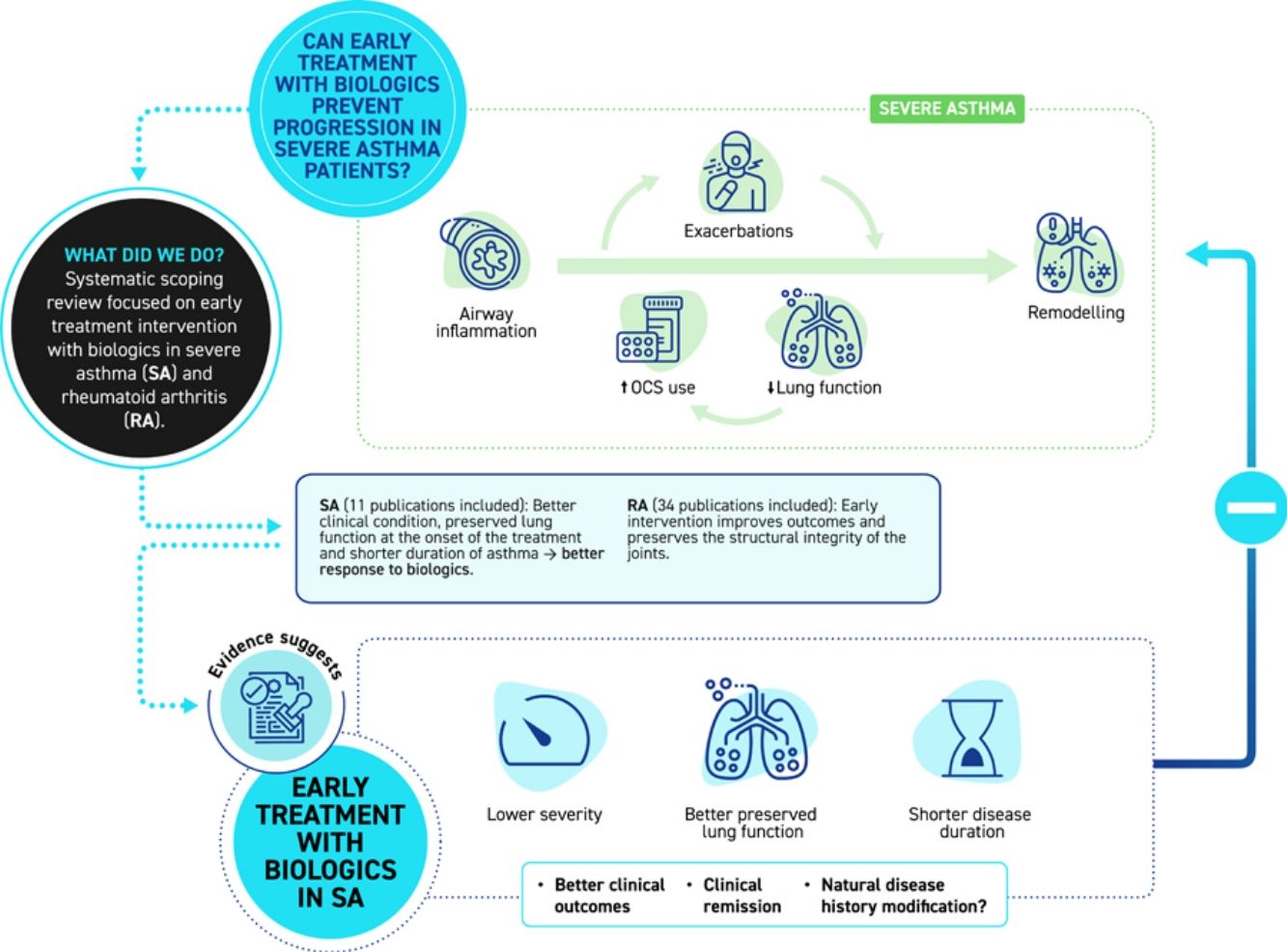
Theoretically, an early intervention with biologics in severe asthma (SA) patients may attenuate inflammatory processes and potentially halt disease progression and remodeling. Changing the approach to a more preventive one could alter the course of the disease, avoid its progression, and improve the likelihood of achieving clinical remission. The aims of this study were to gather scientific evidence on this topic, to draw a parallel between SA and rheumathoid arthritis (RA) and to analyze the potential benefits of establishing early treatment in SA.
Material and methodsA systematic scoping review, conducted in accordance with the methodological guidance of the Arksey and O’Malley framework and focusing on early treatment intervention with biological drugs in SA and RA is presented.
ResultsEvidence supports the early intervention with biologics in RA to improve outcomes. Evidence regarding early intervention with biologics in SA is scarce. To date, the literature reviewed suggests that better clinical condition of the patient and more preserved lung function at the onset of biological treatment, together with a shorter duration of asthma, are associated with better response to biologics.
ConclusionsData suggests that the more preventive approach may lead to improved results. The scarcity of scientific evidence highlights the importance of pursuing this line of research.
Teóricamente, una intervención precoz con biológicos en pacientes con asma grave (AG) puede atenuar los procesos inflamatorios y potencialmente detener la progresión y remodelación de la enfermedad. Cambiar el abordaje hacia uno más preventivo podría alterar el curso de la enfermedad, evitar su progresión y mejorar la probabilidad de alcanzar la remisión clínica. Los objetivos de este estudio fueron reunir evidencias científicas sobre este tema, establecer un paralelismo entre el AG y la artritis reumatoide (AR) y analizar los beneficios potenciales de instaurar un tratamiento precoz en el AG.
