Patients with severe chronic obstructive pulmonary disease (COPD) are often underrepresented in cohorts, creating uncertainty about the natural history and prognostic factors of this subgroup. Our goal was to describe the SPOCCAT (Severe COPD: Prospective Observational study of COPD in Catalonia) study protocol.
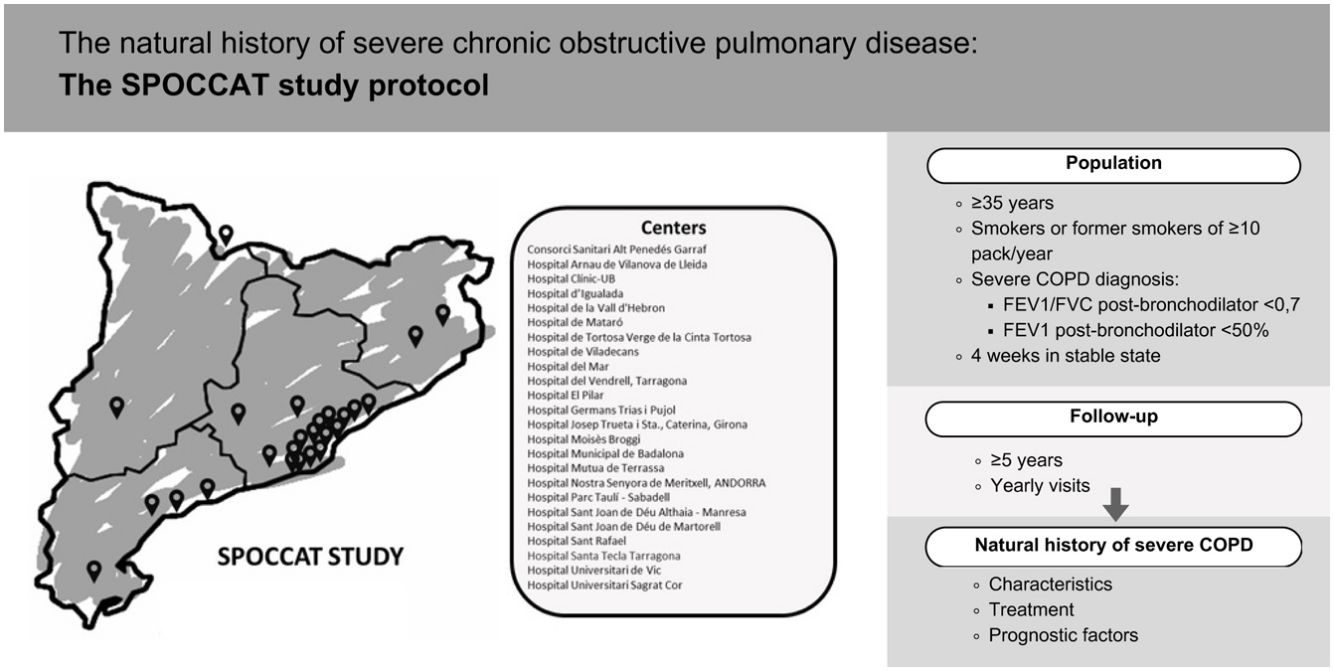
Material and methodsSPOCCAT is a non-interventional, multicenter, prospective cohort study of patients with severe COPD (FEV1% predicted<50%). The study aims to: (1) establish a five-year prospective cohort; (2) identify demographic and clinical characteristics; (3) describe treatment patterns; (4) better understand the natural history of severe COPD, including lung function decline, exacerbation rates, and mortality; and (5) identify prognostic factors for poor outcomes.
Recruitment began in January 2024, and the cohort will be followed for a minimum of five years (or until death or lung transplant) with follow-up visits every 12 months. Baseline data include demographics, laboratory analyses, comorbidities, lung function, respiratory symptoms, respiratory disease exacerbations and etiology, quality of life, physical activity, chest computed tomography, and treatment. Annual follow-up visits will assess changes in treatment, exacerbation frequency and severity, microbiological outcomes, complementary tests, and mortality. Participation requires written informed consent from all patients, with data collected in an anonymized electronic Case Report Form.
ResultsThe results of the SPOCCAT study will provide relevant information about the characteristics, treatment, and prognostic factors of severe COPD.
