Nirsevimab therapy has the potential to revolutionize infant respiratory syncytial virus (RSV) prophylaxis. But other populations suffering RSV, such the elderly or those over 60, may also be protected by using this novel antibody in the infant group. It is true that some studies link the use of nirsevimab to a reduction in the virus's ability to spread by lowering the viral load in infants as a result of the drug's long half-life. However, this protective effect may not be very significant because RSV transmission in the elderly typically comes from other elderly people or from school-aged children. Furthermore, RSV may be transmitted at any time of the year and not just during the period of nirsevimab protection due to its existence in human reservoirs. The reasons made here show that, even though nirsevimab treatment in infants may protect the elderly, this benefit would be limited and testimonial. Therefore, immunizing the elderly with currently licensed and developing vaccines should be a priority.
El uso de nirsevimab puede suponer una revolución en la prevención del virus respiratorio sincitial (VRS) en lactantes. Sin embargo, el uso de este nuevo anticuerpo en dicho grupo de edad podría proteger también a otros grupos que conviven con ellos, como por ejemplo las personas de edad avanzada o grupo de personas mayores de 60años. Si bien es cierto que algunos estudios sugieren una disminución en la propagación del virus con el uso de nirsevimab, al reducir la carga viral en lactantes como consecuencia de la prolongada vida media del fármaco, este efecto protector podría ser de escasa relevancia, ya que la transmisión del VRS en personas de edad avanzada sucede en la mayor parte de los casos desde personas de la misma edad o desde niños en edad escolar. Adicionalmente, la presencia de VRS en reservorios humanos puede permitir que el VRS se transmita en cualquier época del año, no limitándose únicamente al periodo de protección de nirsevimab. Los argumentos aquí expuestos demuestran que, si bien el uso de nirsevimab en lactantes podría tener un efecto protector en las personas de edad avanzada, este solo sería testimonial y limitado. En consecuencia, debe priorizarse la inmunización de los pacientes de edad avanzada con las vacunas actualmente autorizadas y en desarrollo.
Reducing the burden of severe disease and mortality from respiratory syncytial virus (RSV) in infants under one year of age is expected to be greatly impacted by the approval and use of nirsevimab in this age range. Treatment efficacy against medically attended lower respiratory tract infection (MA-LRTI) can reach 78.6% in healthy, term infants1 and 83.2% in pre-term infants,2 according to data from many research and clinical trials. Nevertheless, since a decrease in MA-LRTI does not always imply a decrease in infection – only in cases of severe pathology – it is unclear how nirsevimab affects the spread of viruses.
RSV has a large disease burden in various age groups, particularly in the elderly and those with underlying diseases, despite its obvious effects on children's health. The primary issue with RSV and older persons is that, particularly in high-income countries, our understanding of the disease's true impact is quite limited. RSV in these nations specifically reveals an attack rate of around 1.62% (95% CI: 0.84–3.08) in individuals over 65, a hospitalization rate of 0.15% (95% CI: 0.09–0.22), and an in-hospital death rate of 7.13% (95% CI: 5.40–9.36).3,4 This is in light of the fact that, except from the COVID-19 pandemic, the epidemic sickness only manifests itself in the fall and winter. As a result, emphasis must be placed on preventive treatments that can have a positive effect on lowering the incidence of RSV in the elderly.
When the SARS-CoV-2 Omicron variant first appeared in 2022, it was already clear that humans may alter the behavior of respiratory viruses.5 Omicron's great divergence since its emergence has been influenced at different periods by the immune system's pressure, which is applied through immunizations, natural infections, and the use of monoclonal antibodies. In fact, computer modeling accounting for the widespread use of monoclonal antibodies to treat COVID-19 has allowed for the description of some mutations in the Spike protein, such as those that emerged during the emergence of the EG.5 (Eris) subvariant of interest, before they appeared.6 It was also feasible to see how non-pharmaceutical intervention strategies (NPIs) significantly reduced the spread of the majority of respiratory viruses during the COVID-19 pandemic.7
Thus, we might speculate that the adoption of preventive measures against RSV, like monoclonal antibodies, may have some effect on how this virus evolves and how it affects different age groups, like the elderly. Based on earlier research that other researchers have detailed, the goal of this work is to outline the reasons for and against the potential effects that nirsevimab use in infants may have on the elderly.