Material y métodosSe presenta una revisión sistemática de alcance, realizada de acuerdo con las orientaciones metodológicas del marco de Arksey y O’Malley y centrada en la intervención terapéutica precoz con fármacos biológicos en AG y AR.
ResultadosLa evidencia apoya la intervención temprana con fármacos biológicos en AR para mejorar los resultados. Los datos sobre la intervención precoz con fármacos biológicos en el AG son escasos. Hasta la fecha, la literatura revisada sugiere que un mejor estado clínico del paciente y una función pulmonar más preservada al inicio del tratamiento biológico, junto con una menor duración del asma, se asocian a una mejor respuesta a los biológicos.
ConclusionesLos datos sugieren que el abordaje más preventivo puede conducir a mejores resultados. La escasez de evidencias científicas pone de manifiesto la importancia de continuar con esta línea de investigación.
Asthma is a chronic inflammatory respiratory disease that affects millions of individuals worldwide, causing substantial morbidity and imposing a significant burden on healthcare systems.1 Approximately 5–10% of the patients with asthma have severe asthma (SA), which accounts for the majority of the morbidity and cost of the disease.2
Recurrent exacerbations in patients with SA can impact lung function, further complicating disease management and impacting patient's quality of life.3,4 Also, research reveals that adults with asthma who exhibit more significant baseline airway remodeling are at higher risk of experiencing lung function decline.5 Moreover, the management of SA often involves the use of oral/systemic corticosteroids, which can lead to significant adverse effects, emphasizing the critical need for alternative treatment options to break this vicious cycle.6,7 In addition, some studies highlight the significant risk of systemic adverse effects (osteoporosis, hypothalamic–pituitary–adrenal axis suppression, diabetes, and respiratory infections) associated with high doses of inhaled corticosteroids (ICS).8–11 This consideration becomes crucial as high-dose ICS remains the treatment of choice for patients with SA. We can hypothesize that the longer asthma patients are exposed to such situations, the worse their clinical and functional situation will become, and the lower the chances of achieving an optimal response. Therefore, early diagnosis and intervention may be relevant to mitigate any potential negative impact of asthma on clinical evolution, highlighting the pressing need for timely use of treatment strategies.12,13
Monoclonal antibodies (mAb) have emerged as a promising strategy for the management of patients with SA, offering a potential breakthrough in this complex condition.14 Various mAb have been shown to be effective in reducing exacerbation rates, improving lung function, achieving better asthma control and quality of life,15 and even reaching clinical remission.16 With the aim of delaying the progression of SA, preventing irreversible accumulated damage, and avoiding the adverse effects of treatment, the hypothesis of starting early treatment with biologics as a therapeutic option can be considered for SA patients.
Monoclonal antibodies have shown considerable success in the treatment of various chronic inflammatory conditions beyond SA, such as rheumatoid arthritis (RA). RA is a systemic autoimmune disease, characterized by inflammation and chronicity. It primarily affects the joints, but it can also involve other organs such as the heart, lungs, kidneys, skin, and eyes, among others, as well as the hematopoietic system or neuropsychiatric sphere.17 Notably, RA is considered a severe disease. If not properly treated, it typically progresses to joint destruction, functional impairment, and increased mortality.18 Therapy with disease-modifying antirheumatic drugs (DMARDs) should be started as soon as the diagnosis of RA is made.19 The management of RA has undergone a transformative shift with the incorporation of mAb.20 These mAb, which target molecules such as tumor necrosis factor (TNF) alpha or interleukin-6 (IL-6), among others, have fundamentally changed treatment approaches by specifically inhibiting inflammatory processes contributing to joint degradation and tissue damage.20,21 Remarkably, since the introduction of mAbs, treatment has shown efficacy in modifying disease activity and slowing or halting structural joint damage.22 The synergistic effect between methotrexate (MTX) and mAb, along with an early intervention and a ‘treat-to-target’ strategy, constitutes an effective approach, promoting sustained remission and enhancing the quality of life for RA patients.19 The ‘treat-to-target’ strategy in RA involves a dynamic methodological design, allowing for therapeutic adjustments at each visit if the initially set goal hasn’t been reached.23 According to the European League Against Rheumatism (EULAR) guidelines,19 this approach implies frequent patient monitoring and aims to achieve a relevant improvement in disease activity within three months, striving for remission in early stages, and low disease activity in long-standing cases by approximately six months. EULAR guidance also indicates that “if the treatment target is not achieved with the first conventional DMARD (cDMARD) strategy, when poor prognostic factors are present, a biological DMARD (bDMARD) should be added”.19
We carried out a scoping review focused on early treatment intervention with biological drugs in SA with the aims to compile the available scientific evidence, to identify gaps in the literature and to provide an overview of the existing evidence to understand if this approach can be a suitable future strategy for the optimal treatment of patients with SA. We also wanted to draw a parallel with RA to underscore the potential benefits of establishing early treatment in SA, akin to the approach adopted in managing chronic inflammatory joint diseases. Through a comprehensive review of the current literature and ongoing research, this manuscript strives to shed light on the promise of mAb in revolutionizing the management of SA and aims to invite the scientific community to reflect on early treatment of SA with biological drugs.
Material and methodsStudy designTo collect relevant information, a systematic scoping review was conducted in accordance with the methodological guidance of the Arksey and O’Malley framework, which recommends the following stages: (1) identifying the research question; (2) identifying relevant studies; (3) study selection; (4) charting the data; and (5) collating, summarizing, and reporting the results.24 The report of this scoping review was compiled by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension for scoping reviews (PRISMA-ScR).25
Identifying the research questionTo develop our research question, we used the PCC (population, concept and context) framework,26 which is useful for both qualitative and quantitative (mixed methods) topics, and is commonly used in scoping reviews.
