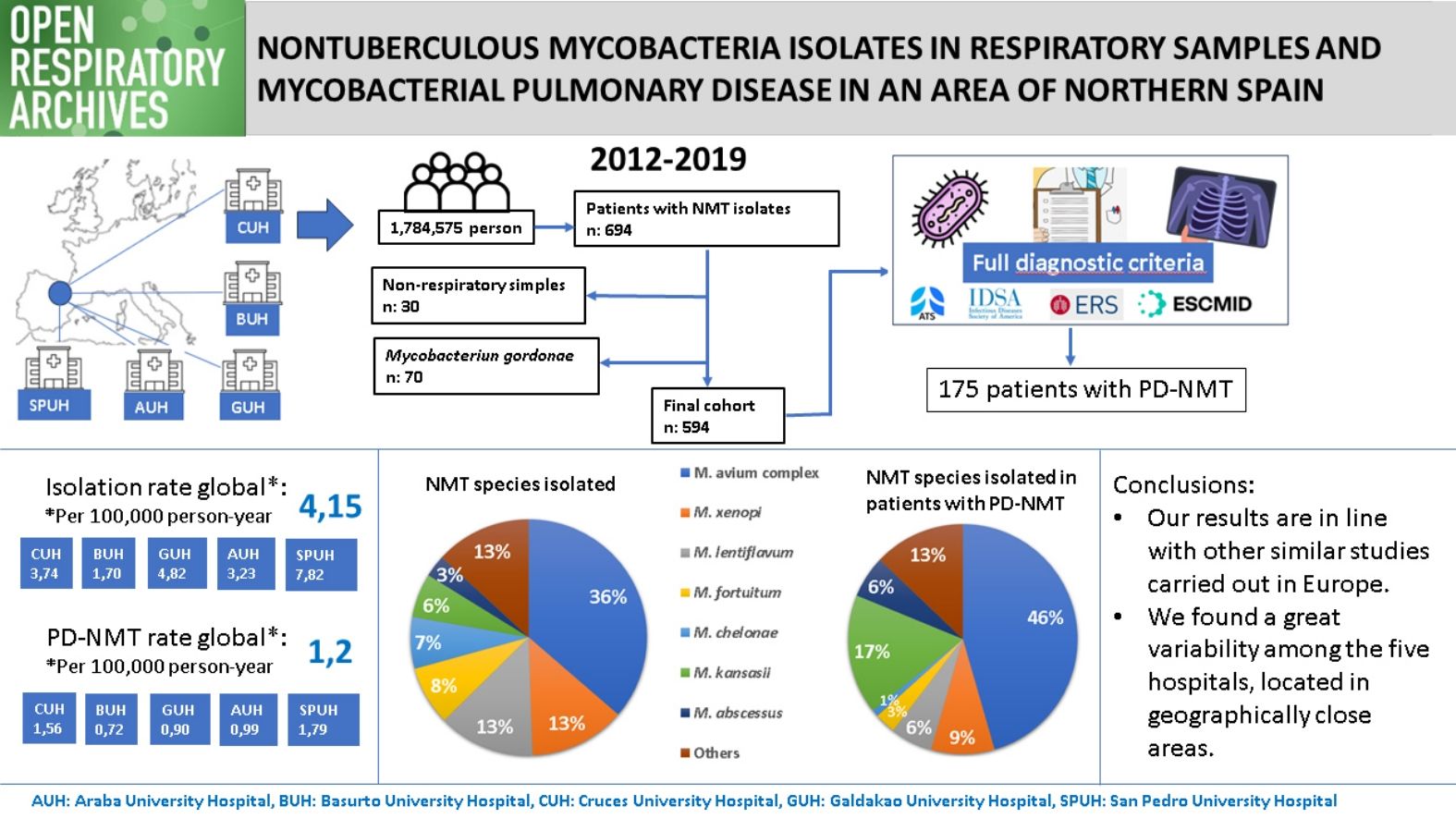
The epidemiology of nontuberculous mycobacteria (NTM) is not well known. In this study, we aimed to determine the incidence of NTM isolates and nontuberculous mycobacterial pulmonary disease (NTM-PD) in five closely located hospitals in an area of northern Spain and analyse differences between them.
Material and methodsDemographic, microbiological, clinical and radiological data were collected retrospectively from all patients with a NTM isolated from respiratory specimens at five hospitals between 2012 and 2019. Mycobacterium gordonae isolates were excluded. Once the data was collected, it was determined which patients met the NMT-PD criteria.