ConclusionsSPOCCAT has the potential to enhance understanding of severe COPD, exploring innovative aspects and establishing a robust research framework for future COPD-related projects.
Los pacientes con enfermedad pulmonar obstructiva crónica (EPOC) grave a menudo están infrarepresentados en las cohortes, lo que genera incertidumbre sobre la historia natural y los factores pronósticos de este subgrupo. Nuestro objetivo fue describir el protocolo del estudio de EPOC grave: estudio observacional prospectivo de la EPOC en Cataluña (SPOCCAT)
Material y métodoEl SPOCCAT es un estudio de cohorte prospectivo, multicéntrico y no intervencionista de pacientes con EPOC grave (volumen espiratorio forzado en un segundo [FEV1] % previsto<50%). El estudio tiene como objetivo: 1) establecer una cohorte prospectiva de cinco años, 2) identificar características demográficas y clínicas, 3) describir patrones de tratamiento, 4) comprender mejor la historia natural de la EPOC grave, incluida la disminución de la función pulmonar, las tasas de exacerbación y la mortalidad, y 5) identificar factores de pronóstico de malos resultados. El reclutamiento comenzó en enero de 2024 y se seguirá a la cohorte durante un mínimo de cinco años (o hasta la muerte o el trasplante de pulmón) con visitas de seguimiento cada 12 meses. Los datos basales incluyen datos demográficos, análisis de laboratorio, comorbilidades, función pulmonar, síntomas respiratorios, exacerbaciones y etiología de enfermedades respiratorias, calidad de vida, actividad física, tomografía computarizada de tórax y tratamiento. Las visitas de seguimiento anuales evaluarán cambios en el tratamiento, frecuencia y gravedad de las exacerbaciones, resultados microbiológicos, pruebas complementarias y mortalidad. La participación requiere el consentimiento informado por escrito de todos los pacientes, con datos anonimizados recopilados en un cuaderno de recogida de datos electrónico.
ResultadosLos resultados del estudio SPOCCAT aportarán información relevante sobre las características, el tratamiento y los factores pronósticos de la EPOC grave.
ConclusionesEl estudio SPOCCAT tiene el potencial de mejorar la comprensión de la EPOC grave, explorando aspectos innovadores y estableciendo un marco de investigación sólido para futuros proyectos relacionados con la EPOC.
Although several studies have been published to date, there are important gaps in our knowledge about the natural history of chronic obstructive pulmonary disease (COPD). One of the most renowned studies was published by Fletcher and Peto in the 1960s,1 providing a pivotal contribution to the knowledge of this disease. Subsequent comprehensive studies with longer follow-up periods have reported significant insights, including the important association between tobacco and the decline in pulmonary function.2,3
Following these initial investigations, several research groups were established with the objective of not only examining pulmonary function but also adopting a multidimensional approach, encompassing various facets of the disease. Among these studies, cohorts of note include ECLIPSE4 and PAC-COPD5 which were aimed at characterizing the phenotypes of COPD. Additionally, the Hokkaido cohort in Japan6 was focused on the study of emphysema, while the SAPALDIA 17 and 28 studies evaluated the impact of environmental pollution and gender differences. Subsequently, two of the most pivotal cohorts emerged: the BODE cohort (Body-Mass Index, Airflow Obstruction, Dyspnea, and Exercise Capacity)9 involving multidimensional assessment, and the COPDGene (Genetic epidemiology of COPD) study,10 aimed at exploring the genetic, functional, and radiological determinants of the disease. Along this line, the CHAIN study11 was launched in Spain a decade ago with the objective of carrying out comprehensive, long-term assessment of COPD progression (minimum 5 years). This study included a control group consisting of smokers or former smokers without COPD. The findings of this cohort analysis have been extensive and highly influential.12–14
However, despite substantial advancements in understanding this disease, numerous questions remain regarding its natural history. This is particularly critical for the most severe patients, who are often underrepresented in existing cohorts and have a bleaker prognosis.15 It is of note that only one third of the COPD patients included in the CHAIN cohort had severe or very COPD.11 The SPOCCAT (Severe COPD: Prospective Observational study of COPD in Catalonia) study is a prospective observational registry of patients with severe COPD aimed at characterizing the population of patients with severe COPD and better understand the natural history and prognostic factors of the disease. Here, we describe the characteristics of the SPOCCAT study protocol.
