Global clinical guidelines recommend limiting the use of oral corticosteroids (OCS) in the management asthma in order to avoid potentially serious adverse outcomes.1 Since their effectiveness was initially demonstrated, OCS have been routinely used for decades to treat asthma exacerbations and severe asthma. However, recent years have seen a global move towards reducing their use in the management of this condition.2 Indeed, evidence has strongly suggested that their use is associated with significant adverse events and long-term complications. The more serious side effects noted with systemic corticosteroid therapy include osteoporosis, adrenal suppression, hyperglycemia, dyslipidemia, cardiovascular disease, Cushing's syndrome, psychiatric disturbances, and immunosuppression, particularly when these products are used at high doses for prolonged periods.3,4 Furthermore, the therapeutic arsenal to treat asthma has grown considerably, from newer generation inhaled corticosteroids and bronchodilators to the most relevant addition of biological therapies for severe asthma. It is, therefore, time to control and reduce the use of OCS.2
In October 2022, a group of specialists in the management of severe asthma published the Spanish Multidisciplinary Consensus for the use of OCS in asthma,5 a compilation of scientifically supported recommendations for the reduction of OCS use. It was conducted using a Delphi process involving a panel of 48 allergy and pulmonology experts in severe asthma treatment. The Spanish Society of Pulmonology and Thoracic Surgery (SEPAR), together with the Spanish Society of Allergology and Clinical Immunology, have now launched a small initiative to assess, as a primary endpoint, the impact, level of knowledge and implementation of those recommendations. To this end, a questionnaire was administered to asthma specialists during 2023 (October 10th–November 7th), and a total of 112 interviews were completed by 55 allergists and 57 pneumologists. Full results are provided in the supplementary material and Table 1 summarizes answers that provide interesting insight. All participating specialists provided their consent; no patients were involved.
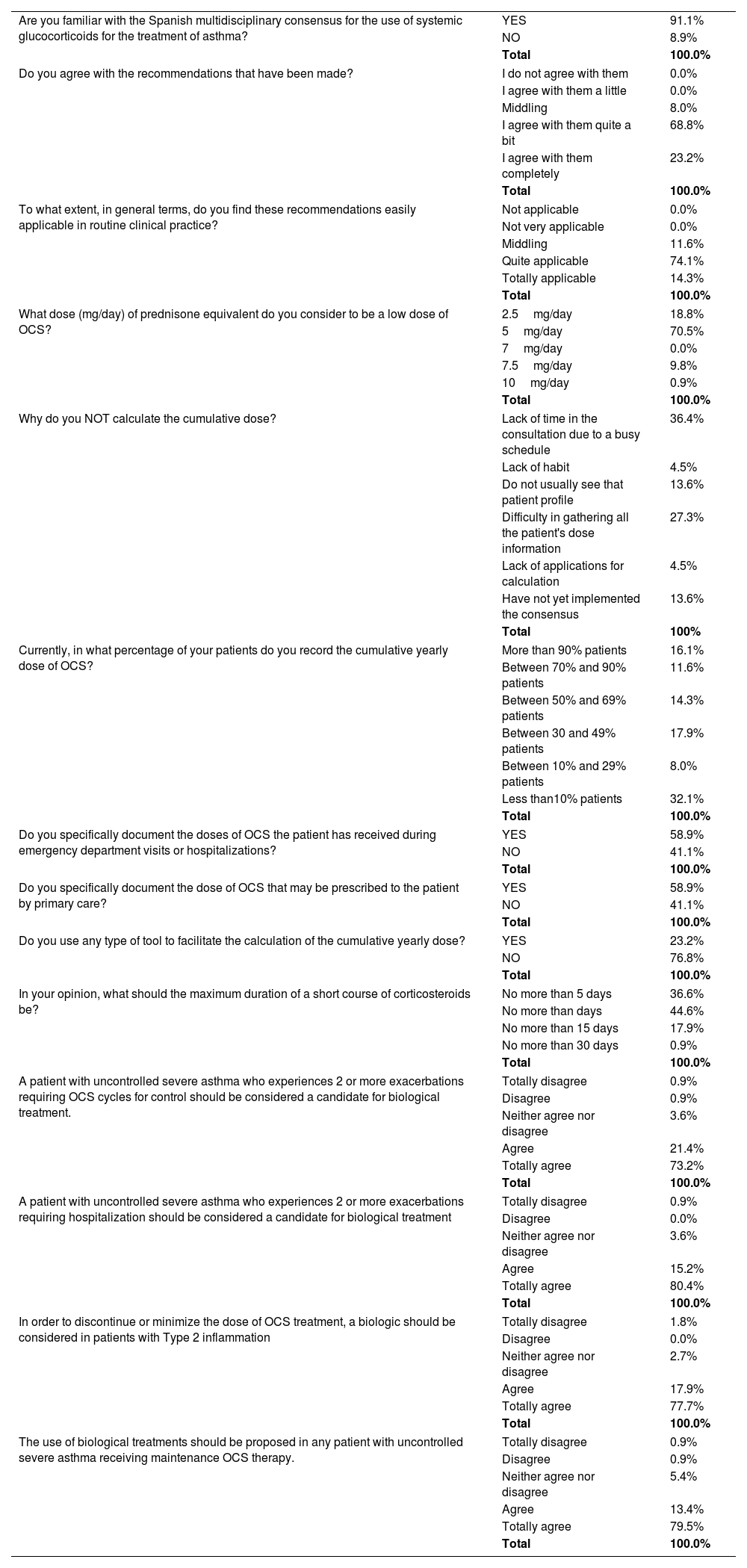
Selection of survey results (complete results in l).