Factors by which the use of nirsevimab could modify the pattern of attack in older adultsReduction of viral load in infants immunized with nirsevimabThe use of an immunizing mechanism that lessens the severity of infection may, in some way, positively impact the reduction of viral load in immunized infants, even though the goal of nirsevimab is not the reduction of viral load but rather the reduction of hospitalization and mortality in infants who contract RSV.
Regarding adults, RSV vaccinations in various stages of clinical trials (for those between the ages of 18 and 50) have occasionally demonstrated a lower viral load in respiratory samples from human challenges as compared to those who were not vaccinated.8,9 However, since parenteral delivery is not explicitly intended to induce mucosal immunity, it is unknown what function conventional vaccinations (subunit protein, split vaccines, etc.) may have in restricting viral shedding. Nevertheless, some research indicates that the processes behind this kind of protection are still not well understood.10
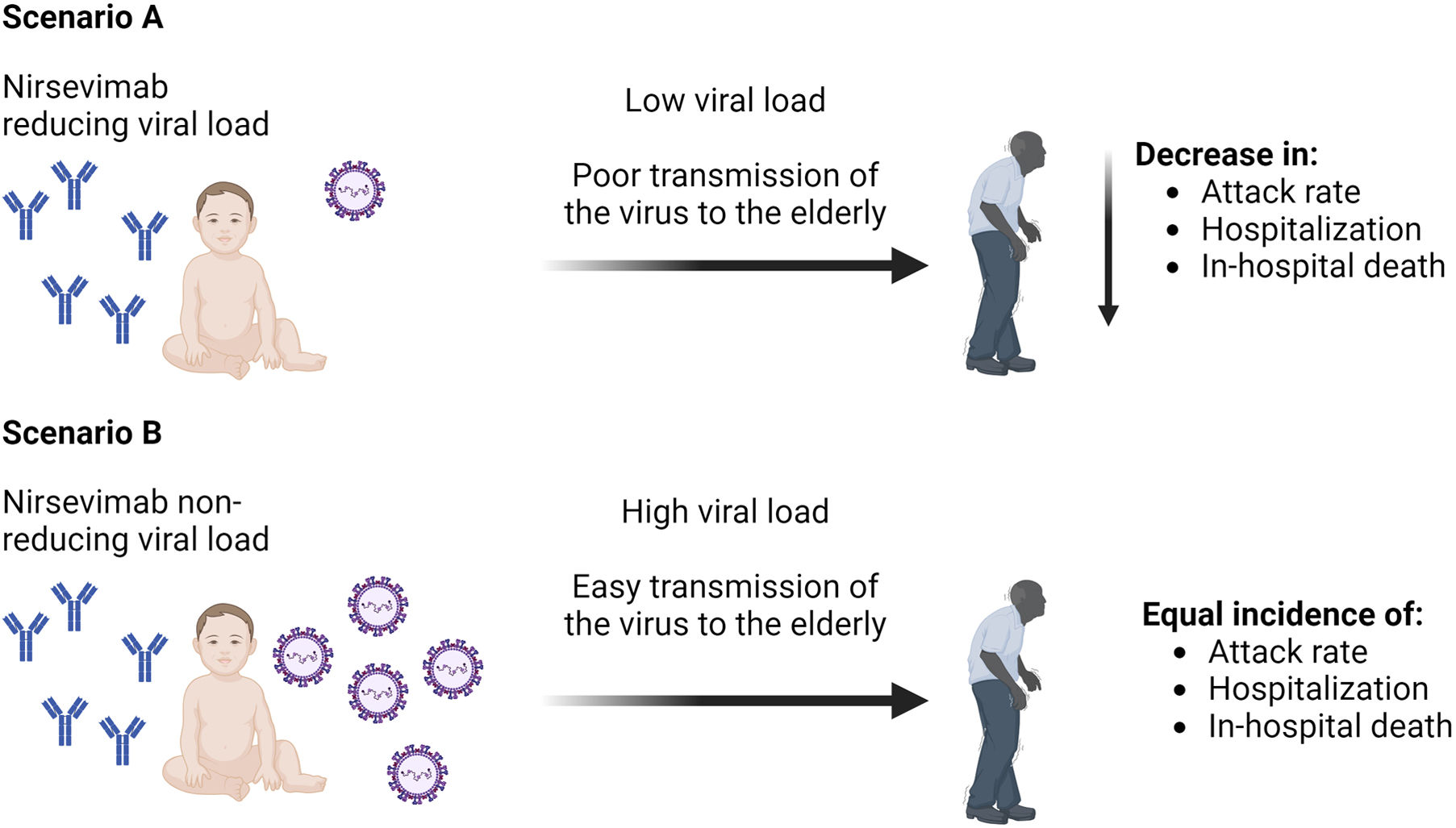
The effectiveness of nirsevimab in reducing the viral load in immunized infants has not been thoroughly studied, but recent unpublished data from the 5th ReSViNET Conference 2019, RSVVW’19, indicates that the drug does, in fact, lower the viral load at the pulmonary level, which would account for the decrease in severe disease.11 However, sterilizing immunity does not seem to be produced by the drug. The ability of nirsevimab to lower viral load in the upper respiratory tract and hence influence RSV transmissibility is not evident from these data. There is currently very little information, yet some studies seem to indicate that infants inoculated with nirsevimab versus placebo had exactly the same viral load in their nasopharynx.12 Nirsevimab, however, appears to reduce viral load in the upper and lower respiratory tract by inhibiting F-protein activity, which leads to virus neutralization and ultimately virus eradication13 (Fig. 1). But, this is only supported by research conducted in animal models. Regarding that, one group of authors described that giving nirsevimab to a sizable portion of infants could clearly reduce the incidence of disease not only in that age group but also in older children, young adults, and older adults through studies that performed mathematical modeling in various scenarios (no viral load reduction and an arbitrary 50% reduction).14