Material and methodsStudy objectivesThe objectives of the SPOCCAT study are:
- 1.
Establish a cohort of severe COPD patients who will be followed for at least 5 years.
- 2.
Identify the demographic and clinical characteristics of patients with severe COPD.
- 3.
Describe the patterns of treatment of patients with severe COPD.
- 4.
Better understand the natural history of the disease in terms of decline of lung function, exacerbations and mortality in patients with severe COPD.
- 5.
Identify prognostic factors of poor outcomes (decline in lung function, exacerbations and mortality) in patients with severe COPD.
SPOCCAT is a non-interventional, multicenter, prospective cohort study of patients with severe COPD followed in outpatient pulmonary clinics in Catalonia, Spain.
Patients will be managed according to the local procedures and policies without any clinical intervention from the study team. All patients will provide written informed consent to participate, and participating investigators will collect data prospectively. The following domains will be covered: demographics, laboratory analyses, comorbidities, lung function, respiratory symptoms, exacerbations of respiratory disease and their etiology in the previous year, quality of life, physical activity, chest computed tomography (CT) (when applicable) and treatment.
The cohort will be followed for a minimum period of five years with follow-up visits every 12 months. Changes in treatment, the number and severity of exacerbations, as well as microbiological outcomes and other complementary tests, will be evaluated during the yearly follow-up visits. In the event of death, the date and cause of death will be documented.
Data collection started in January 2024 in a secure electronic case report form (eCRF) developed in the REDCap (Research Electronic Data Capture) tool, sponsored by the Spanish Society of Pneumology and Thoracic Surgery (SEPAR-acronym in Spanish of the Sociedad Española de Neumología y Cirugía Torácica). SPOCCAT has been registered in www.clinicaltrials.gov number NCT06252805.
PopulationThe study population will consist of patients greater than or equal to 35 years of age, smokers or former smokers of at least 10 pack-years diagnosed with severe COPD demonstrated by spirometry showing a post-bronchodilator forced expiratory volume in 1 second (FEV1)/Forced vital capacity (FVC)<0.7 and an FEV1(% predicted)<50%. Patients will be recruited in stable state, at least 4 weeks after resolution of an exacerbation.
The exclusion criteria are: (1) Medical conditions that make it impossible to perform the usual diagnostic procedures or follow-up; (2) Another severe chronic respiratory disease that justifies impaired spirometry, such as lung fibrosis or severe bronchiectasis; (3) Active neoplasm or a diagnosis of cancer in the previous 5 years; and (4) Lack of informed consent.
Patients will be removed from the study if the informed consent is withdrawn by the patient or their legal guardian. For patients who are lost to follow-up (i.e. patients whose status is unclear because they fail to appear for study visits without stating an intention to withdraw), the investigator should report the steps taken to contact the patient, e.g. dates of telephone calls, registered letters, etc. in the eCRF.
Sample sizeThe sample size calculation was based on the prevalence of COPD (17%) in Catalonia among individuals older than 35 years of age,16 with approximately 10% being classified as severe. Therefore, our target population would encompass around 130,000 individuals. Taking into account a 95% confidence interval with a standard deviation of 1.96 and a margin of error of 5%, and factoring in an expected loss of 15–20%, the final study population should include a minimum of approximately 460 patients in order to obtain valid estimates for relevant variables such as frequency of exacerbations.
Center selectionThe study is promoted by the Catalan Foundation of Pneumology (FUCAP in Catalan – Fundació Catalana de Pneumologia) and will initially be implemented in Catalonia and Andorra, but if successful, it will be extended to other areas of Spain or even other countries, providing that ethical approval is obtained in all participating sites and that the appropriate data processing agreements are established between the promoter (FUCAP) and the candidate sites.
The FUCAP extended invitations to all centers listed in its roster of hospitals before the beginning of patient recruitment. The invitation noted that participation was voluntary and unpaid. By the end of January 2024, 31 pulmonologists from a total of 24 secondary and tertiary centers (Fig. 1 and Appendix) demonstrated interest and were invited to participate in an online investigators meeting. During this launch meeting, specific topics regarding the database, logistical considerations, project initiation, and potential challenges in patient recruitment were addressed.
Baseline data collection and follow-upThe outpatient visit schedule includes a baseline visit for patient recruitment in a stable state and annual clinical visits for a minimum follow-up period of 5 years or until death or lung transplant.