The research questions from the study were as follows:
- -
What are the clinical implications of the early use of monoclonal/biologic therapies in the treatment of severe asthma?
- -
What are the clinical implications of the early use of monoclonal/biologic treatments in the management of rheumatoid arthritis?
In our literature search, we selected all studies published until April 2023 that used mAbs or biologic treatments for SA and RA that included the concept of early treatment. Studies focused on other types of outcomes (such as cost-effectiveness and prognosis) were excluded. Inclusion and exclusion of the final publications for the review was conducted according to expert's judgment. We kept our search broad and we narrowed the search results in accordance with our predetermined PCC framework, which is listed in Table 1.
PCC framework.
| Criteria | Description |
|---|---|
| Severe asthma | |
| Population | Patients with SA initiating or receiving treatment with biologics |
| ≥18 | |
| Any sex | |
| Concept | Early treatment implications |
| Early intervention | |
| Context | Worldwide. No limits on ethnicity or gender |
| All settings considered with the use of mAb (omalizumab, mepolizumab, reslizumab, benralizumab, dupilumab, tezepelumab, others) for SA | |
| Rheumatoid arthritis | |
| Population | Patients with RA |
| ≥18 | |
| Any sex | |
| Concept | Early treatment implications |
| Early intervention | |
| Context | All settings considered with the use of biological drugs for the management of RA |
mAb: monoclonal antibodies; PCC: population, concept and context; RA: rheumatoid arthritis; SA: severe asthma.
In this comprehensive review, various study designs were considered, including both experimental and quasi-experimental approaches. This encompassed randomized and non-randomized controlled trials, observational studies and systematic and non-systematic reviews that adhered to the inclusion criteria. Reviews were considered due to the limited availability of original studies. In addition to these, a variety of other study types, post hoc analyses or clinical practice guidelines were incorporated to ensure a comprehensive and robust analysis.
Our goal was to identify all published studies concerning the implications of early treatment with mAb or biologics. To achieve this, we developed a search strategy based on the terms included in the Supplementary Material and applied it to the following databases:
- •
MEDLINE via PubMed
- •
Cochrane Library – including the Cochrane Database of Systematic Reviews (CDSR) and CENTRAL (clinical trials)
- •
Epistemonikos
- ∘
Clinical Practical Guideline
- ∘
National Guidelines Clearinghouse-NGC: www.guidelines.gov
- ∘
GuíaSalud: www.guiasalud.es
We were limited to the English and Spanish languages, and our literature search had no time constraints. We also conducted a brief search in Google Scholar to identify possible studies not found in the chosen databases. Additionally, we performed a quick search using the references of the included studies and we incorporated gray literature into the review.
Study selectionTwo reviewers independently assessed articles by title and abstract based on inclusion criteria defined by the PCC framework. After this initial screening stage, the full texts of the articles meeting these criteria were obtained and a second review was conducted to confirm inclusion based on the full text. Disagreements were resolved by discussion or consensus with a third reviewer. The final selection was validated by two SA experts and one RA expert. We used Rayyan QCRI® as data management software for both screening phases.
Charting the dataA data extraction table was developed to compile relevant data for this review. Extraction variables included: Author(s), study design, population, and objectives related to the review question, among others. This information was added to enhance the understanding of the literature included in this review.
Collating, summarizing, and reporting resultsA narrative synthesis was conducted by integrating the results into a narrative summary and tabulating the information in evidence tables, including an explanation of the characteristics, objectives and key relevant points of the studies. The narrative synthesis also emphasized the gaps in the evidence.
Ethics committee that approved the researchIt was not required, as this work corresponds to a systematic scoping review and no human subjects were involved.
Informed consent of the patient/participantNo informed consent was required, as this work is a result of a systematic scoping review and no patients/participants were involved.
ResultsSearch results and study selectionIn response to the question regarding the clinical implications associated with the early use of monoclonal/biologic therapies for SA treatment, a total of 157 publications were initially identified across all selected databases (Medline, Epistemonikos, and the Cochrane Library). After eliminating duplicates (n=7), 150 publications obtained through the various search strategies were manually screened. From these, 133 records were excluded based on titles and abstracts, leading a total of 17 reports assessed for eligibility. From this, 7 reports were excluded. In parallel, an additional 20 articles were identified through citation searches and gray literature, but just one was considered. Thus, a total of 11 articles were included in the review (Fig. 1).
Additionally, to address the question of the clinical impact of early monoclonal/biologic treatments in the management of RA, our database search, citation searches, and review of the gray literature yielded 603 publications. Of the 36 studies assessed for eligibility, 2 were excluded as they did not specifically involve biologic treatments, leading to a total of 34 articles included in the review. Fig. 2 illustrates the flow of articles included for the aforementioned questions.
As a result, a total of 45 studies were included in the review.
Description of the studies from the research question “What are the clinical implications of the early use of monoclonal antibodies/biologic therapies in the treatment of SA?”In our scoping review, no studies that primarily focused on early intervention with biologics in SA treatment were found. However, some of them included pertinent information in relation to this topic, so they were documented and listed in Table 2.
