Microglia are glial cells of the mononuclear phagocytic system of the central nervous system (CNS). They originate from myeloid precursor cells derived from the embryonic mesoderm, and migrate to the CNS to become microglia. These cells continuously modulate and remodel neuronal circuits throughout a person’s life. They represent between 10% and 20% of all CNS cells, with the highest density in the hippocampus, basal ganglia, substantia gelatinosa, and spinal cord. They are responsible for a large proportion of neuroplasticity, and participate in the cellular processes occurring during and after a nerve injury, one of the pathognomonic characteristics of neuropathic pain.1–3
When a nerve is injured, microglia undergo microgliosis or activation and migration, enabling these cells to perform several responses, including the protection of injured nerves, phagocytosis of cell debris, and the release of cytokines and other inflammatory modulators to trigger and propagate an immune response. Persistent pain models have shown that hyperalgesia is associated with microglial activation in the dorsal horn of the spinal cord.2,3
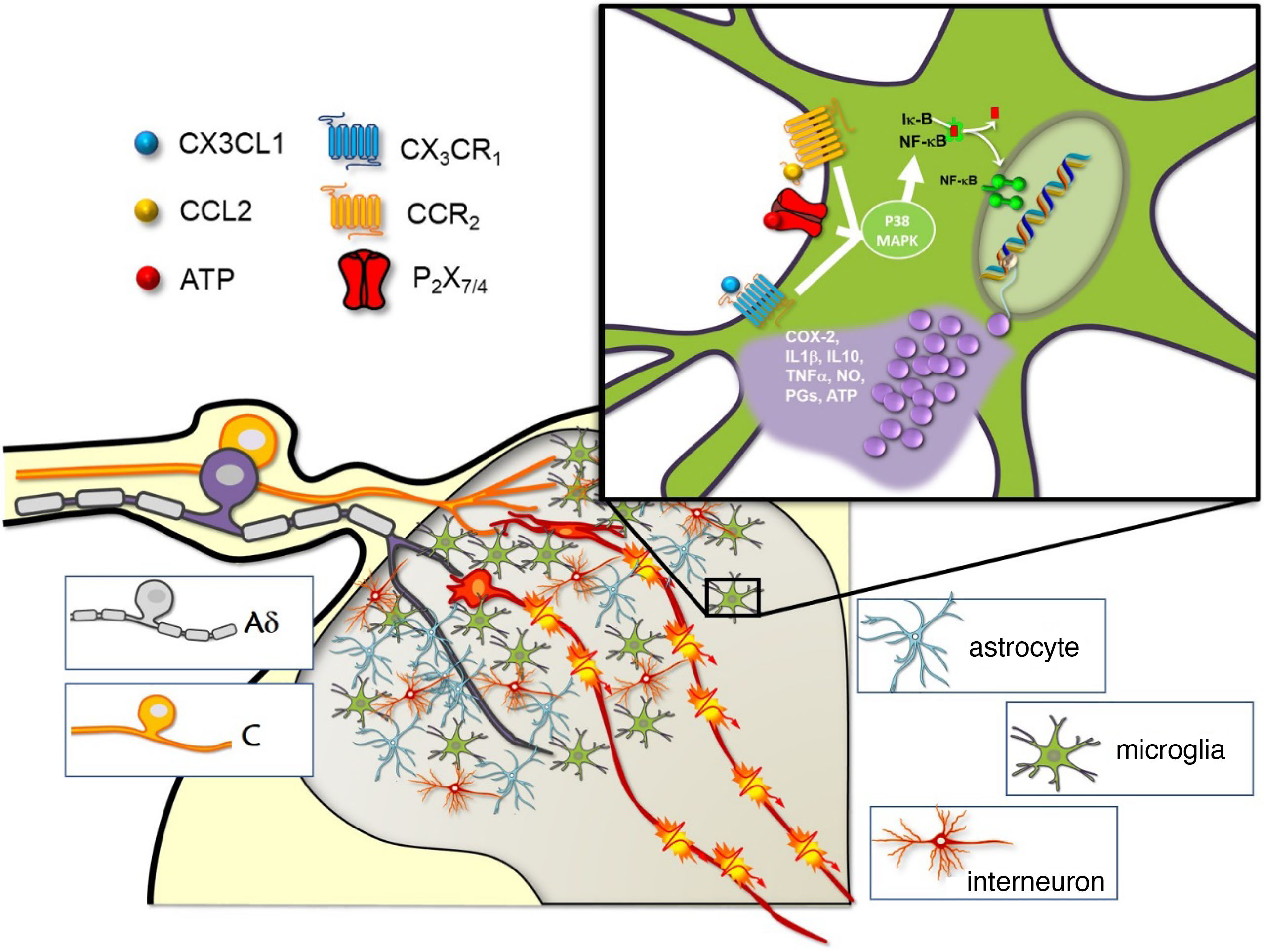
Microglial activation occurs through several pathways, with the most important probably being the mitogen-activated protein kinase (MAPK) pathway.1,2 These systems affect intracellular transcription and play an essential role in inflammation. The MAPK pathway includes several signalling chemokines and cytokines, including the kinases regulated by extracellular signals, protein p38, and c-Jun N-terminal kinases.2
The p38 MAPK signalling pathway is activated through the phosphorylation of many proinflammatory cytokines and mediators, including the tumour necrosis factor-α (TNF-α), interleukins (such as IL-1β), and adenosine triphosphate (ATP). These cytokines are released by injured afferent neurons themselves, to activate the p38 MAPK signalling pathway, and ultimately to induce the transcription of other inflammatory mediators, including cyclooxygenase-2, brain-derived neurotrophic factor, IL-6, TNF-α, and IL-1β. These mediators then activate the surrounding astrocytes, increase microglial recruitment, and promote CNS sensitisation and neuronal transmission (Fig. 1). Glial TNF-α also increases synaptic efficacy in propagating pain signals, increases endocytosis of gamma-aminobutyric acid receptors, and enhances the activity of the N-methyl-D-aspartate receptor through phosphorylation of kinases, which is regulated by extracellular signals. This downregulates the inhibitory modulation of pain, leading to increased signalling of thermal and mechanical nociception, and to hyperalgesia.2
Role of microglia in spinal pain processing.
The synapse with the spinal neurons that transmit the nociceptive signal to higher centres of the central nervous system occurs in the dorsal horn of the spinal cord, where C and A-delta nociceptive afferent fibres terminate. There are two types of glial cells in the dorsal horn (astrocytes and microglia), with mainly GABAergic interneurons. The window shows some of the different receptors and ligands with capacity to stimulate microglial cells and trigger the release of proinflammatory substances through activation of the p38 MAPK pathway.