| Are you familiar with the Spanish multidisciplinary consensus for the use of systemic glucocorticoids for the treatment of asthma? | YES | 91.1% |
| NO | 8.9% | |
| Total | 100.0% | |
| Do you agree with the recommendations that have been made? | I do not agree with them | 0.0% |
| I agree with them a little | 0.0% | |
| Middling | 8.0% | |
| I agree with them quite a bit | 68.8% | |
| I agree with them completely | 23.2% | |
| Total | 100.0% | |
| To what extent, in general terms, do you find these recommendations easily applicable in routine clinical practice? | Not applicable | 0.0% |
| Not very applicable | 0.0% | |
| Middling | 11.6% | |
| Quite applicable | 74.1% | |
| Totally applicable | 14.3% | |
| Total | 100.0% | |
| What dose (mg/day) of prednisone equivalent do you consider to be a low dose of OCS? | 2.5mg/day | 18.8% |
| 5mg/day | 70.5% | |
| 7mg/day | 0.0% | |
| 7.5mg/day | 9.8% | |
| 10mg/day | 0.9% | |
| Total | 100.0% | |
| Why do you NOT calculate the cumulative dose? | Lack of time in the consultation due to a busy schedule | 36.4% |
| Lack of habit | 4.5% | |
| Do not usually see that patient profile | 13.6% | |
| Difficulty in gathering all the patient's dose information | 27.3% | |
| Lack of applications for calculation | 4.5% | |
| Have not yet implemented the consensus | 13.6% | |
| Total | 100% | |
| Currently, in what percentage of your patients do you record the cumulative yearly dose of OCS? | More than 90% patients | 16.1% |
| Between 70% and 90% patients | 11.6% | |
| Between 50% and 69% patients | 14.3% | |
| Between 30 and 49% patients | 17.9% | |
| Between 10% and 29% patients | 8.0% | |
| Less than10% patients | 32.1% | |
| Total | 100.0% | |
| Do you specifically document the doses of OCS the patient has received during emergency department visits or hospitalizations? | YES | 58.9% |
| NO | 41.1% | |
| Total | 100.0% | |
| Do you specifically document the dose of OCS that may be prescribed to the patient by primary care? | YES | 58.9% |
| NO | 41.1% | |
| Total | 100.0% | |
| Do you use any type of tool to facilitate the calculation of the cumulative yearly dose? | YES | 23.2% |
| NO | 76.8% | |
| Total | 100.0% | |
| In your opinion, what should the maximum duration of a short course of corticosteroids be? | No more than 5 days | 36.6% |
| No more than days | 44.6% | |
| No more than 15 days | 17.9% | |
| No more than 30 days | 0.9% | |
| Total | 100.0% | |
| A patient with uncontrolled severe asthma who experiences 2 or more exacerbations requiring OCS cycles for control should be considered a candidate for biological treatment. | Totally disagree | 0.9% |
| Disagree | 0.9% | |
| Neither agree nor disagree | 3.6% | |
| Agree | 21.4% | |
| Totally agree | 73.2% | |
| Total | 100.0% | |
| A patient with uncontrolled severe asthma who experiences 2 or more exacerbations requiring hospitalization should be considered a candidate for biological treatment | Totally disagree | 0.9% |
| Disagree | 0.0% | |
| Neither agree nor disagree | 3.6% | |
| Agree | 15.2% | |
| Totally agree | 80.4% | |
| Total | 100.0% | |
| In order to discontinue or minimize the dose of OCS treatment, a biologic should be considered in patients with Type 2 inflammation | Totally disagree | 1.8% |
| Disagree | 0.0% | |
| Neither agree nor disagree | 2.7% | |
| Agree | 17.9% | |
| Totally agree | 77.7% | |
| Total | 100.0% | |
| The use of biological treatments should be proposed in any patient with uncontrolled severe asthma receiving maintenance OCS therapy. | Totally disagree | 0.9% |
| Disagree | 0.9% | |
| Neither agree nor disagree | 5.4% | |
| Agree | 13.4% | |
| Totally agree | 79.5% | |
| Total | 100.0% | |
Overall results have shown that the Spanish consensus was well known by the specialists who responded to the poll (Table 1): 91% were familiar with the Spanish multidisciplinary consensus, 92% manifest agreement with the recommendations, and 88% believe that they are quite applicable. However, it should be noted that participants are members of the asthma interest groups in their respective societies, so it remains to be clarified whether specialists and general practitioners attending asthma patients but not dedicated to this condition are also sufficiently aware of OCS tapering initiatives. Initiatives to extend this consensus to emergency departments and primary care would, therefore, be of great interest, in order to ensure a holistic and standardized approach to severe asthma management across all healthcare levels.
