The excellent results for monoclonal antibodies in the treatment of severe uncontrolled asthma (SUCA) represent a milestone in current treatment of asthmatic disorders. Remaining, however, are several subsidiary areas for improvement in which new biologics are expected to make a decisive contribution. These biologics include tezepelumab, a monoclonal antibody that blocks thymic stromal lymphopoietin (TSLP). TSLP is an epithelial-release cytokine (alarmin) that plays a key role in initiating both the innate (group 2 innate lymphoid cell (ILC) pathway) and the acquired (T helper 2 (Th2) pathway) immune responses by activating the type 2 (T2) asthma inflammatory pathway through both. It is also thought that it may additionally intervene in the neutrophilic non-T2 inflammatory pathway (via interaction with ILC3 and interleukin-17). Six clinical trials that included 2187 patients with uncontrolled asthma, with 2 or more exacerbations in the previous year, on medium/high-dose inhaled corticosteroids and at least 1 other controller, have demonstrated – irrespective of T2 endotype (and possibly also non-T2 endotype) – the efficacy and safety of tezepelumab, as it significantly reduces exacerbations (61.7%–66%) and bronchial hyperresponsiveness, and improves lung function, disease control, and quality of life. Tezepelumab could be indicated for the treatment of patients with, independently of the T2 phenotype (eosinophilic and non-eosinophilic), and may even be the only biologic available for treatment of non-T2 SUCA.
Los excelentes resultados de los anticuerpos monoclonales en el tratamiento del asma grave no controlada (AGNC) constituyen un hito en el tratamiento actual de los trastornos asmáticos. Sin embargo, aún quedan varios aspectos complementarios susceptibles de mejorar para los que se esperan contribuciones decisivas de los nuevos biofármacos, entre los cuales se encuentra el tezepelumab, un anticuerpo monoclonal que bloquea la linfopoyetina estromal tímica (TSLP). La TSLP es una citocina de liberación epitelial (alarmina) que desempeña una función clave en el inicio de las respuestas inmunitarias tanto innata (vía de las células linfocíticas innatas [ILC] del grupo 2) como adaptativa (vía de los linfocitos T cooperadores 2 [Th2]), activando la vía inflamatoria del asma del tipo 2 (T2) mediante ambas. También se cree que puede intervenir en la vía inflamatoria neutrofílica con T2 baja (mediante la interacción con los ILC3 y la interleucina 17). En seis ensayos clínicos que incluyeron a 2.187 pacientes con asma no controlada, dos o más exacerbaciones en el año anterior, a tratamiento con corticosteroides inhalados en dosis medias o altas y con un mínimo de un tratamiento preventivo adicional, se ha demostrado la eficacia y seguridad del tezepelumab sin importar el endotipo T2 (y posiblemente tampoco el endotipo no T2), ya que reduce significativamente las exacerbaciones (61,7-66%) y la hiperreactividad bronquial y mejora la función pulmonar, el control de la enfermedad y la calidad de vida. El tezepelumab puede estar indicado para tratar a pacientes con asma grave, independientemente del fenotipo T2 (eosinofílico y no eosinofílico), y tal vez sea incluso el único biofármaco existente para el tratamiento del AGNC no T2.
Severe asthma, a heterogeneous syndrome that clinically presents in various ways, is defined as asthma that requires treatment with multiple drugs at high doses to maintain disease control.1,2 However, even at high doses – as outlined in therapeutic steps 5–6 of the Spanish Asthma Management Guidelines (GEMA) and 5 of the Global Initiative for Asthma (GINA) – some patients are unable to manage what we know as severe uncontrolled asthma (SUCA). Spanish SUCA prevalence is estimated to be approximately 3.9% of the adult population3 and 2%–5% of the child population.4 Poorly controlled asthma accounts for 70% of the economic cost of the disease.5
Managing severe asthma requires a proper understanding of underlying aetiopathogenic mechanisms, comorbidities, and the natural history of the disease, as well as knowledge of the different drugs available and their possible side effects. In view of this complexity, the Spanish Society of Pneumology and Thoracic Surgery (SEPAR) launched an initiative to accredit specialist asthma units in hospitals,6 which was also subsequently adhered to by the Spanish Society of Allergy and Clinical Immunology (SEAIC). By now there are around 100 accredited asthma units (75 corresponding to SEPAR and 25 to SEAIC of varying complexity in Spain).
