In Spain, 2 million people are treated for obstructive sleep apnoea. Continuous positive airway pressure, the gold-standard therapy, requires regular follow-up and periodic evaluation of the efficacy of the treatment via a titration examination, i.e. autoCPAP test. Telemonitoring use is increasing and this study aims to evaluate the cost impact of its use for therapy evaluation instead of the standard ambulatory autoCPAP test.
Material and methodsThis prospective observational study includes 100 OSA patients under CPAP therapy who volunteered to test telemonitoring as an alternative therapy control tool. Costs for both the patients and the Sleep Unit were calculated and compared for the standard of care (ambulatory autoCPAP (SoC)), vs alternative telemonitoring option (TM).
ResultsMore than half (54%) of the patients preferred the TM option vs only 47.5% of the SoC patients. Patients inclining towards telemonitoring option were mainly reported to be more than 10 years youngers, mainly active workers (63%), travelling more distance to the Sleep Unit (16 vs 8km) and spending more expenses in travel than those who preferred SoC (median 30€). 29% of active workers left their jobs to attend the SoC. The costs related to the use of the Sleep Unit resources were found to be lower in the TM option compared to the SoC option (0.47 vs 3.09 euros per patient attended).
ConclusionsThe use of TM for follow-up CPAP therapy enables the patient to save travel costs and to reduce absenteeism but also to save assistential burden and therefore to reduce the Sleep Unit workload and optimize the care activity.
Obstructive sleep apnoea syndrome (OSAS) is a prevalent chronic pathology with a significant impact on health characterized by the occurrence of recurrent episodes of partial or complete limitation of airflow during sleep, resulting from an anatomical and/or functional alteration of the upper airways leading to its collapse.1 Repeated episodes can result in oxygen desaturation and sleep fragmentation.1 Daytime sleepiness, loud snoring, and gasping or choking sensation during sleep are part of the patient's experience. OSAS also favour the development of cardiac, neurologic or psychiatric disorders but also lead to occupational and traffic accident, impaired quality of life and excess mortality.
In Spain, 5–7 million people suffer from OSAS, with approximately 2 million presenting significant symptoms and requiring a treatment.2 Moreover, studies have shown that untreated patients consume two to three times more healthcare resources than the general population,3 designing OSAS a major public health issue.
The gold-standard therapy for OSAS is the administration of continuous positive airway pressure (CPAP) during the sleep to circumvent apnoea/hypopnea events that have been widely shown to have beneficial effects such as symptoms reduction, quality of life improvement and lowering of the comorbidities.2,4 Treatment initiation as soon as possible is crucial to ensure its best efficacy and to regularly monitor the control of the disease and the compliance over time.5,6
Indeed, some patients may not be fully aware of OSA associated consequences and that CPAP therapy, as a chronic or indefinite treatment, come with some unpleasant characteristics (noise, pressure applied, etc.) which can result in a poor compliance from the patients.7
Some CPAP devices allows to record parameters and to objectively measure the level of control achieved with the treatment. AutoCPAP devices adjust the optimal pressure level to apply in order to eliminate a maximum of the respiratory events and lower the residual apnoea/hypopnea index (AHIr). Unintentional air leak in the circuit is also measured as they can diminish the effectiveness of the therapy and be a factor of discomfort for the patient.
Telemonitoring (TM) is defined by digital/broadband/satellite/wireless transmission of physiologic and other non-invasive data to the healthcare professional (HCP) and is widely used to manage sleep disordered breathing.8,9 CPAP telemonitoring systems are able to record the same parameters than the autoCPAP (such as AHIr, leakage and treatment compliance) but also to directly transmit them to healthcare professional through a reliable secure and private web platform. Numerous studies demonstrated its efficacy and cost-effectiveness in improving patient compliance to the therapy, notably through message feedback.10,11 Moreover, TM allows to increase the number of patients followed, anticipate adverse effect, reduce consultation times and reduce the costs (maintenance visits and travels associated), without compromising the treatment effectiveness.12 Studies have shown that compliance and treatment success was improved in the first 30 days of treatment in patients that initiates CPAP with TM.13
Typically, in our Sleep Unit, the patients diagnosed with OSAS who require CPAP treatment are monitored by clinical assessment and recording of an autoCPAP test (autoSet S9, ResMed®). This is the standard of care (SoC) method. The patient come to the Sleep Unit to pick up the autoCPAP device and bring it home for the 2 or 3 nights of test and then bring it back to the Unit to have its data analyzed by the physician. Therefore, the SoC pathway involves some costs for both the patient (travel to the Unit and possible need for a day off at work) and the Sleep Unit (time and resources for the autoCPAP record analysis using ResScan®). To note that the CPAP devices (autoSet S10, ResMed®) usually supplied to patients for their day-to-day therapy have an integrated telemonitoring system (TM) that records continuously monitoring data (such as AHIr and leaks) and compliance. This data is available from Airview® platform through the home care provider platform (Oxinet®). The ResScan® and Oxinet® systems use the same algorithms to analyze and show data. Ergo, the telemonitored CPAP could be used to monitor the patient instead of performing the autoCPAP test in the hospital.