The selection of variables to be collected was initially structured by the Steering Committee and agreed upon with the other participating investigators. Subsequently, all researchers interested in participating in the SPOCCAT study were invited to provide comments and make relevant changes to the database before initiating recruitment.
Given the study objectives and the multidimensional approach, the number of collected variables is extensive. Overall, the variables chosen were categorized into the following domains: sociodemographic data, comorbidities, characteristics of COPD, radiological findings on chest CT, respiratory functional tests, blood samples and gasometrical results, additional tests, and vital status.
In the section on sociodemographic data, the following are specified: year of birth, gender, height, weight, smoking history (including years of cumulative exposure and the year of cessation), vaping consumption, employment status, occupational risk exposure, and educational level. The list of comorbidities is shown in Table 1.17
Baseline demographic and clinical data.
| Demographic data |
| Sex |
| Year of birth |
| Education level |
| Employment status |
| Risky occupational exposure |
| Height (cm) |
| Weight (kg) |
| Smoking status |
| Pack-years |
| Year of smoking cessation |
| Vaping |
| Comorbidities |
| Arterial hypertension |
| Atrial fibrillation |
| Hypercholesterolemia |
| Ischemic heart disease |
| Heart failure |
| Peripheral vascular disease |
| Dementia |
| Gastrointestinal ulcer |
| Gastroesophageal reflux |
| Anxiety |
| Depression |
| Periodontal disease |
| Diabetes mellitus |
| Organ damage due to diabetes |
| Stroke |
| Connective tissue disease |
| Liver disease |
| Hemiplegia |
| Renal insufficiency |
| History of cancer treated more than 5 years ago |
| Type of cancer |
| HIV |
| Obstructive sleep apnea |
| Pulmonary hypertension |
| Osteoporosis |
| COPD characteristics |
| Asthma in childhood |
| Asthma today (according to clinical criteria) |
| Dyspnea mMRC |
| Cough |
| Expectoration |
| CAT |
| Number of days per week usually walked |
| Minutes a day walked |
| Chronic bronchial infection |
| Chronic hypoxemic respiratory failure |
| Chronic hypercapnic respiratory failure |
| Exacerbations in the last year |
| Microorganism |
| Type and severity |
| Admission to ICU |
| NIMV |
| IMV |
| Microorganisms isolated |
| Pneumonia |
Abbreviations: HIV=human immunodeficiency virus; mMRC=modified medical research council; CAT=COPD assessment test; ICU=intensive care unit; NIMV=non-invasive mechanical ventilation; IMV=invasive mechanical ventilation.
In relation to the characteristics of COPD, this section provides a comprehensive description of the disease (Table 1), outlining symptoms (including the COPD Assessment Test questionnaire – CAT–),18 prognostic scales such as the BODE10 and BODEx19 indexes, physical activity measured by the Physical Activity Vital Sign (PAVS)20 and the mean time walked per day.21 Furthermore, detailed information is provided for each exacerbation in the previous year, including severity, tests conducted, microorganism isolated, treatment administered, and the need for hospitalization (including intensive care unit) and respiratory support.22 The severity of exacerbations was determined based on the treatment received as follows: mild exacerbations, those treated with short-acting bronchodilators; moderate exacerbations, those treated with short-acting bronchodilators plus antibiotics or oral corticosteroids; or severe exacerbations, those requiring visits to the emergency department or hospitalization.23
Information regarding spirometry results, lung volumes, lung diffusing capacity and the 6-min walking test will also be collected in each visit (Table 2). Information from other tests or examinations such as blood analysis (including blood gas analysis), exhaled nitric oxide, and echocardiogram will also be collected if performed prior to the baseline visit and during follow-up (Table 2). In relation to radiological findings, data will be gathered on the presence, distribution and severity of emphysema and/or bronchiectasis if the patient has undergone a CT scan within the 5 years preceding the baseline visit or during follow-up.