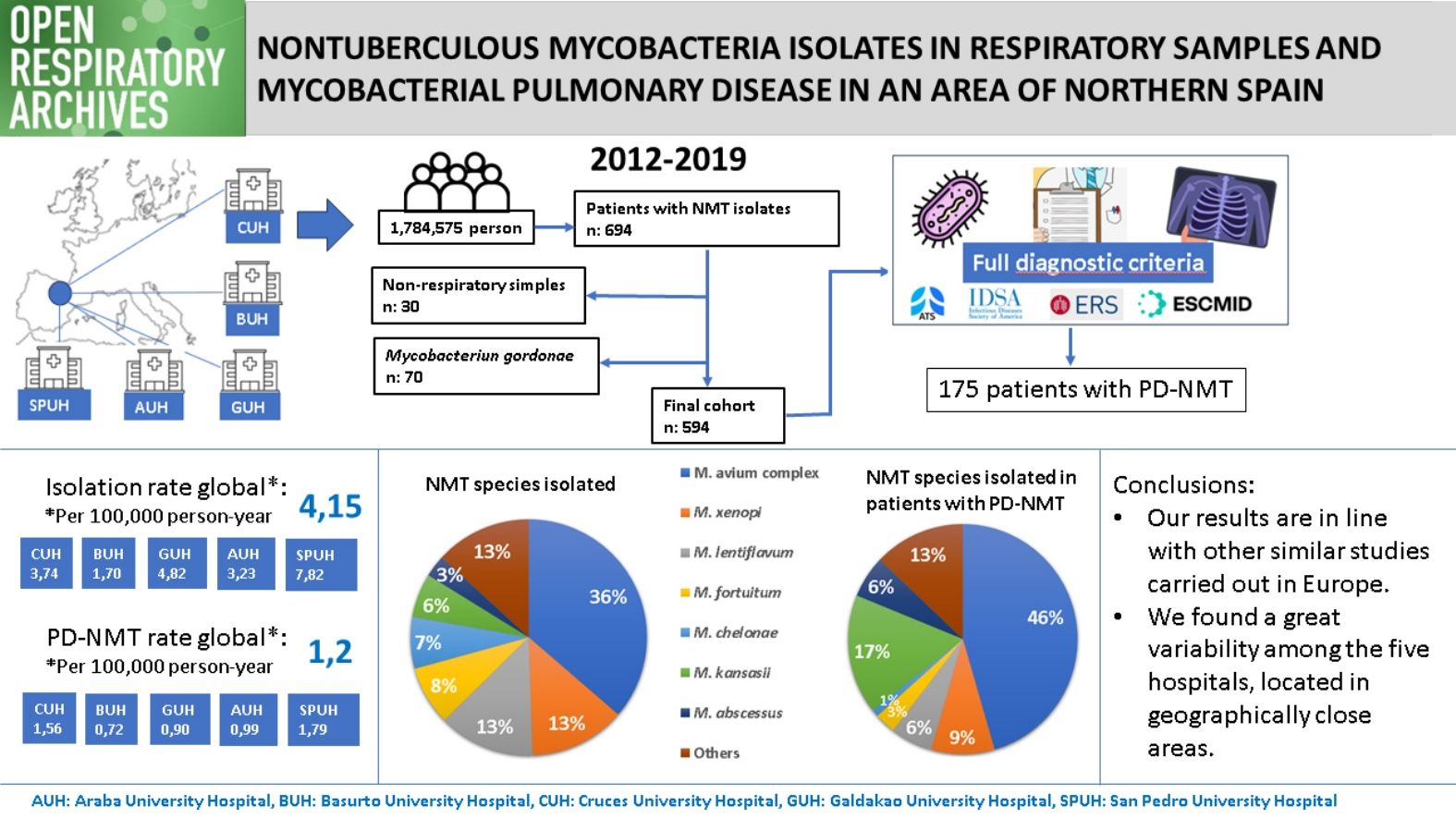
Results594 patients were included in the study. The mean incidence rate of NTM isolates across all five hospitals in the period studied was 4.15 per 100,000 person-year, while the rate of NTM-PD was 1.2. The annual number of isolates showed an upward trend over this period, but the same did not occur in the number of cases of NTM-PD. The species most frequently isolated were Mycobacterium avium complex (MAC) and Mycobacterium xenopi and those most frequently causing disease were MAC and Mycobacterium kansassi. There were significant differences between the five centres.
ConclusionsOur results are in line with similar studies in Europe in terms of NTM isolation and NTM-PD incidence and species isolated; however, we do not see the upward trend in NTM-PD rates described elsewhere. The great variability in isolation and disease rates, as well as in species isolated in geographically close areas, underlines, in our opinion, the importance of local environmental factors.
La epidemiología de las micobacterias no tuberculosas (MNT) no se conoce bien. Nuestro objetivo fue determinar la incidencia de aislamientos de MNT y de enfermedad pulmonar por micobacterias no tuberculosas (EP-MNT) en cinco hospitales del norte de España, próximos entre sí, y analizar las diferencias entre ellos.
Material y métodosSe recopilaron retrospectivamente datos demográficos, microbiológicos, clínicos y radiológicos de todos los pacientes con una MNT aislada en muestra respiratoria entre 2012 y 2019 en los cinco hospitales. Se excluyeron los aislamientos de Mycobacterium gordonae. Una vez recogidos los datos, se determinó qué casos cumplían los criterios de EP-MNT.
ResultadosSe incluyeron 594 pacientes. La tasa de incidencia media de aislamientos de NTM en los cinco hospitales fue de 4,15 por 100.000 personas-año y la tasa de EP-MNT fue de 1,2. El número anual de aislamientos mostró una tendencia creciente durante este periodo, pero no así el de EP-MNT. Las especies aisladas con mayor frecuencia fueron Mycobacterium avium complex (MAC) y Mycobacterium xenopi y las que causaron enfermedad con mayor frecuencia fueron MAC y Mycobacterium kansasii. Hubo diferencias significativas entre los cinco centros.
ConclusionesNuestros resultados están en línea con estudios similares en Europa en términos de aislamiento de MNT, incidencia de EP-MNT y especies aisladas; sin embargo, no vemos la tendencia ascendente en las tasas de EP-MNT descrita en otros lugares. La gran variabilidad entre los cinco hospitales en las tasas de aislamiento y de enfermedad y en las especies aisladas resalta la importancia de los factores ambientales locales.