Another method to determine the potential effect of nirsevimab on lowering viral load and, consequently, respiratory transmission of RSV, is to use previously released information on palivizumab, another monoclonal antibody that has been used for a while in high-risk newborns. Unlike nirsevimab, palivizumab is administered monthly during the RSV circulation season. It is a monoclonal antibody that is licensed for use in children under 6 months of age at fewer than 35 weeks of gestation and for children under 2 years of age with at-risk pathologies.15 The fact that palivizumab is an antibody that is targeted to the F protein's epitope II is one of the primary distinctions from nirsevimab.16
Palivizumab use significantly reduced RSV detection in nasopharyngeal samples during the years of follow-up, according to several studies, including INSPIRE,17 MAKI,18 and TCRI.19 In contrast, the prevalence of detections in the placebo groups was similar to pre-study levels.20 Consequently, these three earlier studies demonstrate that the use of monoclonal antibodies likely does allow an appreciable reduction in the incidence of RSV in infants (or, at least, detections) and, consequently, a concomitant reduction in viral load, given the current lack of evidence due to the scant scientific work on the subject for nirsevimab.
Beyond the mathematical modeling mentioned above, it is entirely unknown what possible effects this reduction would have on other infants, children of different ages, adults, and people over 60.14 In order to achieve this, it is imperative to capitalize on the widespread use of nirsevimab starting in the 2023–2024 season, particularly in countries like Spain,21 and to start prospective studies that assess the drug's potential effects on both the reduction of viral load and the reduction of severe disease in other age groups using actual data.
Lifetime of nirsevimabAssuming that the previously described factors contribute to nirsevimab's ability to lower viral load, the lifetime of the monoclonal antibody in the inoculated infant may be a factor in favor of protecting other age groups, such as the elderly.
