wide variety of mutations distributed throughout the genome.55 The mutation load per individual was higher in the SUDEP group. Curiously, the mutations identified were not limited to small, circumscribed groups of genes; this suggests that SUDEP is a polygenic and multifactorial phenomenon.
Electroencephalographic biomarkersCould any specific EEG patterns indicate higher risk of SUDEP? This may be the case with PGES, which consists in a reduction of EEG amplitude to below 10 μV, frequently occurring after generalised tonic-clonic seizures.37 PGES has been observed in several cases of SUDEP, leading to the hypothesis that PGES is a marker of suppressed brain activity and may therefore inhibit cardiovascular and respiratory activity, ultimately resulting in SUDEP. The fact that 11 video-EEG recordings of cases of SUDEP in the MORTEMUS study showed PGES demonstrates the importance of the phenomenon.12 PGES duration was also correlated with the severity of the postictal respiratory disorder.56 A recent case-control study also reports a greater frequency of SUDEP among patients with PGES of more than 50seconds duration; therefore, PGES has been proposed as the EEG marker with the greatest associated risk of SUDEP.37 However, this relationship was not confirmed in a subsequent study with a larger patient group.57 From a critical perspective, it should be noted that PGES is a feature of at least 50% of non-fatal generalised tonic-clonic seizures, and occurrence and duration are highly variable in individual patients.57,58 Furthermore, intracranial EEG recordings detect local brain activity during PGES on surface EEG59; PGES is consequently of very little value as an EEG biomarker of future SUDEP.
Neuroimaging biomarkersThe occurrence of SUDEP after generalised tonic-clonic seizures, together with PGES, indicates that the pathological mechanism is centrally mediated. This conclusion gave rise to a search for functional and structural cerebral correlates, which in recent years has resulted in a focus on neuroimaging studies in SUDEP research. In a recent brain magnetic resonance imaging (MRI) study comparing resting functional MRI patterns between epileptic patients with low versus high risk of SUDEP, the latter group displayed reduced functional connectivity of the right thalamus with the pons, midbrain, anterior cingulate, and left thalamus.60 Another study reports significantly reduced dorsal midbrain volume in 2 cases of SUDEP.61 However, a larger study comparing MRI findings in 12 cases of SUDEP and 34 patients with high risk of SUDEP, 19 patients with low risk, and 15 healthy volunteers identified increased grey matter volume in the right amygdala, hippocampus, and parahippocampal gyrus in the high-risk group compared to the low-risk and control groups.62 According to these findings, refractory epilepsy may be associated with functional or structural changes in brain regions involved in the central autonomic nervous system. These changes may cause autonomic disorders that increase the risk of SUDEP. However, these studies do not identify clear functional or morphological MRI markers for use in clinical practice.
Prevention of sudden unexpected death in epilepsyCan understanding of SUDEP risk factors and pathophysiology help to protect patients? As poor seizure control is the most significant risk factor, and given that SUDEP is associated with seizures, improving seizure control constitutes the main preventive measure.63 Indeed, a recent meta-analysis of 112 clinical trials demonstrated that adjuvant therapy with AEDs in patients with refractory epilepsy reduced the odds ratio of SUDEP 7 times versus placebo.64 Unfortunately, one-third of epileptic patients continue to present seizures despite such treatment.2 Surgical treatment achieves seizure freedom in a considerable percentage of patients.65 Nonetheless, the extent to which epilepsy surgery modifies the risk of SUDEP remains unclear.66 Some case-control studies have shown that surgery with positive outcomes significantly reduces mortality.67,68 For patients who are not eligible for this treatment, other non-pharmacological alternatives are available; these include vagus nerve stimulation.69 This should be considered a palliative treatment, as it rarely achieves seizure freedom70; however, it has been shown to reduce seizure frequency in patients with refractory epilepsy.71 Theoretically, improving seizure control should reduce the risk of SUDEP. However, the results of previous studies on the topic are contradictory; we are therefore unable to conclude that this treatment has a protective effect.72,73
Maintaining a vigil after seizures probably reduces the risk of SUDEP in patients with nocturnal seizures.13 The results of the MORTEMUS study suggest that resuscitation initiated early by family members or carers can prevent SUDEP.12 However, it is unclear whether resuscitation by untrained individuals can influence SUDEP rates. Nonetheless, there is evidence that simple nursing interventions shorten the duration of postictal respiratory disorders and PGES.34,74 Cardiac pacemaker implantation would appear to be a logical therapeutic step in SUDEP prevention, as it has been demonstrated to be effective for treating ictal asystole.75 However, no effect on the risk of SUDEP has been demonstrated to date. In fact, one case of SUDEP in a case-control study occurred in a patient who had previously had a pacemaker implanted due to peri-ictal bradycardia.25
Communication with patients and their families regarding sudden unexpected death in epilepsyShould patients and family members be informed about SUDEP? No effective treatments (besides AEDs) are currently available for SUDEP prevention.64 It therefore seems reasonable to question whether physicians and nurses attending patients with epilepsy should educate them regarding the risk of the phenomenon. Education about SUDEP may confuse patients, especially those with refractory epilepsy, who feel very powerless against their seizures.
Poor or partial treatment adherence is common in patients with epilepsy.76 In one study, post mortem toxicology analysis of 74 cases of SUDEP found that only 26 patients had detectable levels of AEDs; this indicates that failure to take the medication was probably a factor in the patients’ deaths.43 Therefore, informing patients about the risk of SUDEP may improve treatment adherence, particularly in patients with uncontrolled seizures.77 For example, one study reported better treatment adherence in 7 of 27 patients who were informed about SUDEP.78 According to various surveys, around one-third of patients or family members would wish to be informed about SUDEP at the time epilepsy was diagnosed.79,80 Despite this, only 12% of neurologists in Austria, Germany, and Switzerland regularly speak with patients about SUDEP, according to another recent survey.81 Furthermore, 61% to 86% of patients treated at epilepsy units have never heard of the phenomenon.79,80 Fear of negative psychological effects in patients may explain many physicians’ caution about raising the subject of SUDEP, although it is yet to be established whether this fear is justified.82 With relation to this, in one study parents of children with epilepsy were informed about SUDEP and interviewed 3 months later.83 Over 90% of parents stated that they wanted to be informed about SUDEP; no negative consequences were reported.83 Another challenge is how to approach the families of patients who died with SUDEP. The majority of family members were not informed about the phenomenon before it occurred.84 They would have preferred to have had SUDEP explained to them before the patients died.84
In conclusion, it is our opinion that physicians should appropriately discuss SUDEP with patients and their families, treating it as a rare but severe complication. Unlike in the United Kingdom, where communication about SUDEP is included in current clinical guidelines, no recommendations regarding SUDEP are available for patients and their families in Spain. The best time (eg, if treatment adherence is poor) and way to broach the subject should be decided individually according to each patient’s specific circumstances.
ConclusionsSUDEP, a rare but severe complication of epilepsy, is probably caused by seizure-induced cardiorespiratory failure. Controlling generalised tonic-clonic seizures appears to be a crucial part of SUDEP prevention; however, effective pharmacotherapy is the only preventive measure demonstrated with sufficient scientific evidence. In patients with refractory epilepsy, such non-pharmacological treatments as epilepsy surgery should be considered at an early stage. The majority of patients and their family members should receive personalised education about SUDEP.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Hampel KG, Rocamora Zuñiga R, Quesada CM. Desentrañando los misterios de la muerte súbita en epilepsia. Neurología. 2019;34:527–535.