Overview of the studies included in response to the question regarding the clinical implications associated with the early utilization of monoclonal/biologic therapies for severe asthma treatment.
| Author (year) | Journal | Design | Population/sample | Objective | Key relevant points for our scoping review |
|---|---|---|---|---|---|
| Eger27 (2021) | J Allergy Clin Immunol Pract | Retrospective Observational Study | Patients with SEA | To assess prevalence and predictors of super, partial, and non-responders to long-term anti–IL-5 treatment; frequency and reasons for switches between anti–IL-5 biologics, and nature of residual disease manifestations. | It is observed that certain baseline characteristics such as shorter duration of asthma (6 years in super-responders vs 16 and 21 years in partial responders and non-responders, respectively) and better lung function (higher FEV1% predicted and lower percentage of patients with FEV1<80%)) implies a higher probability of achieving a super response “Super response was predicted by shorter asthma duration and higher FEV1.” |
| Graff33 (2022) | The journal of allergy and clinical immunology | Observational study | Patients with SA | To evaluate risk factors for accelerated lung function decline in patients on anti-Interleukin-5 treatment. | The study shows that there are some patients who experience greater lung function decline than others and a multivariable linear regression model suggests that factors such as higher ACQ at baseline, having late-onset asthma, or the addition of anti-IL-5 are associated with attenuated FEV1 decline. |
| Kavanagh28 (2021) | Chest | Observational study | Patients with SEA | To evaluate the real-world effectiveness of benralizumab and identify any baseline characteristics associated with response to therapy. | It is observed that patients with less severe disease (strongly eosinophilic phenotype, lower OCS dose, annual exacerbation rate, higher FEV1 post BD and better ACQ-6 and AQLQ scores) are more likely to achieve a complete response or even become super responders. |
| Kavanagh29 (2020) | Chest | Observational study | Patients with SEA | To evaluate the efficacy of mepolizumab in a real-world setting; to perform a detailed assessment of which baseline clinical characteristics predict response to mepolizumab; and to define an exacerbation-free and OCS-free “super-responder” to mepolizumab. | It is observed that responders had better asthma control and lower OCS dose at baseline than non-responders. In addition, a higher baseline FEV1 was observed in super responders compared with non-responders. |
| Kroes34 (2022) | The European respiratory Journal | Observational study | Patients with SEA | To compare the cumulative OCS exposure over a 2-year period before and after anti-IL-5/5Ra initiation, and secondarily to investigate whether duration and cumulative OCS exposure prior to anti-IL-5/5Ra influence the ability to discontinue OCS within 2 years of anti-IL-5/IL-5Ra therapy. | This real-world study showed that the use of anti-IL-5/5Ra therapy allows the reduction of cumulative OCS exposure, with better results observed in patients with lower and shorter exposure to OCS. “Since cumulative exposure increased progressively prior to anti-IL-5/IL-5Ra initiation, data suggest that early intervention might lead to a better long-term prognosis in patients with severe eosinophilic asthma.” |
| Lanz37 (2019) | Pediatr Pulmonol | Non-systematic review article | Children with asthma | To assess the current understanding of childhood asthma treatment and progression in patients with airflow obstruction in adulthood. | Frequent asthma exacerbations in childhood can negatively impact development of proper lung function. The resulting airway remodeling leads to reduced FEV1 and fixed obstruction in some adult individuals. |
| Miralles-López30 (2022) | Allergol Immunopathol (Madr) | Observational study | Patients with SA | To describe super-responders to benralizumab in a series of 79 patients who completed at least 1 year of treatment, and to compare super-responders with non-super-responders. | It was observed that super-responder patients have a better baseline clinical situation (e.g., fewer exacerbations, lower OCS dose or better ACT and AQLQ scores), higher FEV1% (71 vs 62%, NS) and a shorter disease evolution time (17.14 vs 22.8 years, NS) vs non-super-responders. “It is perceived that patients with milder disease may meet super-responder criteria more easily than those with more severe disease.” |
| Oishi31 (2023) | J Clin Med | Retrospective Observational study (RWE) | Patients with SA | To assess the achievement rate and predictors of CR and DR using long-term biologics. | Patients who achieve “deep remission” (no symptoms, exacerbations, use of OCS, normal lung function and low T2 biomarkers) have a shorter duration of asthma (5 vs 19 years) or a higher FEV1% (91.5 vs 71.5) vs patients who do not achieve deep remission. “This finding suggests that early initiation of biologics in severe asthma could modify the clinical course and prognosis.” |
| Pérez De Llano35 (2024)a | Am J Respir Crit Care Med | Longitudinal Cohort study: International Severe Asthma Registry (ISAR) | Patients with SA | To examine the association between different definitions of remission and selected patient characteristics. | Initiating biologics earlier in the disease course, before the development of severe impairments, or in patients with less severe baseline clinical condition or better lung function is associated with an increased likelihood of clinical remission following biologic initiation. “Patients with less severe impairment and shorter asthma duration at initiation had a greater chance of achieving remission after biologic treatment, indicating that biologic treatment should not be delayed if remission is the goal”. |
| Thomas32 (2022) | The European Respiratory Journal | Review article | Patients with SA | To provide insight into the criteria for defining remission, potential therapeutic interventions, and the overall understanding of achieving a state where asthma symptoms are absent or significantly reduced over an extended period of time. | This review determines that factors such as better lung function, asthma control or shorter duration of asthma act as predictors of asthma remission in several studies. This points in the direction of early and timely intervention to achieve better outcomes, although it acknowledges an evidence gap to prove this hypothesis. “Conceptually, timely introduction of biologics therapy may weaken the inflammatory process at the earlier stages of disease activity, restricting the exposure of inflammatory mediators to the airway wall, lowering the potential for airway remodeling and halting the disease progression.” |
| Varricchi36 (2022) | Allergy | Review article | Patients with SA | To examine and analyze the relationship between biologic therapies and airway remodeling in individuals with SA. | Airway remodeling is a major feature of SA, and some experimental studies indicate that there is a limited window of opportunity to stop the inflammation that leads to structural damage and fibrosis. Biologics have even been shown to normalize airflow obstruction. “Biologics might prevent and possibly even revert “fixed” remodeling due to structural changes.” |
ACT: asthma control test; ACQ: asthma control questionnaire; AQLQ: asthma quality of life questionnaire; BD: bronchodilator; CR: clinical remission; DR: deep remission; FEV1: forced expiratory volume in one second; SA: severe asthma; SEA: severe eosinophilic asthma; OCS: oral corticosteroids.