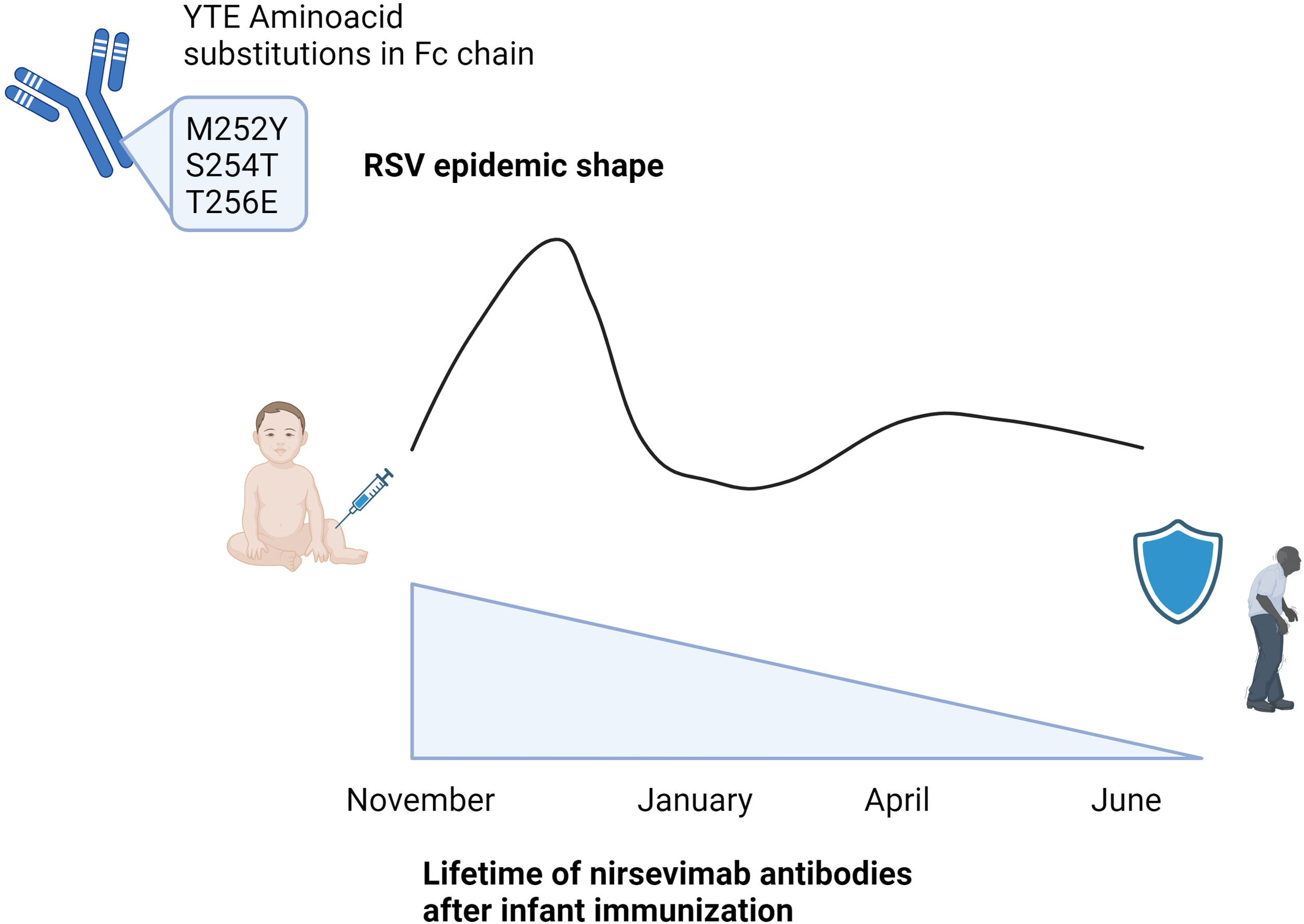
The structure of the nirsevimab antibody directed against the Ø epitope of the F protein in the pre-fusion state has been modified in the laboratory through three amino acid changes that stabilize the antibody and extend their half-life.13 The antibody's half-life is extended by 71–90 days or more as a result of these three amino acid changes, which are found at positions M252Y, S254T, and T256E (YTE substitution) of the Fc chain.13,22 By accelerating endothelium recycling, YTE substitution slows down the rate of antibody degradation and lengthens the antibody's half-life. Some clinical trials, including one carried out in South Africa in 2021,1 have demonstrated that the antibodies remain detectable even after 150 days, with 6.1% of infants exhibiting detectable levels of the antibodies one year later (Fig. 2).
