After the SARS-COV-2 pandemic, the scientific community has shown special interest in the use of non-invasive respiratory support devices for the management of mild to moderate acute respiratory distress syndrome (ARDS) specifically high flow oxygen (HFO). According to consensus1 severe ARDS should be managed with invasive mechanical ventilation, establishing parameters for pulmonary protection. These strategies involve close monitoring of many mechanical values, but this refined approach is not possible with HFO. So far, pressures generated by HFO have been only registered at nasopharynx.2 With the aim of understanding more about HFO mechanics and in order to improve and personalize HFO future settings we carried out a physiological study measuring pressures beyond the conducting zone of the respiratory system with a high-precision ultra-thin catheter3 under HFO therapy.
With local ethical committee approval five subjects recovering from a COVID19 ARDS were randomly selected after completing a weaning, decannulation and stoma healing procedure in our Intermediate Respiratory Care Unit (IRCU). We obtained written informed consent from all these patients.
We monitored them following standard clinical practice in IRCU: electrocardiogram, invasive arterial pressure, and oxygen saturation (SatO2). Likewise, a surface electromyography was placed in the lower right axillary region aimed to assess respiratory effort.
Under local anesthesia and light sedation an electronic Millar® pressure catheter3 that can reach the limits of the conducting zone (1.2mm diameter) was placed as distal as possible in the posterior segment of the lower right lobe guided with a bronchoscope. Once the catheter was placed and fixed, the bronchoscope was removed. Signal recording started once the patients were completely awake.
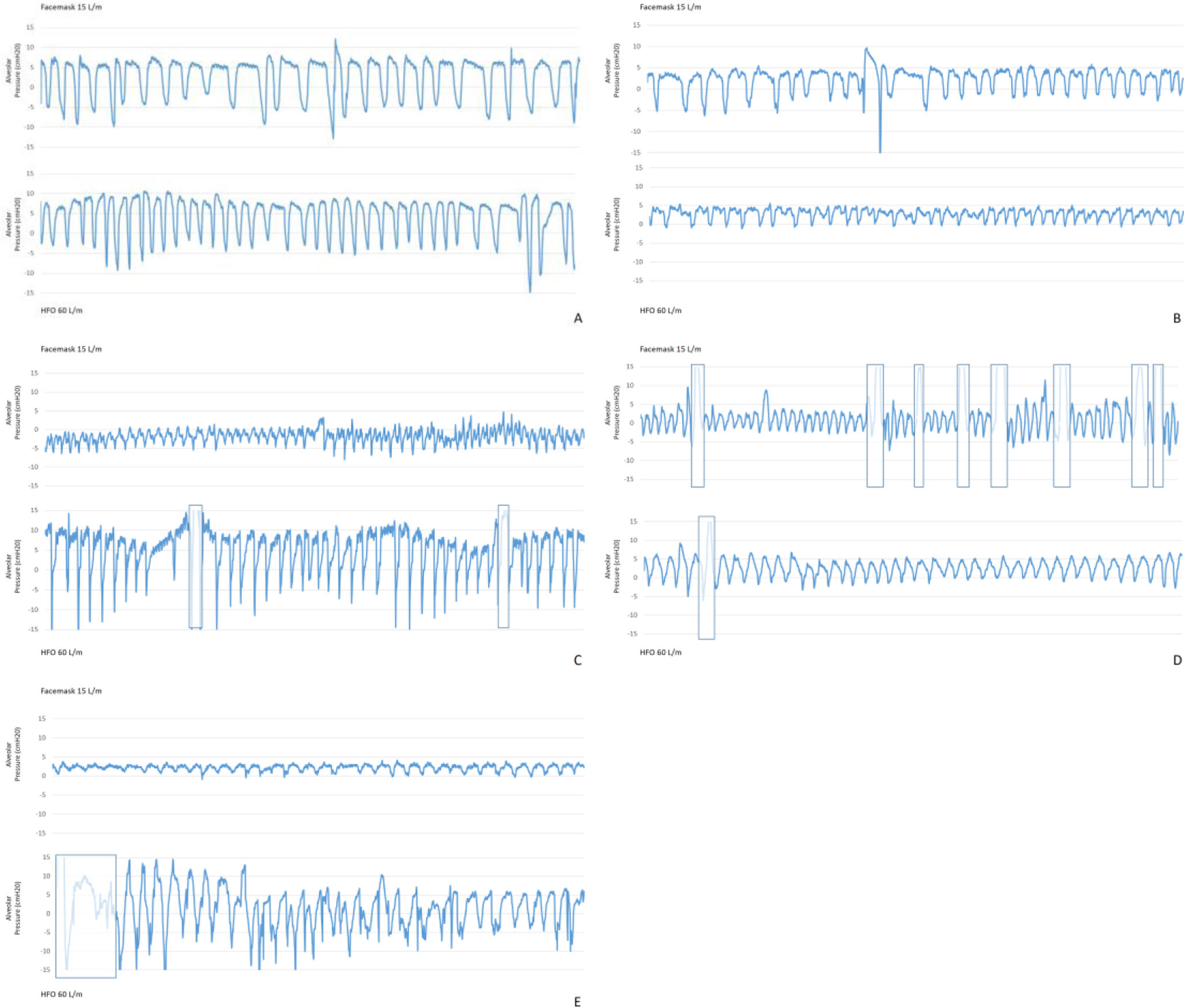
The study was initiated under conventional oxygen therapy (COT) with a facemask delivering 15L/m. Then we switched into HFO administrated through nasal cannula with a fraction of inspiratory oxygen of 1, delivered by an Optiflow® circuit heated by a Fisher & Paykel MR850 humidification system at 60L/m. Recordings lasting two minutes at each condition were saved and read offline. The curves were analyzed manually determining positive end expiratory pressure (PEEP), the minimum peak inspiratory pressure (PIP), the difference between PEEP and PIP or alveolar pressure gradient (APG), respiratory rate (RR), inspiratory time (Ti) and expiratory time (Te). Oxygen saturation (SatO2) was recorded at the beginning and at the end of each of the tests as well as heart rate and mean arterial pressure. Arterial blood gas samples were collected at the end of each scenario.