By the time of the bibliographic search, the information of this publication had been presented as an abstract: Pérez de Llano L, Scelo G, Tran TN, Le TT, Martin N, Fagerås M, et al. Characteristics associated with clinical remission in patients with severe asthma who initiate biologics. Proceedings of the ERS 2023 International Congress; 2023 Sept 9–13; Milan, Italy.
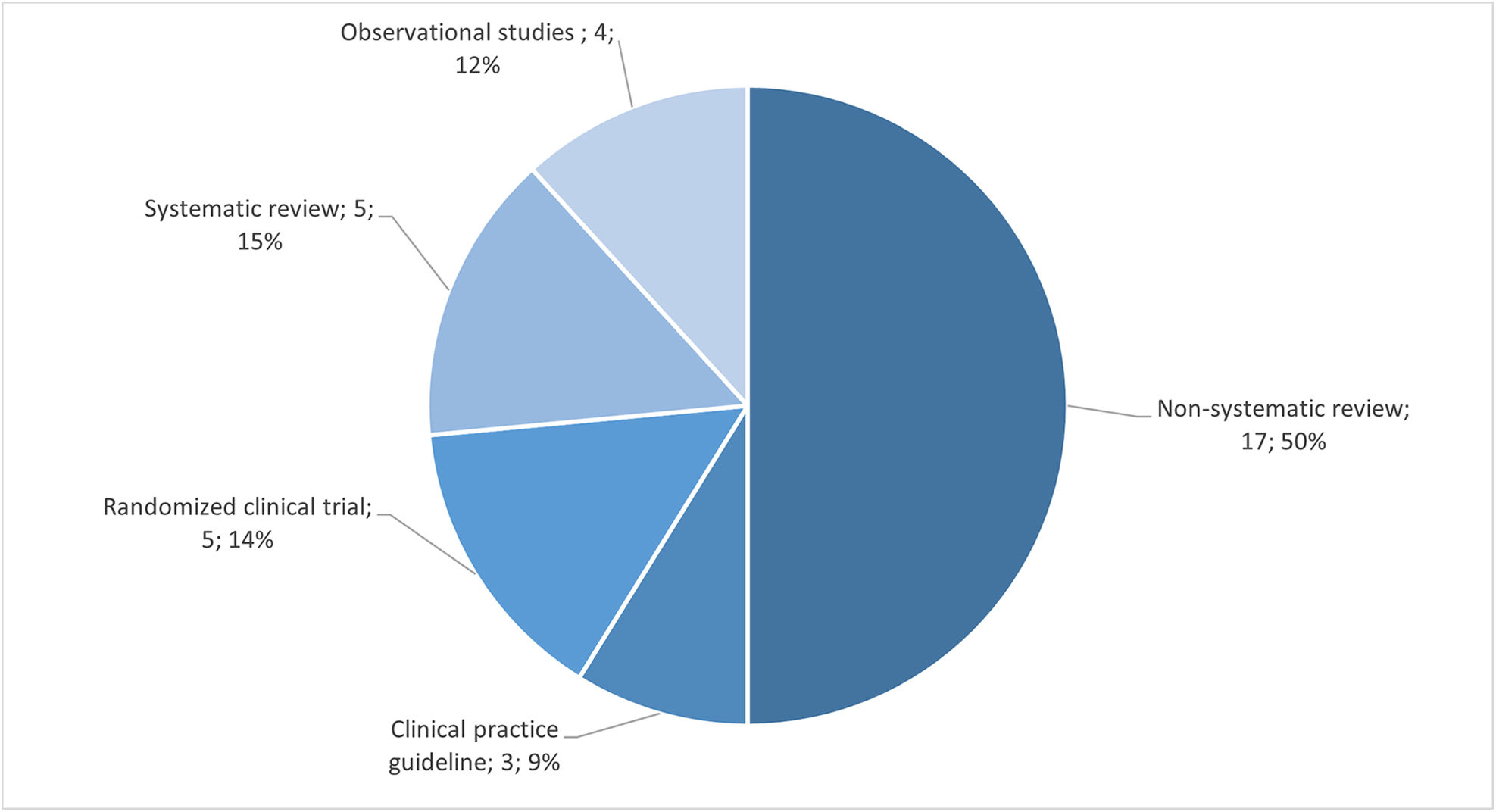
Among the studies included in our analysis, none of them were randomized clinical trials related to the early use of mAb or biological therapies for SA. We identified observational studies, non-systematic reviews, and review articles. Additionally, we identified an abstract of an observational study which provided a concise summary of the relevant research findings. Fig. 3 illustrates the distribution of study types included in this review.