Nontuberculous mycobacteria (NTM), also known as atypical, environmental or opportunistic mycobacteria, constitute a large group of bacteria that, like Mycobacterium tuberculosis complex and Mycobacterium leprae, belong to the family Mycobacteriaceae.1 In recent decades, the number of known NTM species has not stopped growing, from 50 in 19972 to almost 200 in 2023.3 Nonetheless, to date, only a small number have been reported to cause disease in humans.4
NTM are widely distributed in the environment, mainly in soil and water, both natural and treated.5 Patients are likely to acquire their infections from repeated inhalation of bioaerosols from water and soil (enriched with NTM).6
NTM disease is only notifiable in a few settings.7 Given this, together with the absence of large multicentre studies with standardised microbiological methods,8 the epidemiology of NTM infections remains poorly understood.7 It is known that the incidence rates of NTM isolation and disease vary between geographical areas, for example, being higher in North America and Australia than in Europe.9 Increases in the annual number of NTM isolates and cases of nontuberculous mycobacterial pulmonary disease (NTM-PD) have been reported in most studies, but not all.7 Often, increases in NTM-PD are associated with decreases in the number of cases of tuberculosis (TB).10,11 Indeed, in some countries, the rate of NTM disease already exceeds that of TB.11,12 The distribution of different species of NTM also differs from one region to another,9 but globally, Mycobacterium avium complex (MAC), Mycobacterium gordonae and Mycobacterium xenopi are the NTM species most frequently isolated13,14 and MAC is the most common cause of NTM-PD.8
Knowledge of the local epidemiology of NTM-PD is essential for daily clinical practice.8 For that reason, we conducted a study aiming to investigate the incidence of NTM isolation and NTM-PD in five closely located hospitals in the north of Spain and to analyse the differences between them.
Material and methodsDesignWe performed a multicentre retrospective cohort study, including data from all patients with at least one respiratory sample (sputum, bronchoaspirate [BAS], bronchoalveolar lavage [BAL] and/or pleural fluid) yielding NTM, between January 2012 and December 2019, from five hospitals in northern Spain (four in the Basque Country and one in La Rioja). Three of the hospitals, Cruces University Hospital (CUH), Basurto University Hospital (BUH) and Galdakao University Hospital (GUH), are located very close to each other, in the province of Bizkaia (Basque Country), which has a long coastline and a high population density (more than 500people/km2). The other two, Araba University Hospital (AUH) and San Pedro University Hospital (SPUH), are located somewhat further away, inland and in areas with lower population densities. The maximum distance between hospitals is 103km. Fig. 1 shows the location of the five hospitals.
Data collectionWe included all patients with NTM isolates from respiratory samples registered in the database of the corresponding microbiology laboratories. Individual patients were included only once during the period studied. For each patient, data were gathered on the following: demographic characteristics (age at first isolation, sex, nationality, place of residence [rural vs urban]), risk factors (smoking, chronic obstructive pulmonary disease, asthma, bronchiectasis, immunosuppression [transplant recipients, and patients with blood cancer or on immunosuppressive medications such as tumour necrosis factor inhibitors or Systemic steroids]), microbiological results (from sputum, BAS, BAL, and/or pleural fluid including number of isolates), clinical features (cough, expectoration, fever, and/or weight loss) and radiological findings (nodules, cavitation and/or nodular bronchiectatic pattern). Once the data were collected, we applied the 2020 ATS/ERS/ESCMID/IDSA disease criteria to assess whether the patient had NTM-PD15 (Table 1).
Clinical and microbiologic criteria for diagnosis of nontuberculous mycobacterial pulmonary disease proposed in the ATS/ERS/ESCMID/IDSA clinical practice guideline in 2020.18
| Clinical | Pulmonary or systemic symptoms | Both required |
| Radiologic | Nodular or cavitary opacities on chest radiograph, or a high-resolution computed tomography scan that shows bronchiectasis with multiple small nodules | |
| And | Appropriate exclusion of other diagnoses | |
| Microbiologica | 1. Positive culture results from at least two separate expectorated sputum samples. If the results are nondiagnostic, consider repeat sputum AFB smears and culturesor2. Positive culture results from at least one bronchial wash or lavageor3. Transbronchial or other lung biopsy with mycobacterial histologic features (granulomatous inflammation or AFB) and positive culture for NTM or biopsy showing mycobacterial histologic features (granulomatous inflammation or AFB) and one or more sputum or bronchial washings that are culture positive for NTM | |
The samples were processed by routine microbiological procedures for detecting mycobacteria.16,17 A BACTEC® MGIT® 960 automated system (Becton Dickinson, Sparks, MD, US) was used for culture in liquid media and Löwenstein-Jensen and/or Coletsos solid media. Until 2014 all hospitals used line probe assay methods based on specific amplification and subsequent solid-phase hybridisation with genetic probes immobilised on nitrocellulose strips (Speed-oligo® Mycobacteria [Vircell, Granada, Spain] and GenoType Mycobacterium CM/AS [Hain Lifescience, Nehren, Germany]). From 2014 on, all hospitals except SPUH changed to matrix-assisted laser desorption ionisation time-of-flight mass spectrometry (MALDI-TOF MS, Bruker Daltonics, Bremen, Germany) to identify mycobacteria and a reverse hybridisation and amplification test system (GenoType Mycobacterium CM/AS, Hain Lifescience, Nehren, Germany) for subspecies-level identification.