In 2018, SEPAR published an expert consensus indicating that the clinical management of patients with severe asthma should be multidisciplinary and sequential and be carried out in specialist units.7 Other recommendations were to ensure a confirmed asthma diagnosis, the exclusion of comorbidities contributing to poor control, and therapeutic compliance, and to establish differential characteristics (phenotypes) and possible underlying mechanisms (endotypes) with a view to customizing treatment.7
Maintenance treatment for SUCA includes inhaled corticosteroids (ICS) combined with a high-dose long-acting β2-adrenergic agent (LABA), plus another controller drug – usually a long-acting muscarinic antagonist (LAMA) such as tiotropium or glycopyrronium. Patients with SUCA are also frequently treated with oral corticosteroids (OCS), either regularly or in cycles.8 Due to their potential long-term side effects, it is recommended to use OCS at the lowest effective dose and for the shortest possible time.
The phenotypes useful for therapeutic decision-making are based on the inflammatory mechanisms involved, and are as follows: allergic type 2 (T2) asthma (40%–50% of severe asthma cases), eosinophilic T2 asthma (25% of severe asthma cases), and non-T2 asthma. The 2 T2 phenotypes may coexist in some patients.
Biological therapies effective in T2 asthma are now available. These are monoclonal antibodies (MABs) directed against different biomarkers identified in each of the phenotypes, for which GEMA has developed a selection algorithm. Currently available biologics are as follows:
Omalizumab (Xolair). Anti-immunoglobulin E (IgE): reduces exacerbations, slightly improves forced expiratory volume in the first second (FEV1), and effective in treating nasal polyps.9
Mepolizumab (Nucala). Anti-interleukin 5 (IL-5): reduces exacerbations and OCS use, improves quality of life, slightly improves lung function, and effective in treating nasal polyps, eosinophilic granulomatosis with polyangiitis (EGPA), and idiopathic hypereosinophilic syndrome (HES).10
Reslizumab (Cinqaero). Anti-IL-5: reduces exacerbations, improves quality of life and lung function.11
Benralizumab (Fasenra). Anti-IL-5 receptor: reduces exacerbations, improves symptom control, reduces the need for OCS, and improves lung function.12
Dupilumab (Dupixent). Anti-IL-4 and anti-IL-13: reduces exacerbations, reduces OCS use, improves symptoms and lung function irrespective of peripheral blood eosinophil count, and effective in treating nasal polyps.13
No specific treatment is available for non-T2 asthma, although possibilities are azithromycin,14 bronchial thermoplastyl,15 and continuous parenteral corticosteroids.
Justification for new biologics for severe asthmaSince the introduction of omalizumab in 2006, SUCA treatment has changed substantially, with promising new biologics gradually included in the therapeutic arsenal. The current scenario for SUCA is undoubtedly more favourable than 2 decades ago, when patients would have been practically condemned to corticosteroid dependence. Nonetheless, numerous areas for improvement remain:
- –
SUCA is an orphan disease as far as treatment is concerned. All currently available biologics are targeted exclusively at treating the T2 phenotype and there is no specific treatment for non-T2 SUCA.
- –
There needs to be greater insistence on seeking full remission. The percentage of super-responders to current biologics is merely – depending on the definition – 14%–35%,16,17 while only around 69% of patients achieve a partial response.16
- –
Current biologics mitigate T2 asthmatic inflammation, but little information is available on their effectiveness in preventing bronchial remodelling, crucial in the natural history of asthma, as uncontrolled bronchial remodelling contributes to progressive and accelerated bronchial obstruction.
- –
Overlap is common between the 2 T2 asthma phenotypes and has therapeutic consequences, as it affects omalizumab efficacy in patients with eosinophilic asthma irrespective of IgE levels,18 and (conversely), anti-IL-5 efficacy in eosinophilic severe asthma irrespective of atopic state and IgE levels.19 This overlap, in real clinical practice, often determines selection of the best biologic to administer to a specific patient.
- –
There is a growing debate regarding the advisability of administering 2 biologics when response to a single biologic is only partial.20 Various endotypes coexist in the SUCA T2 phenotype. Specific blockade of a particular metabolic step may fail to control the disease, justifying use of a second biologic that acts on another T2 disease mechanism. This logically increases the treatment cost. Consequently, a drug capable of blocking the inflammatory cascade at a higher level would possibly prove more efficacious.