Regarding the study population, six studies focused on patients with moderate-to-severe asthma, while four studies specifically examined SA within the broader context of asthma patients in general. The selected studies pursued a diverse range of primary objectives. These objectives included assessing the efficacy of mAb and characterizing super-responders to this treatment, investigating risk factors associated with accelerated lung function decline, exploring remission patterns, and analyzing prognostic factors related to lung function deterioration. A summary of the study objectives can be found in Table 2.
Different studies show that factors such as a shorter asthma duration, better baseline clinical condition or better lung function (FEV1) are associated with a higher probability to achieve a complete response.27–32 Additionally, the use of biologics is associated with reduced FEV1 decline33 and reduced oral corticosteroids (OCS) exposure,34 leading to better outcomes. Thus, it appears that an early intervention with biologics in the disease course might increase the likelihood of clinical remission35 and normalize airflow obstruction.36 In the pediatric population, asthma exacerbations and lung inflammation can have a negative impact on lung development.37
Description of the studies from the research question “What are the clinical implications of the early use of monoclonal/biologic treatments in the management of RA?”The results of this review have revealed a diverse range of research approaches when it comes to monoclonal/biologic treatment in the context of RA management. A total of 34 studies were identified, including observational studies, reviews (both systematic and non-systematic), clinical practice guidelines and randomized controlled trials (RCTs). Fig. 4 illustrates the distribution of study types included in this review.
In the review, the authors also decided to include the most relevant Clinical Practice Guidelines for the management of RA. The publications are summarized in Table A.1 of the Supplementary Material.
To briefly summarize the results observed in the literature review on RA, early diagnosis and treatment of RA are important for achieving better outcomes and preserving the structural integrity of the joints.38,39 Furthermore, studies have shown that early use of biologics can offer substantial advantages over conventional treatments, highlighting the need to develop treatment algorithms to facilitate the appropriate selection of patients who should be treated with biologics at an earlier stage.40,41 The implementation of a treat-to-target approach to initiate the biologic therapies as soon as cDMARDs prove ineffective increases the probability of improving the course of RA.42
DiscussionChanges in the approach to asthma management have been suggested in recent years. Beyond disease control, there is a call to allocate resources toward asthma prevention and cure. This proposition specifically highlights the need for studies aimed at modifying the course of the disease, including immunotherapy and early administration of mAb. This perspective also includes the identification of risk biomarkers and a deeper understanding of the pathways initiating respiratory illnesses.43 The introduction of different biologics among the treatment options for SA emphasizes the need to assess treatment responses, not only for individual but also for combined goals. This approach involves the concept of “super-responders” and the pursuit of comprehensive responses, even the potential to achieve clinical or inflammatory remission of the disease.44 The evidence highlights the potential of these therapies to extend beyond symptomatic control. It is worth noting that studies collectively underscore the ability of biological therapies to induce clinical remission in a significant proportion of patients.31,45–48 Interestingly, none of the definitions include a reduction in high-dose ICS exposure over time as a key component of remission. In fact, it has been shown that patients controlled with benralizumab can have meaningful reductions in ICS therapy while maintaining asthma control.49
Over the past few decades, some research has focused on early intervention in asthma. Some studies have suggested that early intervention with ICS is effective in achieving optimal asthma control50–54 in non-severe patients when compared with placebo, although the long-term implications of such an approach are inconclusive. However, the potential benefits of early intervention with mAb to modify the course of SA, its progression, the development of bronchial remodeling, and the likelihood of achieving remission, remains a largely unexplored area in clinical research,32 as shown by the present bibliographic review.
Focusing on SA, early intervention could be defined in two ways:
- •
Treatment at a stage of the disease when asthma is not yet severe, to prevent progression to SA.
- •
Treatment as soon as the patient is eligible for biological therapy.
In the first scenario, the absence of comprehensive knowledge regarding the natural history of asthma makes unfeasible to predict which patients will progress to severe forms of the disease. While there is some evidence to suggest the benefits of this approach,55 initiating a biologic “preemptively” seems an unrealistic strategy at this time and is beyond the scope of this review. It should be noted that treating non-severe patients with biologics would increase their treatment-associated costs. Therefore, it would essential to analyze the cost-effectiveness relationship of this approach in light of the economic burden of the disease.56–58
The second scenario seems more feasible, but we must keep in mind that there are other therapeutic alternatives to consider, such as treatment with triple inhaled therapy, which can control the disease in some patients.59 On the other hand, the evidence suggests that escalating treatments (e.g., using high doses of ICS over medium doses) may not have a clear clinical benefit,10,60,61 at least not for all patients, so perhaps the therapeutic approach should be personalized according to patient characteristics.62,63
Beyond definitions, an essential discussion arises: how can we ensure a more robust response to treatment, and could early intervention play a pivotal role in achieving this? Theory suggests that early intervention with biologics in SA patients may attenuate inflammatory processes, reducing exposure to inflammatory mediators and potentially halting disease progression and remodeling.36,43 The effects of biological interventions might be even more evident in pediatric patients. Uncontrolled asthma in childhood may lead to an impaired lung development.37 Exploring the impact of early treatment on treatment response becomes an essential avenue for consideration. This scoping review highlights several relevant key points from the literature that may underscore the importance of early treatment with biologics in SA patients.