Statistical analysisContinuous variables are reported as the mean (standard deviation) for normally distributed data and otherwise as the median (interquartile range), while categorical variables are presented as frequency (percentage). Baseline sociodemographic and clinical characteristics were compared between two groups using Student's t test, for continuous variables that were normally distributed, and otherwise, the nonparametric Mann–Whitney U test. Categorial data were compared using Chi-square or Fisher's exact tests as appropriate. The threshold for statistical significance was set at p<0.05. When results clustered in more than two groups, the threshold for significance was adjusted for multiple pairwise comparisons using Tukey's method for normally distributed data, and otherwise, the Benjamini–Hochberg procedure. Incidence rates were calculated using census data from the National Institute of Statistics of Spain (www.ine.es). To study between-hospital differences in incidence rates, the proportions test was used and two-by-two comparisons were made, correcting multiple comparisons using the false discovery rate method. All analyses were performed using R statistical software (version 4.0.1, R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria).
Ethical considerationsThe study was approved by the local ethics committees (CEIm Euskadi P. I. 2019001 and CEIm La Rioja P. I. 564). Data from patients were collected retrospectively and pseudonymized, and the ethics committee approved the study without requiring informed consent from the patients.
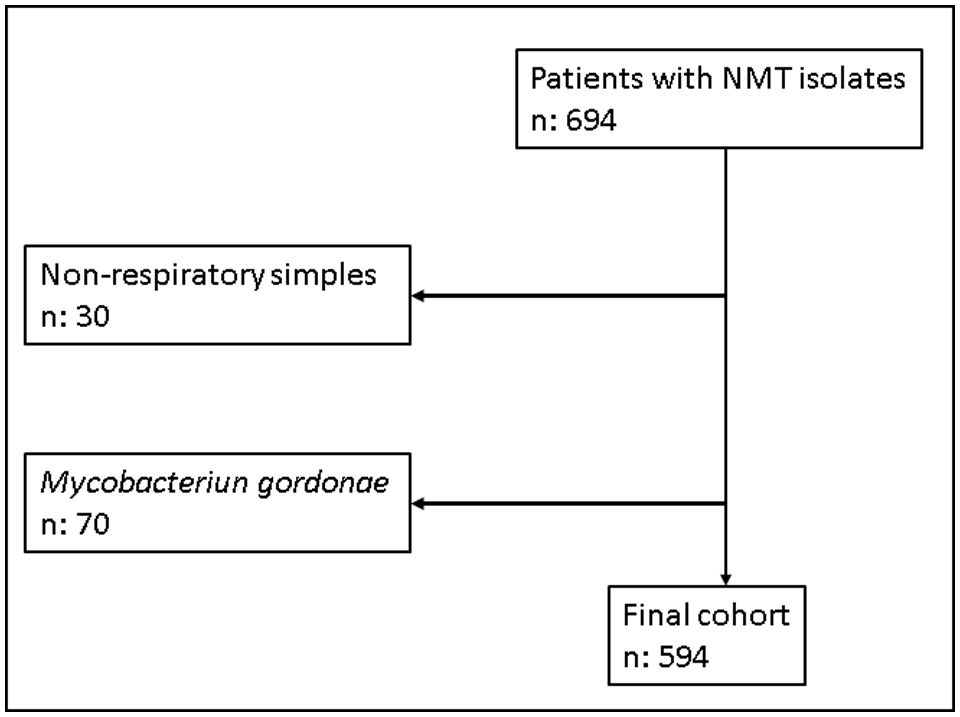
ResultsWe identified 694 patients who had at least one NTM isolated between 2012 and 2019. In 664 cases (95.7%), the sample was of respiratory origin. Microbiology departments from two of the participating hospitals, CUH and BUH, did not report positive results for M. gordonae because it was considered a non-pathogenic NTM. For this reason, we decided to exclude the results for this species from the pooled analysis. Finally, 594 patients were included in the study. Fig. 2 shows the flow of samples through the study.
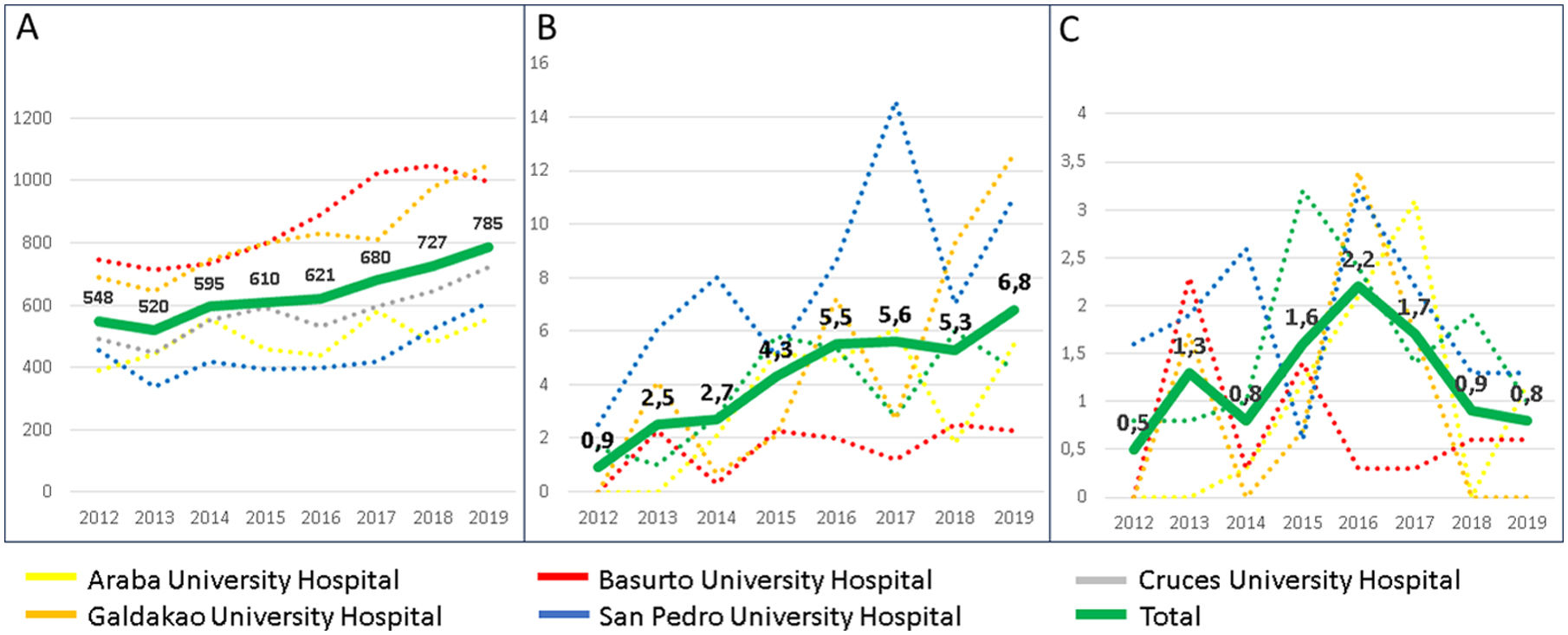