Diagnostic tests performed.
| CT characteristics (within the last 5 years) |
| Emphysema |
| Bronchiectasis |
| Location |
| Type |
| Respiratory functional testing |
| FVC (mL and %) |
| FEV1 (mL and %) |
| FEV1/FVC (%) |
| Bronchodilator test |
| TLC (mL and %) |
| RV (mL and %) |
| FRC (TGV) (mL and %) |
| DLCO (mL/min/mmHg and %) |
| KCO (%) |
| Basal oxygen saturation (%) |
| FeNO |
| Six-minute walking test |
| Blood analysis |
| Hemoglobin (gr/dL) |
| Hematocrit (%) |
| Leukocytes (×10×9/L) |
| Neutrophils (×10×9/L and %) |
| Lymphocytes (×10×9/L and %) |
| Basophils (×10×9/L and %) |
| Eosinophils (×10×9/L and %) |
| Platelets (×10×9/L) |
| Creatinine (mg/dL) |
| Urea (mg/dL) |
| Alpha-1 antitrypsin (mg/dL) |
| IgE (IU/mL) |
| Vitamin D (25-hydroxy) (ng/mL) |
| Fibrinogen (mg/dL) |
| C-reactive protein (mg/L) |
| NT-proBNP (pg/mL) |
| Gasometry |
| pH |
| pCO2 (mmHg) |
| pO2 (mmHg) |
| Standard HCO3 (mmol/L) |
| BE (mmol/L) |
| FiO2 with which it was performed (%) |
| Additional tests |
| Echocardiogram (within the last 5 years) |
| LVEF (%) |
| PAP (mean) (mmHg) |
Abbreviations: CT=computed tomography; FVC=forced vital capacity; FEV1=forced expiratory volume in one second; TLC=total lung capacity; RV=residual volume; FRC=functional residual capacity; TGV=thoracic gas volume; DLCO=diffusing capacity of the lungs for carbon monoxide; KCO=carbon monoxide transfer coefficient; FeNO=fractional exhaled nitric oxide (FeNO); NT-proBNP=N-terminal pro-B-type natriuretic peptide; pH=potential hydrogen; pO2=partial pressure of oxygen; pCO2=partial pressure of carbon dioxide; HCO3=bicarbonate; BE=bases excess; FiO2=fraction of inspired oxygen; LVEF=left ventricular ejection fraction; PAP=pulmonary artery pressure; IU=international units;
Pharmacological treatment (both respiratory and non-respiratory), and non-pharmacological interventions (including the use of oxygen therapy, non-invasive mechanical ventilation, respiratory physiotherapy, etc.),24 and adherence as well as vaccinations will be collected at baseline and at each follow-up visit (Table 3).
Treatments for COPD and comorbidities.
| Maintenance treatment for COPD |
| SABA |
| SAMA |
| LABA |
| LAMA |
| Inhaled corticosteroids |
| Number of devices for maintenance treatment |
| Pressurized device (MDI) |
| Dry powder device (DPI) |
| Respimat device |
| Nebulization device |
| Adherence to treatment |
| Inhalation technique |
| Oral corticosteroids |
| Immunosuppressive treatment |
| Statins |
| Oral antidiabetics |
| Beta blockers |
| Biological treatment for COPD |
| Roflumilast |
| Theophylline |
| Chronic mucolytics |
| Chronic macrolides |
| Chronic hypoxemic respiratory failure |
| Chronic hypercapnic respiratory failure |
| Oxygen therapy |
| NIMV |
| Nebulized antibiotic treatment |
| Flu vaccination last year |
| COVID vaccination last year |
| Pneumococcus vaccination |
| Physiotherapy/respiratory rehabilitation last year |
| Endoscopic or surgical treatment for emphysema |
Abbreviations: COPD=chronic obstructive pulmonary disease; SABA=short-acting β2-agonist; SAMA=short-acting muscarinic receptor antagonist; LABA=long-acting β2-agonist; LAMA=long-acting muscarinic antagonist; NIMV=non-invasive mechanical ventilation; MDI=metered dose inhaler; DPI=dry powder inhaler.
During each follow-up visit, the following events will be recorded: ischemic cardiovascular event, cardiovascular accident, cardiac arrhythmia, pulmonary embolism, cancer and its type. A detailed description of exacerbations including the etiology, severity and treatment will also be noted. Instances in which a patient is lost to follow-up or chooses to withdraw from the study will be recorded. Vital status and the cause of death will be specified with the date and coded as: respiratory, cardiac, lung cancer or other, and other causes. If the patient undergoes lung transplantation due to their condition, it will be reported, marking the conclusion of the patient's participation in the study.
It is important to highlight that there is no specific protocol for conducting additional tests; however, those performed as part of routine clinical practice will be registered.