Baseline clinical situation correlates with clinical outcomes: better response if lower disease severityIn our search, studies exploring factors associated with response and remission after the initiation of biologic therapy provide valuable insights. Specifically, the baseline clinical condition of patients is directly related to the probability of achieving an optimal response to biologics in SA, with less severe initial situations often correlating with better responses and even the possibility of achieving remission.28–30 Real-world data reveal that fewer exacerbations, reduced long-term OCS use and improved symptom control prior to biologic therapy are positively associated with reaching clinical objectives and achieving remission.34,64,65 Regarding the use of OCS, even low doses of this therapy can lead to adverse effects, establishing the need to assess the patient's cumulative exposure over time and to achieve complete withdrawal of OCS.34,64,65 Various studies underscore that higher initial doses of OCS are associated with increased challenges in tapering and discontinuing OCS in patients treated with biologics, and prolonged exposure decreases the likelihood of completing the withdrawal process.34,66 Therefore, initiating biologic treatment as soon as possible in patients with OCS use could be crucial to achieving complete OCS withdrawal. As mentioned, achieving effective steroid-sparing is crucial to avoid undesirable effects of OCS therapy, and could also help to address concurrent issues such as obesity (which usually complicates disease management), as biologic treatment may even lead to weight loss in obese patients.67
Taken together, these observations could suggest that perhaps early intervention with biologics in SA patients, before there has been a high cumulative use of OCS, before exacerbations have caused remodeling, and before control and quality of life are very poor, would increase the likelihood of achieving an optimal response to biologic treatment.
A better preserved lung function is associated with better clinical outcomesAnother relevant objective of biologic treatment, included in most response/remission definitions, is to increase or achieve normal or stabilized lung function.32,44 The evidence suggests that better baseline lung function parameters correlate with greater improvements in lung function and better clinical outcomes after treatment with biologics, and also increases the probability of achieving super-response and remission in patients with SA.27–31,48,68 As an example, the study by Oishi et al. reveals that the deep remission (DR) group exhibited higher FEV1 values compared to the non-DR group (91.5% vs. 71.5%, p<0.001).31 In line with this, the ORBE II study analyzing results with benralizumab in severe eosinophilic asthma (SEA) patients suggests a difficulty in reaching an FEV1≥80% when starting with more compromised lung function.46 This difficulty may stem from the decline in lung function prior to treatment initiation, potentially signaling the need for earlier initiation of biologic treatment in the disease course. Similar results have been observed in other recent studies,35,69,70 where better lung function prior to initiation of biologic therapy was associated with remission, not only at one year35 but also for a longer timeframe.69
Also, some authors highlight the potential of biological therapies not only to improve lung function, but also to revert airflow obstruction and remodeling.32,71 Airway remodeling is a consequence of asthma and leads to structural changes of the airways and lung parenchyma, resulting in airway hyperresponsiveness and the development of fixed airflow obstruction. There is a clinical imperative to distinguish the early and late therapeutic effects of biologics, establishing a new paradigm to assess their impact on airway remodeling in SA and to guide future treatment approaches36 so remodeling can be avoided.
A shorter duration of asthma is associated with better clinical outcomesGiven the insightful findings from various recent studies on SA and biologic treatments, the relationship between the duration of asthma and clinical outcomes has become a subject of considerable interest. Certainly, recent publications further support the association between asthma duration and clinical outcomes in patients undergoing various biologic treatments.71–73 The reviewed evidence highlights how shorter asthma duration correlates with better responses to biologic treatments, resulting in higher rates of clinical remission.35,72,73 Moreover, investigations into responses to anti-IL-5/IL-5Rα biologics reveal distinct patterns among super-responders, partial responders, and non-responders, with shorter asthma duration again emerging as a significant predictor of response.27,30,31,35,66
It can be hypothesized that these findings are related to the fact that longer disease duration is associated with more exacerbations and airway remodeling, resulting in more impaired lung function. In fact, different studies demonstrate how patients with shorter asthma duration have better preserved lung function,68 and that although treatment with biologics leads to notable improvements in lung function in most cases, differences are observed depending on the duration of asthma. Patients with shorter asthma durations tend to show a higher likelihood of achieving normal lung function [pre-BD (bronchodilator) FEV1≥80%] compared to those with longer asthma durations.68,74 Additionally, a study in children aged 6–11 years with moderate-to-severe asthma reveals that patients treated with anti-IL-4R biologic therapy achieve smaller improvements in FEV1 when treatment is started one year later,75 warning about the effects of delaying therapy, especially in younger asthma patients.
These collective findings suggest the potential impact of asthma duration on treatment response and support the notion that a shorter duration of asthma may be associated with more favorable clinical outcomes and a better preserved lung function in patients undergoing biologic therapies, which is also associated with an increased likelihood of achieving clinical objectives as previously described. Therefore, this supports the hypothesis of the benefits of early initiation of biologic therapy, particularly to prevent lung function deterioration. However, studies are needed to show that earlier initiation of biologic therapy favorably modifies the natural history of asthma.