YTE (M252Y, S254T and T256E) aminoacid substitutions in Fc chain extends life of nirsevimab antibodies in the infant, enlarging their protection for at least 150 days, and possibly the protection of other co-living age groups. This protection would lead to extend the protection for all the RSV epidemic and also for the months after when RSV is also detected but causing limited outbreaks. Created with Biorender.
The extension of nirsevimab's half-life may imply that, if the decrease in RSV viral load is real, the corresponding decrease in transmissibility from infants to other age groups would also be lowered, if not only during the RSV season, then at least for a minimum of six months following administration. This could have a long-term effect on the spread of the virus to other age groups.
Factors why the use of nirsevimab might not modify the pattern of attack in older adultsEntry of the virus into the home environmentRSV community transmission is the most common among the elderly, however some research suggesting that nosocomial infections during hospital stays or transmission by social and healthcare staff in nursing homes can also result in RSV transmission in older persons.23
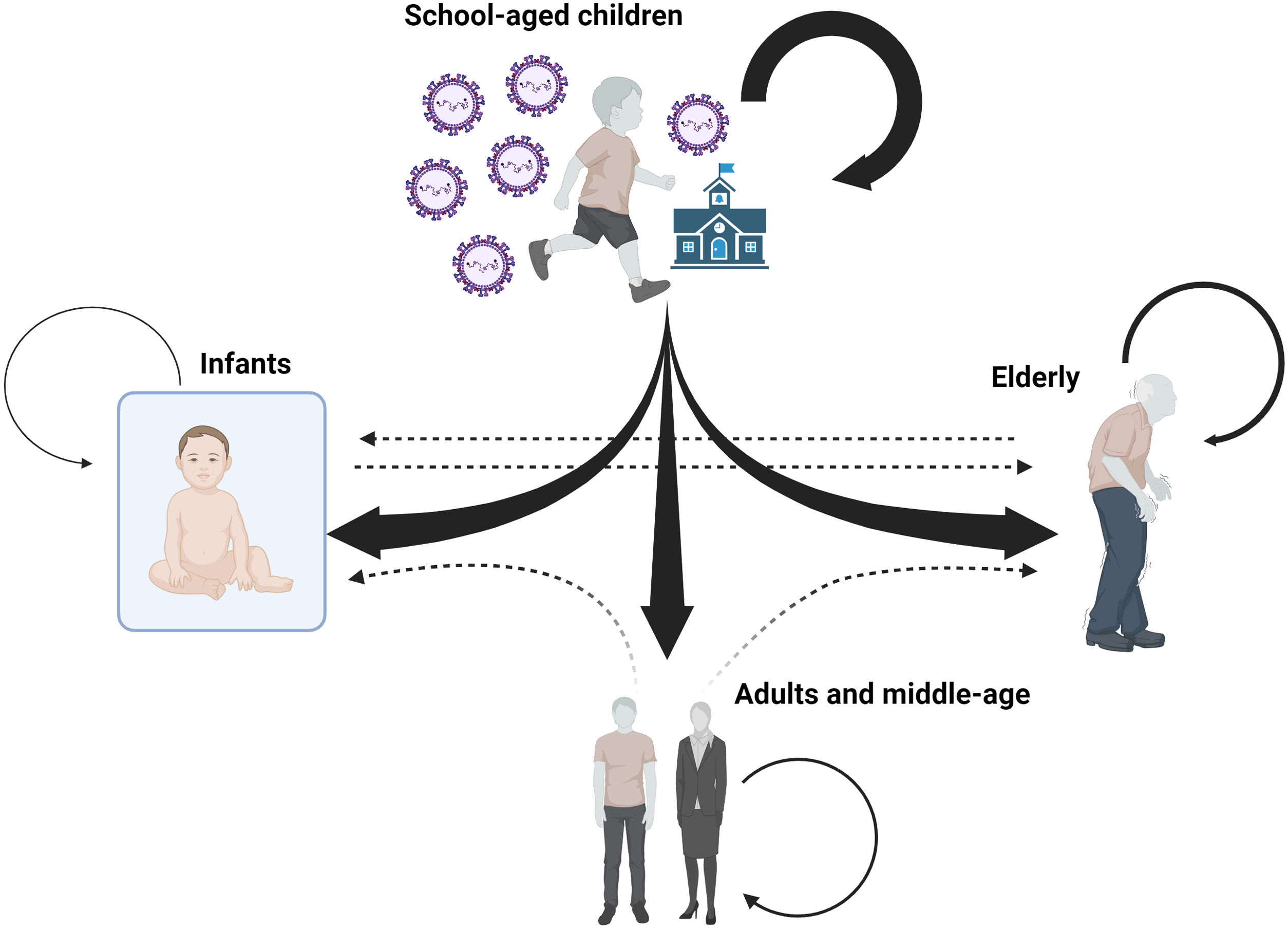
School-age children are the most common carriers of RSV into the home, according to certain research, and as such, they start outbreaks that can impact other age groups that live together.24,25 Certain phylogenetic studies indicate that school-age children frequently start family environment outbreaks. A very significant resemblance between viruses obtained from members of the same family and those obtained from other family outbreaks whose relationship was, precisely, the children who attended the same school, has been revealed by phylogenetic analysis of viruses found in family outbreaks.26 This has also been shown, for instance, with influenza viruses in outbreaks where the virus spreads to additional family members after entering the home through a school-age child.27
The results of these studies thus indicate that a significant portion of the community transmission of RSV that affects the elderly is probably caused by cohabitation with schoolchildren, either because they live together regularly in the same home or because they look after them in households where both parents work, which is a common circumstance in high-income countries. However, since the infant's social life is typically limited to the home setting due to obvious reasons, the schoolchild would also serve as the primary transmitter to the infant. As a result, the likelihood of the virus spreading to the infant from outside the family is significantly reduced (Fig. 3).