ATP: adenosine triphosphate; CCL2: monocyte chemoattractant protein 1; CCR2: CCL2 receptor; COX2: cyclooxygenase-2; CX3CL1: fractalkine; CX3CR1: fractalkine receptor; IL1β: interleukin 1 beta; IL-10: interleukin 10; NO: nitric oxide; PG: prostaglandins; P2X7/P2X4: purinergic receptors; TNFα: tumour necrosis factor alpha.
Image courtesy of Carlos Goicoechea García.
In terms of therapeutic potential, intrathecal administration of such p38 inhibitors as minocycline, an antibiotic drug from the tetracycline group, inhibits microglial activation and decreases pain activity by diminishing neuropathic pain.2,3 Studies conducted to date only use animal models, and few analyse the adverse effects. It has been suggested that intrathecal administration may minimise the systemic effects of minocycline; one study showed no evidence of neurotoxicity.4 Spinal cord stimulation (SCS) using differential target multiplexed (DTM) programmes has been studied, considering that the spinal cord presents greater density of glial cells, especially in the T8–T11 segments. This stimulation system aims to optimise the effects of electrical pulses in neuroinflammatory processes that lead to pain chronification. Until recently, traditional stimulation was limited to the neurons of the posterior funiculi, but this technology expands its action for the first time to glial cells. The preliminary results of ongoing human studies into SCS using DTM programmes reveal an improvement in the analgesic effects as compared to conventional SCS in treating back and leg pain.5 SCS is believed to affect interactions between glial cells, including intra- and intercellular communication, signal transduction, phosphorylation of modified proteins, and ion transport.6
In short, our understanding of pain pathophysiology is continuously increasing. Identification of the underlying cellular and subcellular pathways helps us to design specific therapies that may be effective. In this context, the influence of microglia on neuronal plasticity and the ability to modulate this response may have significant implications for the treatment, prevention, and management of chronic pain syndromes. A better understanding of these pathways and the development of pharmacological and neuromodulatory interventions targeting them will enable us to reduce the burden of chronic pain secondary to nerve injuries, including postsurgical pain syndrome.
FundingThis study has not been presented at any of the SEN's Annual Meetings or any other meeting or congress. It has not received funding from any public or private organisation.
Declaration of Competing InterestThe study complies with the ethical standards and authorship criteria of the journal. The authors have no conflicts of interest to declare.