Data review and database managementThe eCRF is provided with filters for plausible ranges in numerical data. Missing data will generate queries to the investigators. Each researcher involved will be granted access to the eCRF and their patients’ records. The SPOCCAT Scientific Committee will have comprehensive access to all the information for quality control purposes.
The services of a data manager will be enlisted to enhance communication with researchers, manage queries that may arise, maintain the database, and ensure quality control. The data manager will contact the researchers for reminders or corrections, as necessary, by email or telephone call. In addition, the data manager will periodically send researchers an email with an update on case recruitment and the status of the project.
EthicsThe study protocol has received approval from the Research Ethics Committee of the Hospital Universitari Mútua de Terrassa, as the reference center, and also from the ethics committees of the remaining participating centers. All participants will be requested to provide written informed consent to be enrolled in the study. The SPOCCAT study will adhere to the principles outlined in the Declaration of Helsinki for research projects. All participants will receive information about the nature and objectives of the study and will provide consent to participate through an informed consent process.
Data will be collected in an anonymized (eCRF), with each participant assigned a unique identifier. No personal data that could identify individuals will be recorded. The data obtained will be maintained with strict confidentiality in accordance with the regulation (EU) 2016/679 of the European Parliament and of the Council of April 27, 2016 on General Data Protection Regulations, along with the Organic Law 3/2018, of December 5, on the Protection of Personal Data and guarantee of digital rights. Only the designated investigator at each center will have access to the data.
DiscussionThe SPOCCAT study has been designed with the objective of filling the gaps in the knowledge about the characteristics, patterns of treatment and prognosis of patients with severe COPD. This study originated from the interaction among pneumologists working in day hospitals caring for severe COPD patients and has evolved into a highly ambitious project, which currently involves a total of 31 investigators across the entire Catalan territory and Andorra. SPOCCAT has the potential to enhance our understanding of the subgroup of severe COPD patients, encompassing the evaluation of innovative aspects, such as non-pharmacological interventions, radiological observations, and chronic bronchial infection. This will facilitate the characterization of the long-term trajectory of these patients, enabling the investigation of predictive factors and diverse clinical outcomes.
The SPOCCAT study has been developed considering previous successful models, such as the CHAIN Spanish cohort11 and the EARCO (European Alpha-1 antitrypsin deficiency Research Collaboration) International Registry,25,26 which is following +2500 patients from 90 centers in 24 countries and is currently in its fourth year of follow-up. We have replicated this structure with an eCRF that collects the most relevant information obtained in routine clinical visits and organizing yearly follow-ups that will collect information about changes in comorbidities, clinical characteristics, treatments, number, severity and characteristics of exacerbations, and eventual lung transplant or death. With this information, we aim to address the remaining gaps in severe COPD, employing a novel and contemporary approach, covering areas that other cohort studies have not explored to date. In addition to the multidimensional assessment previously described in cohorts such as CHAIN,11 various distinct features of COPD will be evaluated. This includes the coexistence of asthma, pharmacological and non-pharmacological treatment, the presence and type of chronic bronchial infection, the etiology of exacerbations, as well as routine complementary tests, such as chest CT, blood analysis, and respiratory function tests.
With this study, we aim to offer an intricate overview of the standard clinical procedures involved in managing patients with severe COPD. The prospective and multicenter design, combined with a substantial number of participants, will enable comprehensive investigation into the progression of these patients, facilitating the identification of risk factors and diverse clinical trajectories. Moreover, it will allow comparative analysis of this cohort with existing cohorts, potentially fostering collaborations on a national and international scale.
One of the most important objectives of the SPOCCAT study, that is not listed in the protocol, is the construction of a network of researchers interested in severe COPD that may participate in other initiatives in the future. The SPOCCAT database should be the basis of a collaboration that may be extended to other smaller derived protocols aimed at investigating particular aspects of patients with severe COPD.