Diagram showing the theoretical directionality of RSV infections within the family environment. Most RSV introductions are from school-aged children to infants, young adults, and older adults. Transmission among older adults also appears to be a relatively frequent route of RSV transmission. The impact or frequency of transmission among household members who are not school children (dashed arrows) is unknown. The size of the arrows is indicative of the infectious capacity from one age group to another based on the scientific articles referenced. The box surrounding the infant represents the safe environment in which the infant lives with respect to interaction with other people outside the family nucleus in the first months of life. Created with Biorender.
All of this points to a scenario where, in contrast to school-aged children, infants would transmit RSV to the elderly less frequently. Furthermore, because nursing requires parents to be with their infants more frequently to respond to their needs, this means that during breastfeeding, infants spend less time with their grandparents, which likely reduces the frequency of transmission.
Therefore, since infants would not be the primary source of virus transmission to the elderly, it is unlikely that the use of nirsevimab, if it reduces the RSV viral load and infectivity in infants, will have a substantial effect on the prevalence of the disease in the elderly population. However, other research using mathematical modeling assuming various scenarios for reducing viral load with nirsevimab does demonstrate reductions in virus incidence in other age groups, including those above 65.14 To be certain, however, real-world data following the onset of this monoclonal antibody's broad use are required to determine whether this is accurate.
The summary offered here ought to offer guidance for creating future vaccination strategies. The development of live attenuated inhaled vaccines that can boost mucosal immunity and thereby lower viral load and transmission,28 would be ideal for reducing viral transmission to infants and the elderly cohabiting with school-aged children, as they are the primary carriers of the virus to other age groups living together.