- –
The lack of biomarkers, in practice, affects choice of biologics, and, furthermore, the available biomarkers are not useful for evaluating efficacy.21
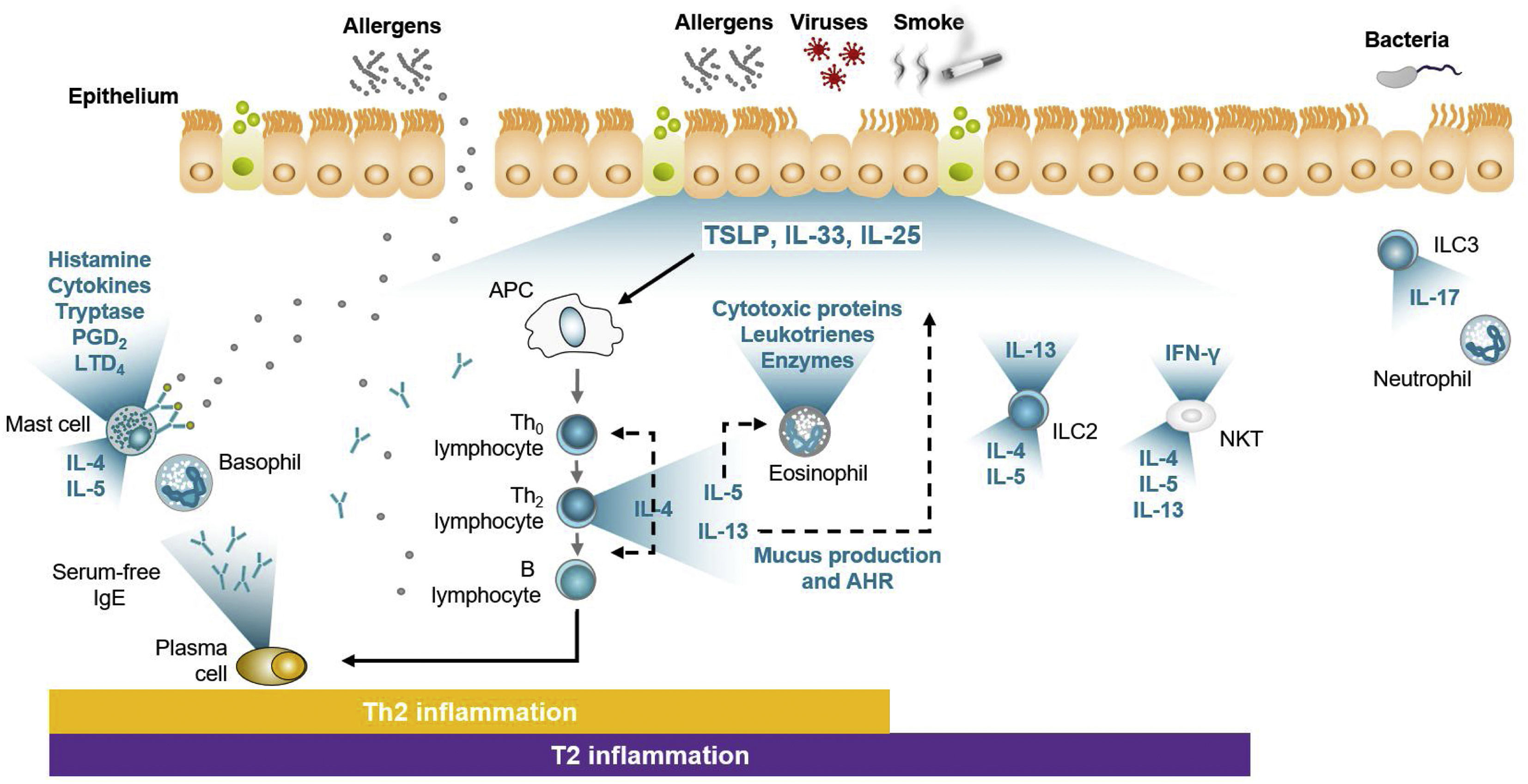
During the 20th century, knowledge of allergic mechanisms expanded, including details of what was known as the allergic cascade. By the early 21st century, this became known as adaptive immunity or – since T helper 2 (Th2) lymphocytes are the main cells involved – the Th2 pathway, while later on in this century, the significance of activation of the other immunity pathway, i.e., innate immunity, came to be better understood. While the Th2 pathway adapts to each allergen and generates a specific response, the innate immunity response is universal. When the bronchial ciliated epithelium is damaged, it becomes secretory, releasing molecules called alarmins (IL-33, IL-25, and TSLP) into the medium that activate innate lymphoid cells (ILC) groups 2 and 3, and natural killer T (NKT) cells (Fig. 1).22 Because TSLP appears to exert antigen presenting cell (APC) control, it is thought that it not only stimulates innate immunity cells (especially ILC2), but also regulates Th2 pathway activity. Both concepts come together in what is known as the T2 pathway (Fig. 1).22
Adapted from Domingo.22 The figure, which may explain clinical results, shows how adaptive immunity (via Th2) is activated by allergens, resulting in synthesized IgE, which enters the medium to bind to effector cells. The right part of the figure shows how damage produced in the bronchial ciliated epithelium causes alarmins to be released that activate ILC2 and NKT cells. In both cases, the ILs typical of T2 asthma (IL-4, IL-5, and IL-13) are synthesized and released. Note how TSLP exerts APC control. TSLP also appears to have some effect on activation of ILC3 belonging to the non-T2 pathway.