Our main results showed that, compared with COT, at HFO 60L/m PEEP increased (4.46 vs 8.25cmH2O p<0.001). On the other hand, PIP decreased (−4.73 vs −5.49cmH2O p<0.05). Consequently, APG was significantly higher at HFO (9.09 vs 13.66 p<0.05). We also noticed that under HFO 60L/m, Ti (1.42 vs 1.12 p<0.001) and RR (20.88 vs 20 p<0.001) decreased, while Te (1.80s vs 2.02 p<0.023) lengthened. Pressure records of each patient can be seen in Fig. 1.
Regarding gas exchange variables, only PaO2 was increased with HFO. No significant changes in hemodynamics were noticed. EMG did not show any activity.
We demonstrated for the first time similar PEEP values beyond the conducting zone of the respiratory system to those observed in previous nasopharyngeal measurements.2 The presence of a basal PEEP of 4.46cmH2O in our patients should be highlighted. Explanations to this finding would include occult auto-PEEP based on COPD diagnosis or diaphragmatic expiratory effort. In absence of specific mechanical variables, our COT Te value 1.8s corresponds with three normal expiratory time constants, not reflecting then an obstructive condition able to generate auto-PEEP. Moreover, only two of our patients had a formal diagnosis of COPD. We speculate that this basal PEEP is attributable to active expiratory activity in lung tissue with high viscoelastic retraction, as all the subjects were recovering from a difficult weaning process due to COVID-19 ARDS. As previously reported these patients usually show an extensive lung damage and low pulmonary compliance in their later phase of evolution.4
On the other hand, the cyclic fluctuation in airway pressure during HFO followed the same trend as spontaneous self-breathing: pressure falls abruptly during the inspiration from an expiratory positive peak. As our APG reflects these swings can be even greater under HFO. The location of the catheter in dependent zones and the fact that we just measure the behavior of a very specific lung region may lead us to interpret our results with caution, as these dependent zones are more proned to suffer the Pendelluft's phenomena harmful effects, as greater tension and higher transpulmonary pressure (TPP). We did not register esophageal pressure to make a real calculation of TPP, but we hypothesize that these alveolar pressure changes could represent stress forces, resulting in a non-trivial continuous lung deformation leading to a possible patient self-inflicted lung injury5 when using 60L/m. HFO is presumed to reduce work of breathing at high rates,6 although some recent studies have also pointed that 30L/m may be enough to achieve this goal, while higher rates would lead to discomfort, dynamic hyperinflation or increased respiratory resistance in some patients.7
HFO generates PEEP by confronting the patient's exhalation against a set air flow, that is, a resistance.8 Vieira et al. probed recently how resistive loads increase as HFO flow is raised.9 Also, classic physiological papers point how expiratory time increases while applying an expiratory resistance, lowering the RR. To date, HFO monitoring depends on composite scores including gas exchange and RR10 with no mention of respiratory system mechanics. Taking into account these observations, physicians may need to pay attention not only to RR, but also by clinically assessing respiratory effort and mechanics to guide HFO setting, as 60L/m or more could create an excessive expiratory load, especially for patients with airflow limitation. Some monitoring options could include of course well-known methods as esophageal pressure or electric diaphragmatic activity, but they are not available in most IRCUs. We suggest direct bedside monitoring of dyspnea and respiratory pattern, as well as diaphragmatic echography, added to ABG, RR and validated scores.
Our limited sample size and the absence of subjects with different respiratory pathologies are some of our study's limitations. We also did not measure esophageal pressure or diaphragmatic function to verify our hypothesis. Ti and Te values were taken from pressure waves as flow measurements were not available.
To conclude, we confirm that HFO can generate PEEP in the alveolar environment. During HFO therapy in addition to RR, careful monitoring of respiratory effort and breathing pattern may be necessary.
FundingThe authors declare that no funding was received for this article.
Authors’ contributionsNPA, PBRG, HFSB, provided study design and guidance of the project. HFSB performed the statistical analysis. NPA, LCC, FOMI, ZCMA, CDMM, HFSB were involved in patient recruitment and data collection. HFSB, NPA, PBRG, RNMJ made the analysis and bibliographic review. All authors contributed in writing of the final paper. All authors read and approved the final manuscript.
Conflicts of interestThe authors declare not having conflict of interest directly or indirectly related to the contents of the manuscript.