Natural reservoir of the virus in the hostAccording to several theories, individuals who serve as RSV reservoirs during non-seasonal periods may retain the virus perpetually.29 As evidenced by a 2008 study, RSV may actually stay latent in immunofluorescence-detected human dendritic cells cultivated in a lab and can subsequently become infectious when exposed to HeLa cell culture.30 The same study demonstrated that endogenous nitric oxide (NO) generation controls the suppression of the viral replication cycle and that viral replication can revive when NO production is blocked by several methods. The authors go on to say that these findings may explain why RSV epidemics start and end at the same times in geographically dissimilar but climate-similar regions of the world,31 presuming that endogenous NO production is affected by climatic conditions that activate RSV in natural human reservoirs (immune sanctuaries) at the same time. This would show that RSV epidemics originate locally rather than spreading across continents.30,32
Although there is not enough evidence to say for sure,33 some studies done in France following the COVID-19 pandemic indicate that RSV regained its circulation after the other respiratory viruses (aside from SARS-CoV-2) were almost completely eliminated from circulation. This happened when NPIs specific to adults (strong restrictions on non-essential travel, night curfews, closing non-essential businesses, and encouraging work from home) were lifted, but not those specific to children (with reinforcement of social distancing and required face masks starting at age six) were lifted.34 As a result, the removal of NPIs for children had little effect on the spread of RSV. This suggests that there were two possible causes for the resurgence of RSV in France following the pandemic, or a combination of the two: either low-level residual transmission from certain adults acting as reservoirs, or the virus being reintroduced from another geographic location. Though some research suggests patients with specific underlying pathologies, such as those with chronic obstructive pulmonary disease (COPD)35 or hematopoietic and solid organ transplant recipients,36–38 who may have detectable virus for more than 80 days,39 no study has identified the exact human reservoirs of the virus when the RSV epidemic ends.
It appears to be true that, in the event that the hypothesis that humans themselves serve as RSV reservoirs is accurate, the majority of these reservoirs would likely be patients with inadequate viral control and incapacity to eradicate the virus. Naturally, immunosuppressive illnesses or conditions that impair respiratory cells’ ability to operate normally in their surroundings (such as transplant recipients and COPD patients) would account for the majority of these patients. Therefore, the use of nirsevimab could not have an effect in limiting such transmission from infants to elderly individuals if RSV is maintained in the population and can be transmitted among adults even in non-epidemic times, since the virus itself could be directly present, albeit latent, in adults.
ConclusionsIn terms of preventing severe RSV disease in infants under a year of age, the use of nirsevimab appears to be a significant advancement. This claim is supported by the statistics that are now available, and the outcomes of the prospective studies that are being conducted will provide empirical information that will enable us to determine the extent of this new preventive strategy.
However, it is important to remember that there are additional preventive measures available for other age groups. The elderly are another population highly affected by RSV, and little is known about the disease's true effects in this regard. It may be tempting to believe that the use of a powerful preventive tool like nirsevimab in one of the groups most affected by RSV will somehow change the virus's circulation or epidemic pattern, lessening its effects on other age groups like elderly, even though there are several approved vaccines and others under development for this age group. The evidence we have compiled in this work suggests that this impact may be very limited because, while there are a number of reasons that lead us to believe that the viral load in infants will decline over time, there are other reasons that demonstrate that older adults are more likely to get RSV from school-age children or even from other individuals who are similar in age to them. Similarly, while nirsevimab may lessen the amount of RSV that infants shed, it does not appear to be enough to stop or lessen the virus's ability to spread from infants to older people in their surroundings; this is, of course, not the primary goal of this novel monoclonal antibody.
In summary, the scientific evidence presented here demonstrates how the elderly should be protected with preventive tools targeted specifically to this age group. While other mechanisms used in other age groups may help to slightly lower the incidence, the elderly should be protected primarily through the use of currently available and future vaccines.
FundingThe authors did not receive any funding to make this work.
Authors’ contributionsISM, JCS and JME recovered the information, wrote the manuscript and revised their final version.
Conflicts of interestThe authors declare non-conflict of interest.