TSLP is a cytokine that exerts its biologic effects by binding to a high-affinity heterodimeric complex composed of the lymphopoietin receptor chain and the receptor for IL-7 (IL-7Rα). TSLP is expressed primarily in activated lung and intestinal epithelial cells, keratinocytes, and fibroblasts. However, dendritic cells (DCs), mast cells, and presumably other immune cells, can also produce TSLP. There are 2 TSLP variants in human tissues: the short form (sfTSLP) plays a homeostatic role and is the main isoform expressed in a stable state,23 while concentrations of the long form (lfTSLP) increase when inflammation occurs. Note that concentrations of messenger ribonucleic acid (mRNA) for TSLP are increased in the cells of patients with asthma24 and appear to be related to asthma severity. Several cellular targets for TSLP have been identified, including immune cells (DCs, ILC2, T cells, B cells, NKT cells, regulatory T (TReg) cells, eosinophils, neutrophils, basophils, monocytes, mast cells, and macrophages) and non-immune cells (platelets and sensory neurons).25
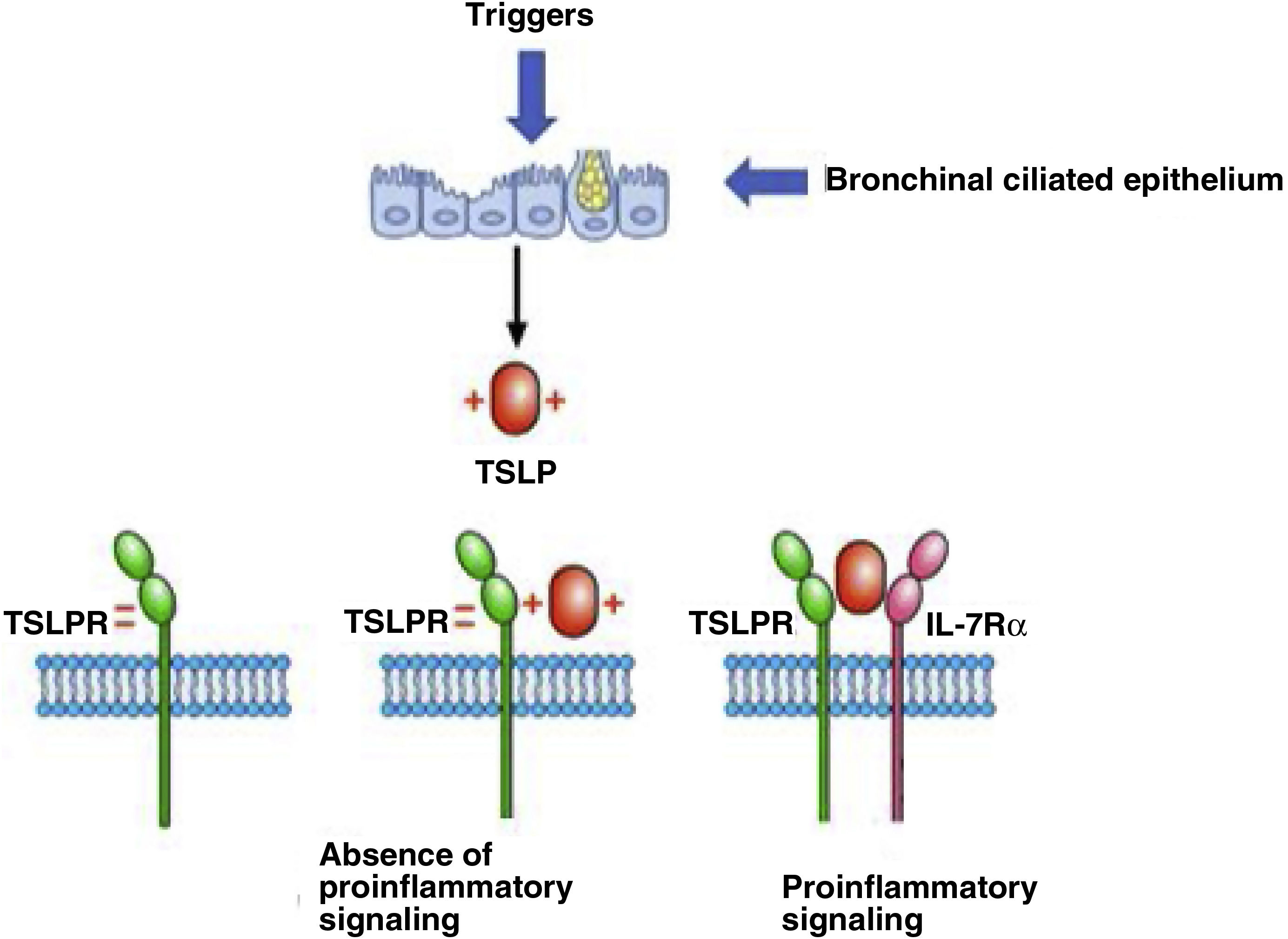
TSLP mechanism of action: binding to its receptorThe positively charged TSLP binds to the negatively charged TSLP receptor (TSLPR). IL-7Rα then binds to the preformed TSLP:TSLPR binary complex, thus forming a ternary complex, TSLPR:TSLP:IL-7Rα, that initiates signalling in cells that co-express TSLPR and IL-7Rα (Fig. 2).
Adapted from Varricchi et al.25 The figure shows how TSLP is produced from a bronchial ciliated epithelium damaged by triggering factors. Once produced and released into the medium, this positively charged TSLP binds to TSLPR. Signalling only occurs when TSLP binds to IL-7Rα.