Answers on the feasibility of recommendations highlight practical issues worth discussing. Indeed, responses on recording OCS use reveal that practical and effective tools are needed for the comprehensive implementation of the Spanish consensus and optimization of the quality of patient care during consultations: 41.1% of specialists reported a lack of specific record-keeping on the OCS doses administered to their patients across different hospital care settings. This is nothing new: scant evidence on acute and long-term OCS use in severe asthma has been previously reported because OCS prescribing occurs across a variety of care settings.1 The doctors participating in the survey report that only 81.8% of them calculate their accumulated dose, 28% mention difficulty in collecting all patient dosage information, while another 38% comment that, due to limited consultation time, the accumulated doses of OCS cannot be registered adequately. From the authors’ point of view, it is relevant to point out that medical care of patients with severe asthma is a complex scenario in which routine visits are intense and demanding. In this context, keeping track of medical records issued in diverse platforms and recording patient history, albeit crucial, can be cumbersome. Across the healthcare system there is a lack of coordination and communication among healthcare practitioners and settings (primary care, emergency room, specialist care, and hospitalization) that must be improved to track OCS use. One significant challenge is the lack of technical tools for documenting and sharing OCS usage information across various levels of care, resulting in the under-recording of relevant patient information needed for effective follow-up. The treatment of severe asthma has improved in the last few years, and it is mandatory for doctors to register different treatments to make therapeutic decisions. Measurement tools that can capture the data needed to reduce OCS use (76% of specialists report not using specific tools to calculate cumulative doses) and improved communication pathways between the different hospitals and care settings will be important factors in addressing these issues.
Regarding the use of OCS over time, there was almost unanimous agreement (98%) in defining the use of OCS for 6 months over a year as chronic treatment, regardless of the dose. Moreover, almost 90% consider >5mg/day a low dose (Table 1). This underlines the consensus achieved in the previous document.5 Asthma control has greatly improved with the advent of biologic therapies directed towards the regulation of airway inflammation, especially in the more severe forms of the disease. In fact, biologics are highly effective in reducing exacerbations, diminishing symptoms and improving lung function in well-defined asthma populations.6 Remarkably, up to 95% of participants agreed on starting biologics when a patient presents 2 exacerbations that require OCS or hospital admission, with suspension of OCS in patients with T2 inflammation. As a general rule, 92% of the specialists believe that biological treatment should be proposed in any patient with severe asthma on treatment with OCS whose condition remains uncontrolled. That is, they suggest that biologic treatment should be considered at the point of a second exacerbation in a patient with severe asthma treated with conventional therapies, without further delay. Supporting evidence for this approach is increasing, as treatment with biologic medications has been shown to provide OCS-sparing effects, which improve patient outcomes and reduce the risk of serious OCS-triggered side effects.7 It is known that patients who have used OCS less frequently and for shorter durations are more successful in achieving a complete discontinuation of OCS. This observation underscores the importance of earlier intervention with biologics to avoid the cumulative OCS exposure. Possibly this topic will come under the spotlight in months to come, and evidence will be needed to assess the appropriateness of this approach.
These results reflect our initial exploration into physicians’ perspectives on the implementation of the Spanish multidisciplinary consensus.5 Our findings identified limitations in the execution process that need to be addressed for a more judicious use of OCS. We also found unanimous agreement regarding the early use of biologics, prompting further investigation. We plan to conduct a second questionnaire to continue assessing the implementation of this consensus and to evaluate emerging limitations.
FundingThis project was promoted and executed by the scientific societies SEPAR and SEAIC through a non-restrictive grant from AstraZeneca.
Authors’ contributionsAll authors participated in the design of the questionnaire, in the interpretation of its analysis, and the critical review; all authors approved the final version.
Conflicts of interestJDO has received fees as a speaker, scientific advisor, or investigator in clinical studies from AstraZeneca, BIAL, Chiesa, GlaxoSmithKline, LETI-Pharma, Novartis, Sanofi, and Teva. XMG has received fees as a speaker, scientific advisor, or investigator in clinical studies from AstraZeneca, Boehringer Ingelheim, Chiesi, Faes, GlaxoSmithKline, Menarini, Mundifarma, Novartis, Sanofi, and Teva. JDR has received fees as a speaker, scientific advisor, or investigator in clinical studies from AstraZeneca, Bial, Chiesi, Faes, GlaxoSmithKline, Novartis, Sanofi, and Teva. FCM has received fees as a speaker, scientific advisor, or investigator in clinical studies from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, CSL-Behring, FAES, Grifols, GlaxoSmithKline, Menarini, Novartis, Sanofi, Teva Respiratory, and VERTEX. MBA has received fees as a speaker, scientific advisor, or investigator in clinical studies from AstraZeneca, Chiesi, GlaxoSmithKline, Menarini, Novartis, Sanofi, and Teva.
Medical writing support was provided by M. Julia Lamborizio Melero MD and Blanca Piedrafita PhD de MSC, Medical Science Consulting, Valencia, Spain.