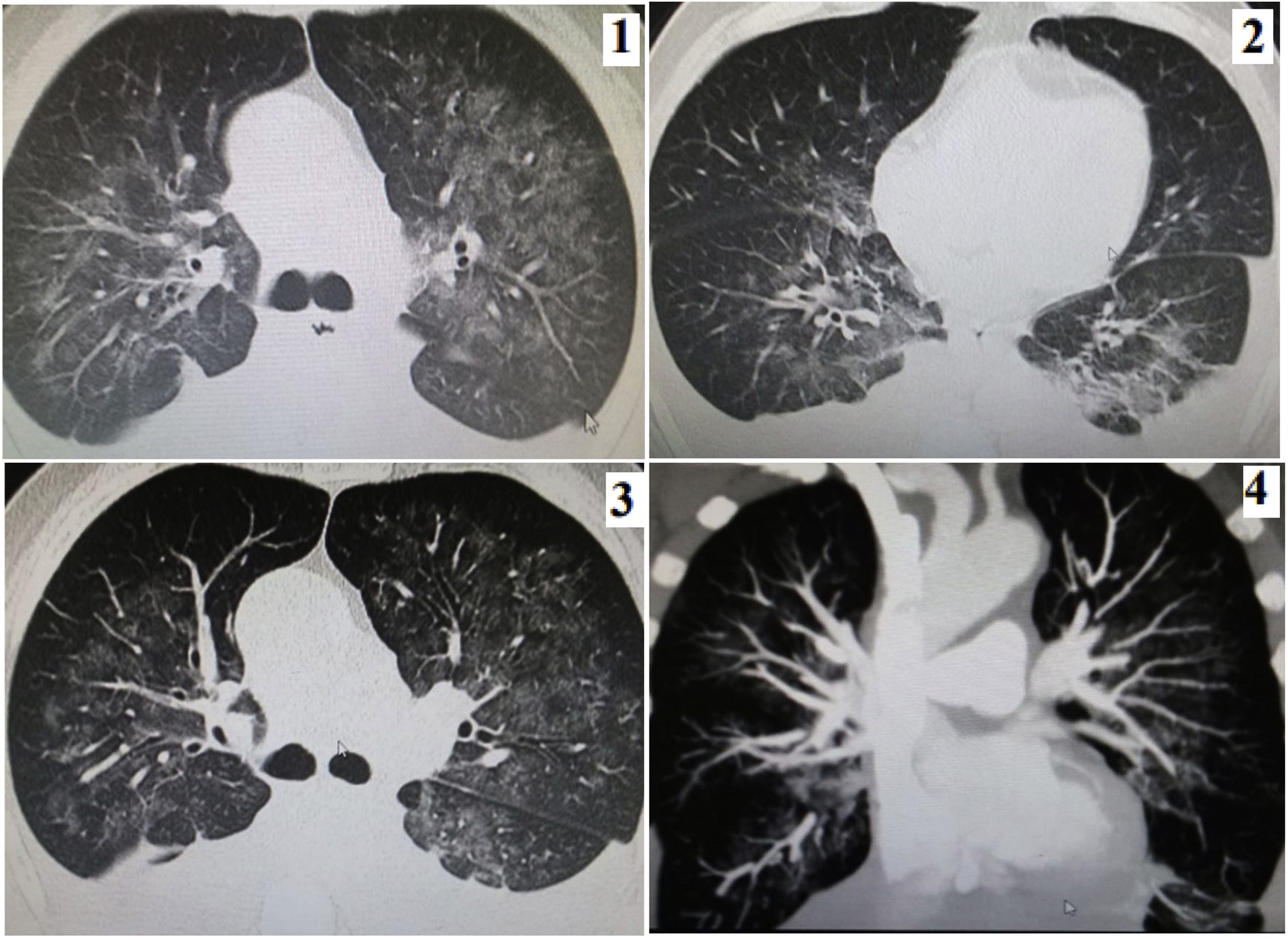
During 2021, a 66-year-old man was admitted to the emergency room with 2 month of weight loss, cough with bloody sputum, dyspnea and fever. With IgM and IgG reactive to COVID-19 and the chest CT scan shown (Fig. 1), COVID-19 pneumonia with bilateral pleural effusion was diagnosed; he had normal vital signs; at lungs: bibasal egophony. He had 3 negative sputum smears for tuberculosis, and negatives ELISA and nasal swab antigen for HIV and COVID-19, respectively. He received dexamethasone 10mg/24h for 5 days, subcutaneous enoxaparin and ceftriaxone 2g/24h for 5 days1; 2000cm3 of thick pink fluid was drained, suspecting chylothorax: cholesterol: 250mg/dl and triglycerides: 420mg/dl, negative adenosine deaminase and Pap Block cell; 2 chest tubes were placed with 1000cm3/day through both tubes. The tomographic evolution can be seen in Fig. 1(1–4). Three days later he passed away.
(1) August: mild pleural effusion and ground glass: 35–40%. (2) Early September: increase in pleural effusion and decrease in ground glass. (3) End of September: similar characteristics. (4) Reconstruction of coronal section of angio tomography without evidence of vascular thrombotic lesions.
This is the second report of chylothorax in a patient with a previous infection by COVID-19. Satriano et al. reported chylothorax due to thrombosis in the superior vena cava.2 The chest tomography in our case did not show thrombi. Turkdogan et al. found a frequency of chylothorax in COVID patients of 3.2%.3 Neither tuberculosis nor lymphoma were found. Chylothorax is a potential complication of this disease.
Informed consentDuring hospitalization, verbal informed consent was requested from the patient for a potential publication of his case.
FundingSelf-financed.
Authors’ contributionsFLJ: devised the publication, collected the information, conducted a bibliographic search, prepared the first version, and reviewed and approved the final version.
SRR: collected the information, conducted a bibliographic search, prepared the first version and reviewed and approved the final version.
SCR: reviewed and approved the first version and reviewed and approved the final version.
ADR: collected the information, conducted a bibliographic search, prepared the first version and reviewed and approved the final version.
Conflicts of interestFLJ and SRR were the patient's treating physicians.