Various cytokines, e.g., tumour necrosis factor alpha (TNFα) and IL-1 beta (IL-1β),26 as well as respiratory viruses,27,28 bacteria and fungi, mechanical injury,29 allergens,30 cigarette smoke,31 and tryptase32 can induce TSLP expression in different cells.
Tezepelumab mechanism of actionThe variable heavy chain fragment, but not the variable light chain fragment, of the human anti-TSLP MAB (tezepelumab) binds to TSLP,33 and as a consequence, all TSLP effects are blocked via both the Th2 pathway and innate immunity. In other words, by blocking free TSLP, tezepelumab prevents TSLP from binding to its receptor and so blocks the T2 pathway.
Principal tezepelumab clinical trial resultsSix important clinical trials of 2187 patients have been conducted with tezepelumab.
The PATHWAY study (ClinicalTrials.gov number NCT02054130)34 was the first clinical trial to demonstrate tezepelumab efficacy in patients with SUCA. This phase 2b, randomized, double-blind, placebo-controlled clinical trial analysed subcutaneous (SC) administration of 3 different doses of tezepelumab over a period of 52 weeks. The study, conducted in 108 centres in 12 countries, included 584 adult patients aged 18–75 years, non-smokers or with <10 pack-years exposure, with SUCA despite medium/high-dose ICS and LABA treatment. Inclusion criteria were to have had at least 2 exacerbations and have required OCS administration in the previous year. The study demonstrated that tezepelumab led to a significant reduction in exacerbations: an annual rate of 0.26, 0.19, and 0.22 for 70mg/4 weeks, 210mg/4 weeks, and 280mg/2 weeks, respectively, versus 0.67 for the placebo. Tezepelumab thus reduced exacerbations by 61.7%–66% in relation to placebo, and this occurred independently of baseline blood eosinophil count. In addition, FEV1 increased by 0.11–0.15L for tezepelumab in relation to placebo.