The population-adjusted sputum culture performed per year in each centre, as well as the annual incidence rate of NMT isolations and NTM-PD, are summarised in Fig. 3. Regarding the cultures performed, an upward trend was observed, from 558 per 100,000 person-year in 2012 to 785 in 2019. Fewer cultures were performed at AUH and SPUH, with means of 431 and 445 cultures per 100,000 person-year, respectively, compared to values of 869 and 818, respectively at GUH and BUH. An increase was also observed in the incidence rate of NMT isolations, from 0.9 per 100,000 person-year in 2012 to 6.8 in 2019. In contrast, the pattern for incidence rate of NTM-PD showed an upward trend in the first part of the period, from 0.5 per 100,000 person-year in 2012 to 2.2 in 2016, followed by a decline, only 0.8 in 2019.
(A) Mycobacterial cultures performed in respiratory samples; (B) patients with nontuberculous mycobacteria isolates, and (C) patients meeting criteria for nontuberculous mycobacterial pulmonary disease. Numbers each year from 2012 to 2019. All data are shown per 100,000 person-year.
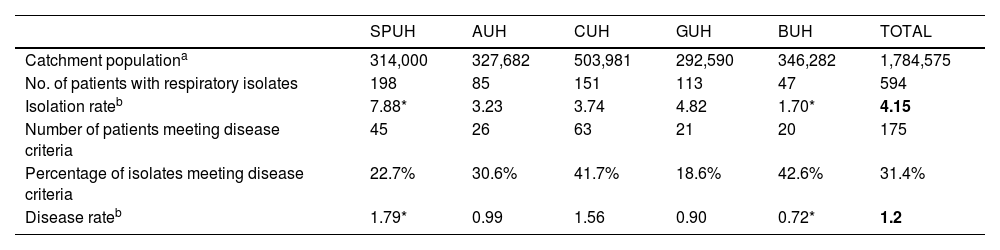
Table 2 shows the average annual incidence rate of isolations and NTM-PD corresponding to each hospital and globally. Notably, the overall isolation rate was significantly higher at SPUH and lower at BUH than at the other three hospitals. Between-hospital differences in the rate of patients meeting criteria for pulmonary disease were not so pronounced, although again the incidence was higher at SPUH and lower at BUH, the latter differing significantly from disease rates at the other hospitals.
Absolute numbers and rates for overall nontuberculous mycobacteria isolation and nontuberculous mycobacterial pulmonary disease.
| SPUH | AUH | CUH | GUH | BUH | TOTAL | |
|---|---|---|---|---|---|---|
| Catchment populationa | 314,000 | 327,682 | 503,981 | 292,590 | 346,282 | 1,784,575 |
| No. of patients with respiratory isolates | 198 | 85 | 151 | 113 | 47 | 594 |
| Isolation rateb | 7.88* | 3.23 | 3.74 | 4.82 | 1.70* | 4.15 |
| Number of patients meeting disease criteria | 45 | 26 | 63 | 21 | 20 | 175 |
| Percentage of isolates meeting disease criteria | 22.7% | 30.6% | 41.7% | 18.6% | 42.6% | 31.4% |
| Disease rateb | 1.79* | 0.99 | 1.56 | 0.90 | 0.72* | 1.2 |
AUH: Araba University Hospital; BUH: Basurto University Hospital; CUH: Cruces University Hospital; GUH: Galdakao University Hospital; SPUH: San Pedro University Hospital.
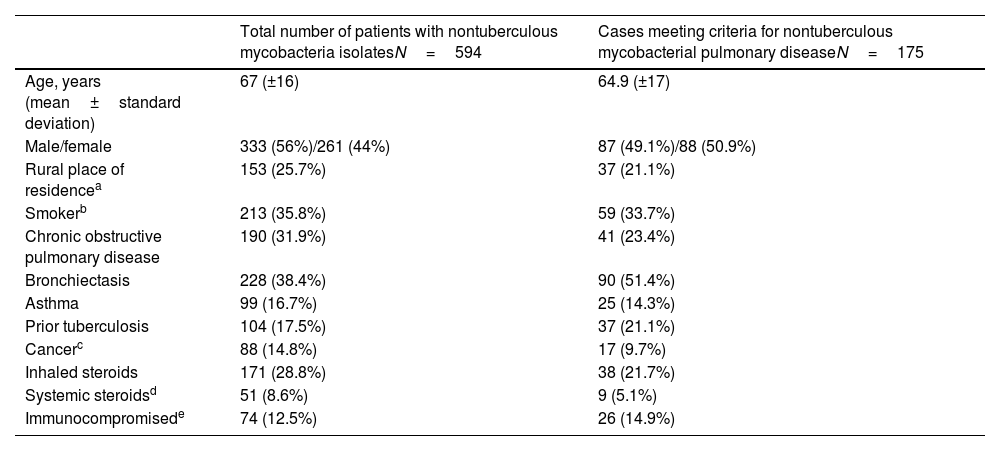
Regarding demographic data, slightly more NTM isolates were obtained in men but the number of cases of NTM-PD did not differ by sex. Bronchiectasis ranks first among predisposing factors, ahead of smoking, chronic obstructive pulmonary disease and asthma, and this is especially pronounced in cases meeting NTM-PD criteria (Table 3).