This study aims to evaluate the impact on cost of a telemonitoring system as a tool for monitoring CPAP treatment compared to the standard of care, i.e. ambulatory autoCPAP titration in the hospital. Telemonitoring does not replace the SoC's ability to titrate CPAP pressure, which can still be performed if necessary.
Material and methodsStudy designThis prospective observational study was performed at the Respiratory Sleep Disorders Unit in compliance with the local ethic committee.
The recruitment was made among the Unit patient's that already followed the SoC pathway. As a result, each patient firstly underwent a SoC autoCPAP test, and was later proposed to be monitored using the TM feature of their own CPAP device for their annual titration. After having underwent the two pathways, the patients were asked about their preferences between these two options. The inclusion criterion was to grant consent to participate voluntarily and anonymously. The exclusion criterion was not granting this consent. No randomization and no controlled selection were made.
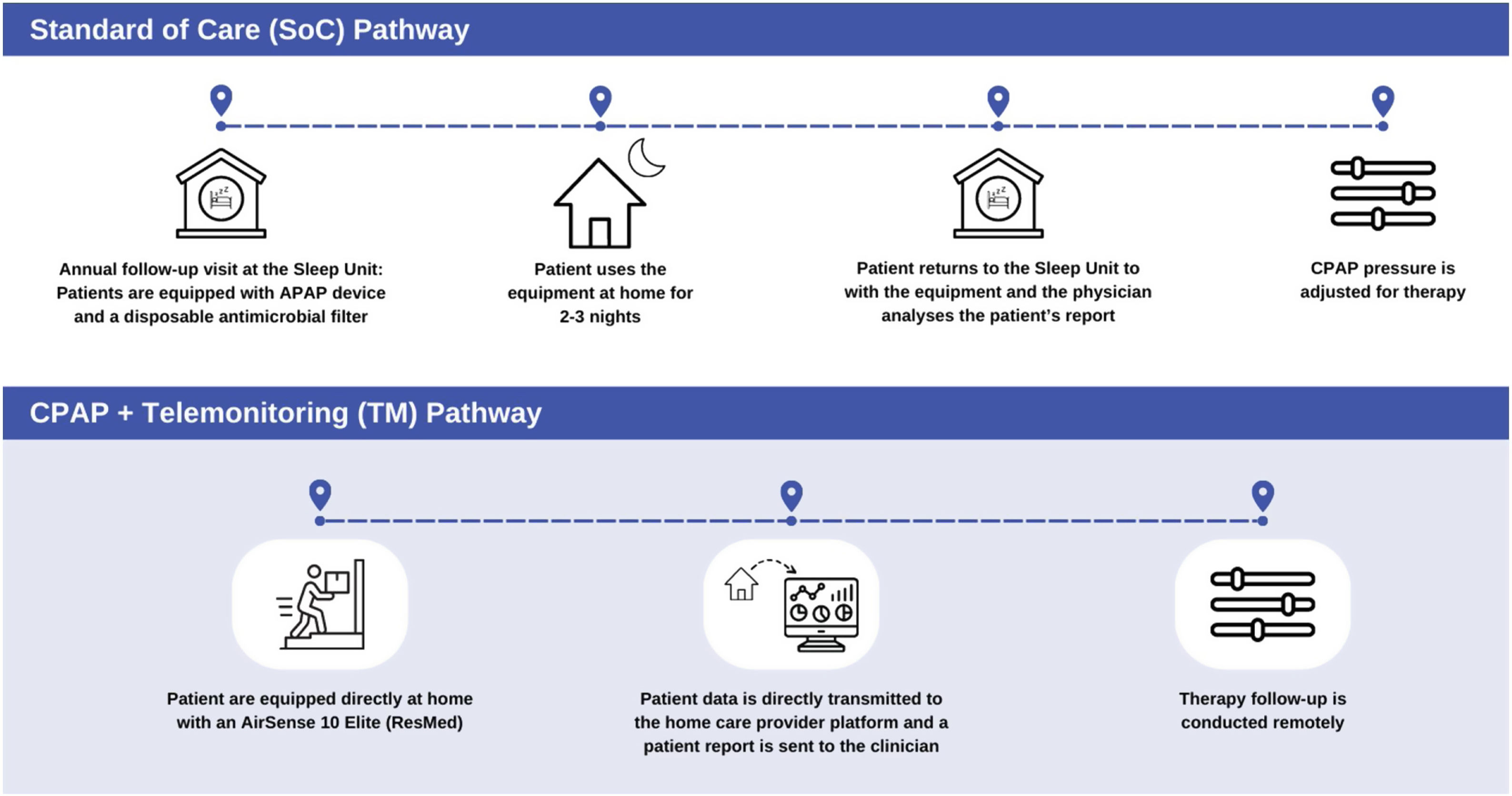
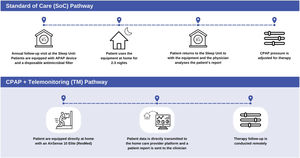
The SoC and TM pathways are explained in Fig. 1.
SoC and TM pathways. SoC pathway: during their follow-up visit, patients are equipped with an autoCPAP device (autoSet S9, ResMed®) and provided a disposable antimicrobial filter (Clear-Guard II Breathing Filter, Intersurgical®). When returning to the Sleep Unit the patients’ data are analyzed using the ResMed® proprietary software (ResScan™). TM pathway: patients’ follow-up is performed using their own AirSense 10 Elite (ResMed®) which directly transmitted the data through AirView™ to the home care provider web platform (Oxinet®). SoC: standard of care; TM: telemonitoring.
Baseline values for each patient were collected through their medical records: demographic and anthropometric data, baseline AHI, baseline oxygen desaturation index (ODI) and baseline CT90 (percentage of time in which the oxygen saturation was below the normal saturation level).