The NAVIGATOR study (ClinicalTrials.gov number NCT03347279)35,36 was a phase 3, multicentre, randomized, double-blind, placebo-controlled clinical trial whose primary objective was to determine the annual exacerbation rate in patients with SUCA after SC administration of 210mg of tezepelumab every 4 weeks. Recruited in 294 centres in 18 countries were 1061 patients aged 12–80 years, non-smokers or with <10 pack-years exposure, treated with medium/high-dose ICS and at least 1 other controller drug, irrespective of whether or not they received OCS. At baseline, patients had at least 2 exacerbations in the previous year (40% had more than 3), all had an ACQ-6 of 1.5 or more, and similar proportions had an eosinophil count above or below 300/μL (25% <150/μL and 25% >450/μL). The results showed that annual exacerbation rates were 0.93 for tezepelumab compared to 2.10 for placebo (p<0.001), and for eosinophils <300/μL, were 1.02 and 1.73, respectively (p<0.001). This reduction in exacerbations was observed, not only irrespective of the eosinophil count (the reduction occurred even for <150/μL), but also of fractional exhaled nitric oxide (FeNO) (<25ppb) and of allergenic sensitization. FEV1 improved by a statistically significant and clinically relevant 0.23L for tezepelumab versus only 0.09L for placebo, as did the ACQ score and quality of life as measured by the AQLQ. Of note was the significant decrease observed in total blood IgE in patients treated with tezepelumab.
Both those clinical trials not only determined the efficacy of tezepelumab, but also its safety, as no adverse reactions of note were reported. In the PATHWAY study, only 6 patients (2 on the medium dose, 3 on the high dose, and 1 on placebo) had to discontinue the study due to adverse reactions. In the NAVIGATOR study, while the number of side effects was high, at 77% in the treated group and 81% in the placebo group, they were only serious in 9.8% and 13.7% of cases, respectively, while study discontinuation rates were 6.8% and 10.7%, respectively. The most frequent adverse reactions reported in both studies were nasopharyngitis, upper respiratory tract infections, headache, and asthma.
SOURCE (ClinicalTrials.gov number NCT03406078)37,38 was a large clinical trial with tezepelumab, designed to assess OCS sparing in corticosteroid-dependent patients with severe asthma. This phase 3, multicentre, randomized, double-blind, placebo-controlled study, conducted in 60 centres in 7 countries, included 150 patients aged 18–80 years, taking, as well as OCS, high-dose ICS and at least 1 other controller drug. Patients were randomized to receive 210mg SC tezepelumab every 4 weeks or placebo over 48 weeks. Although no significant between-group differences were observed in the percentage reduction in daily OCS dose (primary endpoint), an improvement was observed in patients on tezepelumab with an initial eosinophil count >150/μL. The percentage and characteristics of adverse reactions were similar to those reported in previous studies.
The UPSTREAM (ClinicalTrials.gov number NCT02698501)39 and CASCADE (ClinicalTrials.gov number NCT03688074)40,41 studies were designed to evaluate tezepelumab effects on bronchial hyperresponsiveness (BHR). UPSTREAM randomized 40 patients with asthma and BHR to mannitol to receive either tezepelumab (n=20) or placebo (n=20) every 4 weeks for 12 weeks. By the end of the study, patients treated with tezepelumab experienced a 50% reduction in BHR. Parallel to this, pre- and post-treatment bronchoalveolar lavage performed in both groups showed a significant reduction in eosinophil count in the patients on tezepelumab. Similar results were reported for the CASCADE study, which included 116 patients who, in addition to receiving treatment for 52 weeks, underwent bronchoscopy with transbronchial biopsy, with results showing a marked reduction in eosinophils infiltrating the bronchial wall in patients on tezepelumab.