Baseline characteristics and risk factors.
| Total number of patients with nontuberculous mycobacteria isolatesN=594 | Cases meeting criteria for nontuberculous mycobacterial pulmonary diseaseN=175 | |
|---|---|---|
| Age, years (mean±standard deviation) | 67 (±16) | 64.9 (±17) |
| Male/female | 333 (56%)/261 (44%) | 87 (49.1%)/88 (50.9%) |
| Rural place of residencea | 153 (25.7%) | 37 (21.1%) |
| Smokerb | 213 (35.8%) | 59 (33.7%) |
| Chronic obstructive pulmonary disease | 190 (31.9%) | 41 (23.4%) |
| Bronchiectasis | 228 (38.4%) | 90 (51.4%) |
| Asthma | 99 (16.7%) | 25 (14.3%) |
| Prior tuberculosis | 104 (17.5%) | 37 (21.1%) |
| Cancerc | 88 (14.8%) | 17 (9.7%) |
| Inhaled steroids | 171 (28.8%) | 38 (21.7%) |
| Systemic steroidsd | 51 (8.6%) | 9 (5.1%) |
| Immunocompromisede | 74 (12.5%) | 26 (14.9%) |
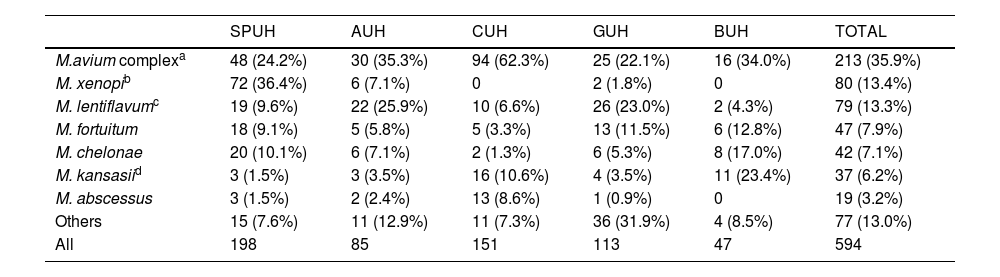
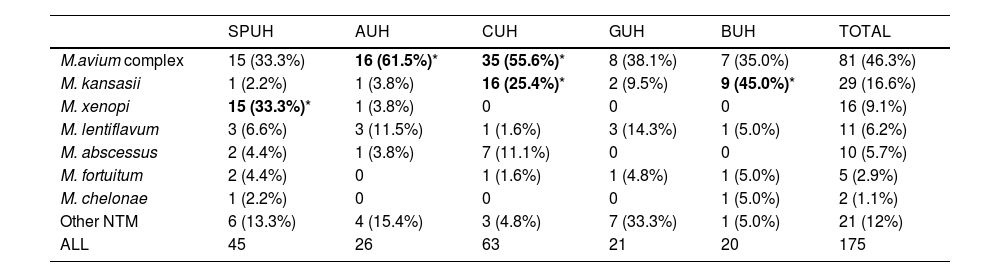
Comparing the different species isolated in each hospital (Table 4), we observed substantial variability, even among the three hospitals closest to each other. We note the large number of isolations of M. xenopi at the SPUH (36.4% of the total) and the high percentages of MAC at the CUH (62.3%) and Mycobacterium kansassi at the BUH (23.4%). On the other hand, Mycobacterium abscessus only accounted for 3.2% of all isolates (N=19) and was mainly clustered in the CUH (13 isolates). Regarding species causing NTM-PD (Table 5), differences between hospitals were less marked. There was a clear dominance of MAC in all cases (46.3% overall), but we note again the high percentages of M. xenopi at the SPUH (33.3%) and M. kansassi at the BUH (45.0%) and the CUH (25.4%).
Nontuberculous mycobacterial species isolated by hospital expressed as number of isolates (percentage).