The clinical data presented in the patient's SoC report (using the ResCan® software) included AHIr, unintentional mask leakage (litres per minute, lpm) and the number of hours of CPAP use. The same data are provided by the TM devices, the main difference being that the data are directly available on the Oxinet® platform.
OSAS is considered well controlled if AHI <5. Good compliance with treatment is defined by device use >4hours per night on >70% of the indicated nights. Regardless of mask type (nasal, oronasal), mask leakage was considered as acceptable if below the 95th percentile of leakage (P95 leakage), i.e. 24 litres per minute.14 No attention has been paid to the differences between mask types.
CostsThe SoC test implied costs related to the use of resources by the patient such as means of transport, distance travelled, total time spent in the journey and stay in the Sleep Unit, need for a companion, absenteeism from work and related costs. To report this information, patients that performed the SoC were asked to fill a questionnaire to collect data regarding costs, affiliation, self-reported residual sleepiness using the Epworth Sleepiness Scale (ESS) and degree of satisfaction using an analogue scale (VAS) ranging from 0 (worst) to 10 (best score). The TM pathway does not involve costs to the patient.
Costs for the Sleep Unit were also collected for both options. SoC costs derived from the consumption of healthcare resources, analysis process, preparation and interpretation of the autoCPAP test report in the Sleep Unit has been established at 0.0572€ per hour for an autoCPAP test (device price included) plus 2.25€ for the disposable antimicrobial filter provided. Telemonitoring direct costs are related to the maintenance costs (autoSet S10, ResMed® and Oxinet® platform) requested from the home care provider, established at 0.0325€ per hour.