The PATH-HOME study (ClinicalTrials.gov number NCT03968978)42 was a phase 3 open-label, multicentre, randomized, parallel-group clinical trial of tezepelumab conducted in 216 patients. Results demonstrated that home self-injection with a pre-filled syringe is feasible, viable, and at least as efficacious as hospital administration. Adverse reactions were broadly similar to those of previous studies and were not affected by whether the drug was administered at home or in hospital.
Finally, DESTINATION (ClinicalTrials.gov number NCT03706079)43 was an extension study that reported, after 2 years of tezepelumab treatment, the same findings as observed in NAVIGATOR and SOURCE in terms of both safety (low incidence of side effects) and efficacy (sustained reductions in exacerbations regardless of initial eosinophil count, FeNO levels, and allergic status).
Tezepelumab positioning in clinical practiceThe candidate to receive tezepelumab is a patient for whom efficacy has been demonstrated in the phase 2 and 3 studies described above,33–35 i.e., patients with uncontrolled asthma, and a minimum of 2 exacerbations in the previous year despite receiving medium/high-dose ICS and at least 1 other controller. In the NAVIGATOR trial, 75% and 25% of patients used high and medium ICS doses, respectively.34,35 This would specifically indicate tezepelumab for patients with SUCA, although it may also be effective for patients with moderate asthma.
Given that the therapeutic target is located upstream in the inflammatory cascade, the candidate profile for tezepelumab treatment is very varied and may differ from the target patient for currently used biologics. Bearing in mind the different sub-phenotypes recommended by GEMA2 for biologic selection to treat SUCA, and also the findings of pivotal clinical trials, the following could be considered in relation to candidates for tezepelumab treatment:
Eosinophilic T2 asthma. The PATHWAY and NAVIGATOR trials34,36 confirmed a significant reduction in exacerbations (61.7%–66%) for SUCA, and similar favourable results for both allergic and non-allergic phenotypes. A PATHWAY43 post hoc analysis found no difference in exacerbation reduction according to the number of allergenic sensitizations. In both trials, tezepelumab reduced eosinophils, FeNO, and total IgE, demonstrating its action on multiple T2 pathways including IL-5, IL-4, and IL-13.
Non-eosinophilic T2 asthma. Findings of the PATHWAY and NAVIGATOR trials34,36 demonstrated that tezepelumab significantly reduced exacerbation rates in patients with an eosinophil count <250/μL (and even <150/μL in NAVIGATOR). Note that the improvement, although favourable, was less than that observed in patients with eosinophilic T2 asthma.
Non-T2 asthma. Both the PATHWAY and NAVIGATOR trials34,36 also confirmed that tezepelumab significantly reduced exacerbation rates in patients with low T2 biomarkers, specifically, eosinophils <150/μL, FeNO <25ppb, and no allergenic sensitization. This finding would suggest that tezepelumab may be the candidate molecule indicated as the first possible treatment for this phenotype, which, to date, has no specific biologic treatment.
Three other scenarios are as follows:
Corticosteroid-dependent asthma. Although the SOURCE study36,37 did not report a significant reduction in daily OCS dose in the tezepelumab versus placebo arms, some improvement was observed in patients with eosinophils >150/μL. However, significance for the between-group results remains unclear, as possible case inclusion bias affects that study, due to uncertainty as to previous adherence to OCS and given the favourable results also reported for the placebo arm. Further evidence is therefore needed to determine tezepelumab efficacy for patients with corticosteroid-dependent asthma.
Asthma with nasal polyps. In asthmatic patients with nasal polyps, tezepelumab reduces exacerbations, improves lung function, and improves nasal symptoms as evaluated using the Sino-Nasal Outcome Test (SNOT).44,45
Bronchial remodelling associated with SUCA. The favourable results reported for tezepelumab's capacity to reduce BHR are suggestive of a capacity prevent to further deterioration of lung function caused by bronchial remodelling.39–41 While this finding needs to be confirmed by other medium- and long-term studies, the hypothesis is nevertheless attractive, since practically no information is available on the possible beneficial effects of different MABs on asthma.
To sum up, a possible candidate to receive tezepelumab – independently of blood eosinophil count – is profiled as a patient aged 12–80 years, with SUCA, experiencing exacerbations despite receiving high-dose ICS/LABA treatment However, response is greater if eosinophils or FeNO are elevated, if there is sensitization to some aeroallergen, or if the patient has nasal polyps. In addition, a favourable response is achieved regardless of body mass index (BMI), the number of perennial aeroallergens to which the patient is sensitized, and IgE level. Response is less strong (but also favourable) when the following 3 factors coincide: eosinophils <150/μL, FeNO <25ppb, and no sensitization to aeroallergens.