| SPUH | AUH | CUH | GUH | BUH | TOTAL | |
|---|---|---|---|---|---|---|
| M.avium complexa | 48 (24.2%) | 30 (35.3%) | 94 (62.3%) | 25 (22.1%) | 16 (34.0%) | 213 (35.9%) |
| M. xenopib | 72 (36.4%) | 6 (7.1%) | 0 | 2 (1.8%) | 0 | 80 (13.4%) |
| M. lentiflavumc | 19 (9.6%) | 22 (25.9%) | 10 (6.6%) | 26 (23.0%) | 2 (4.3%) | 79 (13.3%) |
| M. fortuitum | 18 (9.1%) | 5 (5.8%) | 5 (3.3%) | 13 (11.5%) | 6 (12.8%) | 47 (7.9%) |
| M. chelonae | 20 (10.1%) | 6 (7.1%) | 2 (1.3%) | 6 (5.3%) | 8 (17.0%) | 42 (7.1%) |
| M. kansasiid | 3 (1.5%) | 3 (3.5%) | 16 (10.6%) | 4 (3.5%) | 11 (23.4%) | 37 (6.2%) |
| M. abscessus | 3 (1.5%) | 2 (2.4%) | 13 (8.6%) | 1 (0.9%) | 0 | 19 (3.2%) |
| Others | 15 (7.6%) | 11 (12.9%) | 11 (7.3%) | 36 (31.9%) | 4 (8.5%) | 77 (13.0%) |
| All | 198 | 85 | 151 | 113 | 47 | 594 |
AUH: Araba University Hospital; BUH: Basurto University Hospital; CUH: Cruces University Hospital; GUH: Galdakao University Hospital; SPUH: San Pedro University Hospital.
Species isolated in patients with nontuberculous mycobacterial pulmonary disease.
| SPUH | AUH | CUH | GUH | BUH | TOTAL | |
|---|---|---|---|---|---|---|
| M.avium complex | 15 (33.3%) | 16 (61.5%)* | 35 (55.6%)* | 8 (38.1%) | 7 (35.0%) | 81 (46.3%) |
| M. kansasii | 1 (2.2%) | 1 (3.8%) | 16 (25.4%)* | 2 (9.5%) | 9 (45.0%)* | 29 (16.6%) |
| M. xenopi | 15 (33.3%)* | 1 (3.8%) | 0 | 0 | 0 | 16 (9.1%) |
| M. lentiflavum | 3 (6.6%) | 3 (11.5%) | 1 (1.6%) | 3 (14.3%) | 1 (5.0%) | 11 (6.2%) |
| M. abscessus | 2 (4.4%) | 1 (3.8%) | 7 (11.1%) | 0 | 0 | 10 (5.7%) |
| M. fortuitum | 2 (4.4%) | 0 | 1 (1.6%) | 1 (4.8%) | 1 (5.0%) | 5 (2.9%) |
| M. chelonae | 1 (2.2%) | 0 | 0 | 0 | 1 (5.0%) | 2 (1.1%) |
| Other NTM | 6 (13.3%) | 4 (15.4%) | 3 (4.8%) | 7 (33.3%) | 1 (5.0%) | 21 (12%) |
| ALL | 45 | 26 | 63 | 21 | 20 | 175 |
NTM: nontuberculous mycobacteria; AUH: Araba University Hospital; BUH: Basurto University Hospital; CUH: Cruces University Hospital; GUH: Galdakao University Hospital; SPUH: San Pedro University Hospital.
As mentioned previously, M. gordonae was only recorded at three hospitals. Overall, M. gordanae accounted for 15% of all NTM isolates obtained at these three hospitals.
DiscussionThis multicentre study provides a comprehensive evaluation of microbiological data together with clinical and radiological data over an 8-year period for all patients in whom an NTM was isolated, enabling NTM-PD patients to be correctly identified according to the 2020 ATS/ERS/ESCMID/IDSA criteria.15 In addition to the overall results, data on isolation and NTM-PD incidence rates and the species isolated are presented separately for each of the participating hospitals, allowing comparison between five centres located in a small geographical area. We have not found any studies in the literature providing all these types of data.
Our results show that the upward trend in the annual number of NTM isolates in the period analysed does not translate into an increase in NTM-PD cases. This contrasts with most previous studies included in a systematic review having shown increases in cases of NTM-PD,7 although in at least two no such increase was seen18,19 and one study even reported a decrease in the incidence of the disease.20 Further, our NTM-PD incidence rate is far lower than the incidence of tuberculosis in our area, which was around 9 cases per 100,000 in La Rioja and the Basque Country in 2019.21
We also consider the marked differences in isolation and NTM-PD incidence rates between some of the participating hospitals notable, disparities which cannot be attributed to more proactive searching for cases in the centres with higher rates. In fact, SPUH had the highest rates of isolation and illness, but the lowest mean population-adjusted sputum culture rates (along with AUH), while the opposite was true in BUH.