Can the early treatment approach in RA provide some guidance for severe asthma?The main objective of this scoping review is to identify the scientific evidence on the intervention of early treatment with biological drugs in SA and to draw a parallel analysis with RA. Taking this into account, the primary objectives of RA treatment include the management of synovitis and the prevention of joint damage. The rationale behind adopting an early treatment strategy (i.e., DMARD therapy as soon as RA is diagnosed, ideally within 3 months) is based on the observation that joint damage, which can ultimately lead to disability, begins early in the course of the disease. Furthermore, the longer an active disease persists, the less responsive patients are likely to be to therapeutic interventions.11 Initially, biologic therapies for RA were primarily reserved for patients with advanced and severe cases, after failure of all conventional disease-modifying anti-rheumatic drugs (cDMARDs). However, their use has subsequently been extended to earlier stages of the disease to modify its course, eventually resulting in sustained remission.32,76 Currently, in line with the treat-to-target strategy, if the treatment target is not achieved with the first cDMARDs (MTX is usually the preferred option), a bDMARD or targeted synthetic DMARD (tsDMARD) should be added to maintain tight control over the disease.17,19,77
Our scoping review highlights a disparity in the availability of publications addressing the use of biologics for RA management compared to SA, specifically in the context of “early treatment” and “early intervention”. The higher number of publications in RA can be attributed to the fact that biologic treatments have been used in this disease for a longer period of time than in SA, and therefore research in this area is more extensive. Although asthma and RA are certainly different diseases, insights gleaned from RA experience could provide valuable guidance to asthma clinicians and researchers in delineating the prospective role of biologics in SA treatment.12 There may be certain parallels between asthma and RA beyond their inflammatory nature and subsequent tissue remodeling associated with each condition (see Fig. 5). Much like the decline in joint function characterizing RA, loss of lung function emerges as a significant feature in asthma. In terms of treatment, cDMARDs in RA could be considered equivalent to inhaled treatment in SA. Also, patients in both diseases may experience acute events (flare-ups and exacerbations, respectively), and clinicians seek to minimize the use of systemic corticoids in both diseases.
Parallels between rheumatoid arthritis and severe asthma. ACT: asthma control test; anti CTLA-4: cytotoxic T-lymphocyte associated protein 4 blocking agent; anti-IgE: immunoglobulin E inhibitor; anti-IL: interleukin inhibitors; anti-TNF: tumor necrosis factor blocking agent; bDMARD: biological disease-modifying antirheumatic drug; CDAI: clinical disease activity index; cDMARD: conventional disease-modifying antirheumatic drug; CS: corticosteroids; DAS28: disease activity score 28; FeNO: exhaled nitric oxide, FEV1: forced expiratory volume in one second; HCQ: hydroxychloroquine; JAKi: Janus kinase inhibitor; MTX: methotrexate; OCS: oral corticosteroids; SDAI: simplified disease activity index; tsDMARD: targeted synthetic disease modifying antirheumatic drug; TSLP: thymic stromal lymphopoietin.
Analyzing the management approach in RA and the concepts of early treatment and “treat-to-target”23 and understanding the implications of drawing parallels between disease entities could pave the way for new therapeutic avenues in asthma management, offering patients more effective treatment approaches. In fact, even though patients may suffer from milder forms of asthma, it is important to keep in mind that they could progress to more severe forms.78 Therefore, asthma should be approached as a serious disease, and asthma patients need to be carefully managed regardless of their GINA classification of severity, as is the case with RA or other inflammatory conditions.
Although the early treatment approach in RA can serve as a guide for managing asthma patients and there is a growing body of work emphasizing the need for a strategic shift toward early use of recommended medication in SA management,32,79 unlike RA, there is a scarcity of elements to anticipate risk in asthma. In this context, certain factors could stand out: biomarkers such as FeNO80 and, more importantly, eosinophil count81,82 are predictors of risk of exacerbations and lack of asthma control. Additionally, the use of questionnaires such as AIRQ83 and ORACLE79 could help to predict poor outcomes and identify patients who could most benefit from early intervention with biologics.
Study limitations and future researchThis review is subject to certain limitations. Evidence on “early treatment of asthma with biologics” is limited and indirect, which makes it difficult to draw definitive conclusions. However, the fact that some publications hypothesize that early therapeutic intervention with biologic drugs in asthma patients may lead to better long-term outcomes suggests that this is a relevant area for further research. Additionally, it is important to acknowledge the heterogeneity within the context of “early treatment”. Nevertheless, this review serves as an initial compilation of the literature concerning early intervention in asthma and one of the first steps toward research on early intervention, emphasizing the need for further investigation to explore and consolidate this concept.
ConclusionFrom the available literature, it can be inferred that better patient condition, more preserved lung function decline and shorter duration of asthma (which may in fact be related to the previous two elements) are associated with better clinical outcomes in patients with SA treated with biologics.
Changing the approach to a more preventive one, similar to that which has been adopted in other inflammatory conditions such as RA, could alter the course of the disease, prevent its progression and the development of lung remodeling, and increase the likelihood of achieving clinical remission. This scoping review highlights gaps in the scientific evidence regarding early intervention with biologics in SA and emphasizes the importance of continuing this line of research.
FundingThis review was funded by AstraZeneca Spain.
Authors’ contributionsAll authors had access to and reviewed the publications, contributed to the study conception, and participated in the elaboration of the manuscript. All authors revised and approved the final version of the manuscript.
Conflicts of interestLPL reports grants, personal fees and non-financial support from AstraZeneca, FAES, Sanofi and TEVA, personal fees and non-financial support from Chiesi and GSK, personal fees from Gilead, Leo Pharma, MSD and Techdow Pharma, and grants and personal fees from GEBRO, outside the submitted work. MGB reports personal fees and non-financial support from AstraZeneca, GSK, Boehringer Ingelheim, Bristol Myers Squibb and Pfizer, and non-financial support from Nordit, outside the submitted work. EL and FRL are employees of AstraZeneca. JCM reports personal fees from AstraZeneca, Bial, Chiesi, Gebro, GSK, Menarini, Organon and Sanofi, outside the submitted work.
The authors acknowledge AstraZeneca for the economic support and Marlyn Campo, Laia Robert, Bibiana Pérez and Maria Rodríguez from GOC Health Consulting for their methodological and medical writing support.