Conclusions- (1)
Despite the great improvement implied by biologics for the treatment of SUCA, a number of shortcomings need to be considered, namely: (a) the rate of super-responders (currently low); (b) biologic selection (given the heterogeneity of endotypes that make up the T2 phenotype); (c) the lack of treatment for the non-T2 asthma phenotype; and (d) the limitations of current biomarkers (not valid for monitoring).
- (2)
New biologics could alleviate some of those shortcomings.
- (3)
Tezepelumab is a TSLP alarmin-blocking drug that inhibits initiation of the T2 inflammatory cascade from both the innate (via ILC2) and acquired (via Th2) immune response, and also possibly also the neutrophilic non-T2 pathway (via IL-17).
- (4)
Six clinical trials of 2187 patients that evaluated the efficacy and safety of tezepelumab have demonstrated that tezepelumab significantly reduces exacerbations (66%–71%) and BHR, and improves lung function, disease control, and quality of life for patients with previous exacerbations and uncontrolled asthma despite medium-high doses of ICS and another controller.
- (5)
Unlike current biologics, which are highly selective, tezepelumab exerts its pharmacological action, irrespective of the T2 endotype (eosinophilia and/or high or low FeNO) – and possibly also the non-T2 endotype – considered.
- (6)
Tezepelumab is a safe drug, as suggested by the most frequent side effects reported in clinical trials: nasopharyngitis, upper respiratory tract infections, headache, and asthma.
- (7)
Tezepelumab could be indicated for patients with SUCA and exacerbations despite treatment with high ICS/LABA doses and irrespective of the blood eosinophil count, i.e., for both eosinophilic T2 asthma and non-eosinophilic T2 asthma.
- (8)
In addition, in view of the clinical trial results, tezepelumab may be the only biologic with proven efficacy for the treatment of non-T2 SUCA.
This article was funded by the Barcelona Respiratory Network (BRN) who did not participate in the articles selected or writing of the manuscript.
Authors’ contributionsVP was the project coordinator, writer of part of the article and a major revisor of the final manuscript.
CC participated as a writer of part of the article and revisor of the final manuscript.
CD participated as a writer of part of the article and revisor of the final manuscript.
CM participated as a writer of part of the article and revisor of the final manuscript.
XM participated as a writer of part of the article and revisor of the final manuscript.
Conflicts of interestVP has received fees in the last 3 years for talks at meetings sponsored by AstraZeneca, Chiesi, Gebro, GlaxoSmithKline, and Sanofi, and as a consultant forAstraZeneca, GSK, Menarini and Sanofi, and has received attendance expenses for conferences from AstraZeneca, and Chiesi.
CC has received fees in the last 3 years for talks, participation in clinical trials, attendance at conferences, and as a scientific advisor from AstraZeneca, Boeringher Ingelheim, Chiesi, GlaxoSmithKline, Novartis, and Sanofi.
CD has received financial assistance for attendance at conferences, congresses, and symposia, participation in scientific meetings, and consultancy from Novartis, GlaxoSmithKline, TEVA, Merck Sharpe Dohme, Sanofi, Boehringer Ingelheim, Esteve, Almirall, Astra-Zeneca, Chiesi, Menarini, Takeda, Pfizer, Ferrer, Stallergenes, ALK-Abelló, Allergy Therapeutics, Hall Allergy, and Inmunotek.
CM has received fees and other payments for conferences, presentations, and educational events from AstraZeneca, Chiesi, GlaxoSmithKline, Gebro, Mundipharma, Novartis, TEVA, and Sanofi and for participation as an advisor from AstraZeneca, GlaxoSmithKline, CSL Behring, Mundipharma, and has received research project grants from AstraZeneca, GlaxoSmithKline, and TEVA.
XM has received fees as a speaker, scientific advisor, and clinical trial participant from AstraZeneca, Boehringer Ingelheim, Chiesi, Gebro, GlaxoSmithKline, Menarini, Mundifarma, Novartis, Sanofi, and Teva.