Regarding the species isolated, there was notable variability in the results, even between the three hospitals in the province of Bizkaia, which are geographically close. MAC and M. abscessus are the NTM species most frequently isolated in patients with cystic fibrosis,22 and this probably explains the concentration of M. abscessus cases in the CUH and also the large number of MAC isolates in this hospital, since this hospital has a Cystic Fibrosis Unit that receives patients from other areas. On the other hand, a higher presence of M. kansassi has been related to industrialisation,23 this could partly explain the high rates of M. kansassi in CUH and BUH, since the environment of both hospitals presents a notably greater degree of industrialisation than that of the other three hospitals. Perhaps the most striking result is that 90% of the isolates of M. xenopi occurred in SPUH, with 72 isolates, compared to 0 in two hospitals, but we have found some similar cases in the literature. In a Spanish study carried out between 1976 and 1996, with a large number of samples (11,128) and the participation of 26 hospitals from 17 regions, 91.3% of all the isolates of M. xenopi were obtained from just 3 regions in the north.24 Even within a same region, Tuscany (Italy), M. xenopi represented 9.5% of NTM isolates in Pisa, while in Florence was isolated in 39.5%.8 Initial studies suggested a predominance of M. xenopi in coastal areas, but its high presence in Hungary refutes this.14 Thus, the presence of a specific environmental niche for M. xenopi is a likely explanation and suggests that the quantitative environmental exposure to mycobacteria is associated with the likelihood of isolation in respiratory samples and the progression to clinical respiratory disease.14
For comparing our incidence rates with other regions, we have only used studies that report incidence data. Studies reporting disease prevalence were excluded because NTM-PD is a chronic condition, and hence, prevalence should be higher than incidence,25 as shown in a recent study in the USA.26 On the other hand, only studies published since 2010 have been considered, given that both isolation and NTM-PD rates are tending to increase, although with variable trends in different periods.7
Within Spain, our disease incidence rate is lower than that found in a study conducted in Asturias (2.24 per 100,000 person-year) although it should be noted that the latter included extrapulmonary samples, which accounted for 14.1% of the cases of disease.27 Our incidence rate was also lower than in a study conducted in an area of the Basque Country between 1997 and 2016 that showed a gradual decline, with an NTM-PD incidence of 1.8 at the end of the study.20
Regarding elsewhere in Europe, although our isolation incidence rate was almost twice that in Denmark (2.14/100,000 person-year), NTM-PD rates were very similar (1.2 vs 1.10 in Denmark).18 Our NTM-PD rate is also similar to rates in France (1.0),28 the Czech Republic (1.1)29 and Germany (1.12–1.48).30 In contrast, compared to our rates, studies in the United Kingdom have found a higher incidence of isolates, 5.5–7.631 and disease, 3.4–6.5,11 while in the Balkans, the disease incidence was lower, with rates of 0.23 in Croatia32 and 0.29 in Serbia.33
Beyond Europe, as expected from previous studies,9 our incidence rates were lower than those found in North America (Oregon), with an NTM-PD rate of 4.8–5.6/100,000 person-year25 and Australia (Queensland), with an isolation rate of 12.2 and NTM-PD rate of 3.2.34 Elsewhere, Japan and Taiwan stand out, with NTM-PD rates of almost 15,12,35 much higher than our results, and at the opposite extreme, the rate of NTM isolation found in Tunisia was much lower (0.2),36 while the results from French Guiana were similar to ours, with an isolation rate of 6.17 and NTM-PD rate of 1.07.37
Concerning the percentage of patients with NTM isolates meeting criteria for disease, our figure of 31.4% is intermediate with respect to results in the aforementioned studies: close to the rates of 22% in Serbia33 and 26% in Queensland, Australia,34 higher than that of 17% in Asturias, Spain,27 and substantially lower than the 50% observed in Denmark18 and Oregon, USA.25
The pattern of species most frequently isolated in our study, namely, MAC, M. xenopi, Mycobacterium lentiflavum and Mycobacterium fortuitum (in addition to M. gordonae, in the three hospitals in which this species was counted) largely agrees with the results of several of the aforementioned studies.19,25,27,29,31,35 Although with small differences, overall, MAC and M. gordonae are the two most frequently isolated NTM, followed by M. fortuitum in most studies and M. xenopi in many cases. A distinct characteristic of our series is the large number of M. lentiflavum isolates, which does not appear among the most frequently isolated NTM in any of the research previously mentioned. However, in a study carried out in Spain in 2013–2017, with a large number of isolates (4535), M. lentiflavum was the second most frequently isolated NTM (12%) after MAC (47%).38
In patients meeting criteria for NTM-PD, in our series, and in practically all the other studies cited,13,19,25,27,29,32,33,38 MAC is the most frequently isolated NTM. The other three most frequent NTM in our results (M. kansassi, M. xenopi and M. abscessus) also appear among the most frequent in several previous studies,13,25,29,33–35,38 although with some differences between the series.
Our study has several limitations. First, the data in our study were collected retrospectively, therefore, it presents the limitations inherent to this type of study, although we believe that selection bias is unlikely since all NTM isolates (except M. gordonae) were included by all five participating hospitals and all of these are tertiary referral centres in their corresponding areas. Second, the procedures used in the microbiology laboratories at the five hospitals were not the same, especially for species identification. Specifically, while the hospitals in the Basque Country have preferentially used MALDI-TOF MS techniques, HUSP has used line probe assay methods. Nonetheless, we believe that this is unlikely to have had a significant influence on the results, since both techniques are recommended for identifying NTM.16,17 And third, the data collected all come from public hospitals. In particular, the incidence rates have been estimated taking into account the catchment population of each hospital, despite the fact that part of this population is treated by private healthcare providers, and this might lead to an underestimation of the rates. Nonetheless, given the clear dominance in our setting of public over private healthcare and that the latter focuses its activity on surgery, we consider that the underestimation should be negligible.
ConclusionsOur NTM isolation and NTM-PD incidence rates are intermediate with respect to other European areas. The incidence rate of NMT-PD in our area does not show the increasing trend that is described in most studies and it is still far lower than that of tuberculosis in our area. The most frequently isolated species and those that most frequently cause NTM-PD are similar, with small differences, to those from other areas of the world, being MAC the predominant NTM in both cases. Finally, in geographically close areas, there is great variability in isolation and disease incidence rates, as well as in the species isolated, supporting the view that local environmental factors are important in the transmission of NTM.
FundingThis research received financial support from the Department of Health of the Basque Government (No.: 2018111098).
Authors’ contributionsJUU and ETH take the responsibility of the manuscript as a whole. JUU, ETH, LAB, MVLA and JAGF conceived and designed the study. JUU, ETH, LAB, MVLA and JAGF collected and compiled data. ETH performed the statistical analysis. JUU analysed and interpreted the data. JUU and ETH wrote the manuscript. JUU, ETH, LAB, MVLA and JAGF commented and revised the report. All authors read and approved the final manuscript.
Conflicts of interestsThe authors declare they have no conflict of interest.