The main limitation of this study is common to other cohorts of COPD patients and is related to the complexity involved in collecting data on such a heterogeneous disease. The collection of even more detailed and exhaustive data had to be sacrificed to make the project feasible in different clinical settings. Nevertheless, we firmly believe that all the dimensions of the disease are very specifically represented. The second limitation is the absence of a protocol for conducting complementary tests such as blood tests, respiratory function tests or chest CT scans, due to the non-interventional design of the study. Therefore, we may not have complete information on all patients or tests performed with the same frequency during follow-up. However, since these are patients with severe or very severe COPD, it is highly likely that such tests have often been performed in routine clinical practice, as recommended in guidelines.27,28 To date, we have not planned the collection of biological samples in a biobank for molecular studies, but this will be a future objective after securing funding. Lastly, we employed the fixed ratio for diagnosing COPD primarily to streamline the process, thereby enhancing its applicability. Nonetheless, we have collected data on variables such as age, sex, height, and race for each patient, which will facilitate the application of Global Lung Initiative (GLI) equations29 in future analyses.
ConclusionsIn conclusion, SPOCCAT is a multicenter observational study of a prospective cohort aimed at gaining deeper understanding of the natural history of patients with severe COPD. The context of routine clinical practice will provide a wealth of relevant information to advance our knowledge of this condition. Furthermore, it should contribute to the establishment of a robust structure of researchers, fostering the development of numerous future projects.
FundingThe SPOCCAT study has been funded in part by an unrestricted grant from the Fundació Catalana de Pneumologia (FUCAP).
Authors’ contributionsR. Costa is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to publication of the article. JG, DS, DRM, EM, MM, and RC were responsible for the conception, design, and drafting of the manuscript for important intellectual content.
Conflicts of interestMarc Miravitlles has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Menarini, Kamada, Takeda, Zambon, CSL Behring, Specialty Therapeutics, Janssen, Grifols and Novartis, consulting fees from AstraZeneca, Atriva Therapeutics, Boehringer Ingelheim, BEAM Therapeutics, Chiesi, GlaxoSmithKline, CSL Behring, Ferrer, Inhbrix, Menarini, Mereo Biopharma, Spin Therapeutics, Specialty Therapeutics, ONO Pharma, Palobiofarma SL, Takeda, Novartis, Novo Nordisk, Sanofi, Zambon, Zentiva and Grifols and research grants from Grifols.
Roser Costa has received speaker fees from AstraZeneca, Chiesi, GlaxoSmithKline, Zambon, Bial and Novartis.
Elena Miguel has received speaker fees from Glaxo SmithKline, AstraZeneca, Chiesi, Teva, Boehringer Ingelheim, Bial and Gebro.
Daniel-Ross Monserrate has received speaker fees from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Faes Farma, Gebro, GlaxoSmithKline, Menarini, Novartis, Pfizer, Sanofi, TEVA and Zambon.
Dan Sánchez Berenguer has received speaker fees from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Faes Farma, Gebro, GlaxoSmithKline, Menarini, Novartis, Orion, Pfizer, Sanofi, Teva, and consulting fees from AstraZeneca, Bial and Gebro.
Jessica González has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini and Novartis.
Principal investigator: Roser Costa; Scientific Committee: Dan Sánchez, Elena Miguel, Jessica González, Daniel Ross-Monserrate, Marc Miravitlles.
Investigators: Cristina Aljama, Miriam Barrecheguren and Galo Granados (Hospital Universitari Vall d’Hebron, Barcelona); Nestor Soler (Hospital Clínic, Barcelona); Alicia Marin and Laura Rodríguez (Hospital Germans Trias I Pujol, Badalona); Claudia Guevara and Nuria Rodríguez (Consorci Sanitari Alt Penedés Garraf); Pilar Ortega (Hospital de Mataró); Luis Adolfo Urrelo (Hospital de Tortosa Verge de la Cinta Tortosa) Juan Antonio Lloret (Hospital de Viladecans); Sergi Pascual (Hospital del Mar, Barcelona); Minerva Sofía Ramírez (Hospital del Vendrell, Tarragona); Raquel Viana (Hospital El Pilar); Sònia Belda and Marc Bonnin (Hospital Josep Trueta i Sta., Caterina, Girona); Núria Bruguera (Hospital Moisès Broggi); Víctor Núñez (Hospital Nostra Senyora de Meritxell, Andorra); Noelia Pablos (Hospital de Martorell); Annie Navarro (Hospital Mutua de Terrassa); Eduardo Antonio Vélez and Josep Albert Martos (Hospital Sant Rafael, Barcelona); Irene López (Hospital Santa Tecla Tarragona); Fernando Ruíz (Hospital Universitari de Vic); Danny Zaya (Hospital Universitari Sagrat Cor, Barcelona).