To note that for both ways (SoC and TM), the cost of HCP (doctors, nurses, auxiliary nurse) has not been taken into account as they are permanent staff of the Sleep Unit. However, the time spent on the analysis and reporting of the autoCPAP test and TM therapy has been analyzed.
Statistical analysisA descriptive and comparative analysis of the results obtained were carried out using parametric or non-parametric tests, as appropriate for qualitative and quantitative variables (Kolmogorov–Smirnov). Chi-square tests, Student's t-test and U-Mann–Whitney tests were used for the analyses and values with p<0.05 were considered statistically significant. The analyses were run using the SPSS v20 software.
Ethics statement relatingThis research has been approved by the Valladolid Health Area Drug Research Ethics Committee (CEIm ÁREA DE SALUD VALLADOLID ESTE) at a meeting held on November 21, 2019. Registration number: PI 19-1528 TFG.
The authors of this research have complied with the relevant ethical standards for publication.
The authors of this research have complied with the Spanish regulations related to the data protection law: Organic Law 3/2018, of December 5, on the Protection of Personal Data and guarantee of digital rights.
The authors of this research have the implicit informed consent of the patients who gave their permission to participate freely and voluntarily in this study.
This research work does not include a clinical trial and no drugs have been used during it.
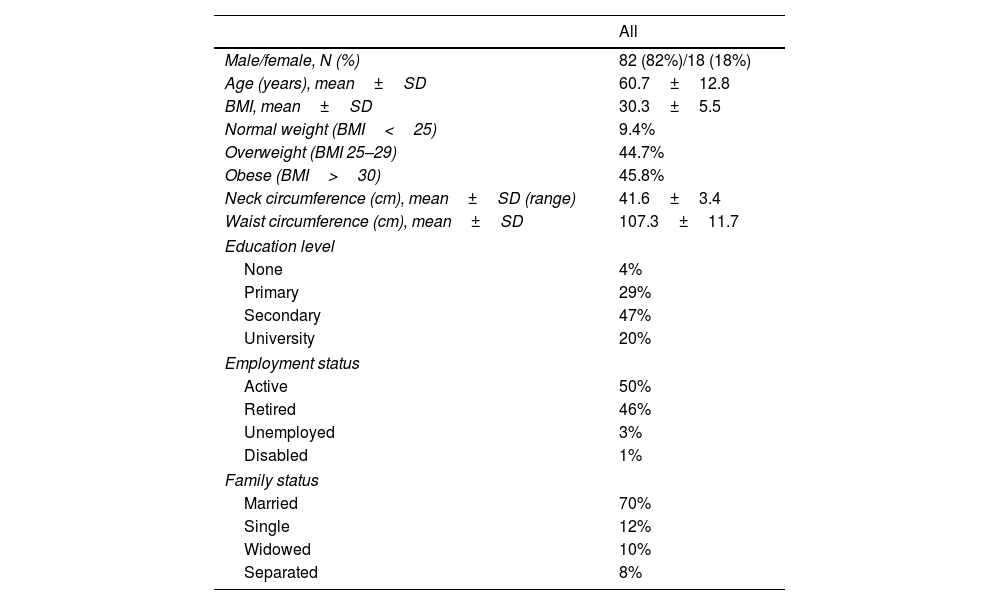
ResultsOne hundred patients were recruited for this study and their characteristics are detailed in Tables 1 and 2.
Patients’ characteristics (I).
| All | |
|---|---|
| Male/female, N (%) | 82 (82%)/18 (18%) |
| Age (years), mean±SD | 60.7±12.8 |
| BMI, mean±SD | 30.3±5.5 |
| Normal weight (BMI<25) | 9.4% |
| Overweight (BMI 25–29) | 44.7% |
| Obese (BMI>30) | 45.8% |
| Neck circumference (cm), mean±SD (range) | 41.6±3.4 |
| Waist circumference (cm), mean±SD | 107.3±11.7 |
| Education level | |
| None | 4% |
| Primary | 29% |
| Secondary | 47% |
| University | 20% |
| Employment status | |
| Active | 50% |
| Retired | 46% |
| Unemployed | 3% |
| Disabled | 1% |
| Family status | |
| Married | 70% |
| Single | 12% |
| Widowed | 10% |
| Separated | 8% |
BMI: body mass index; cm: centimetres.
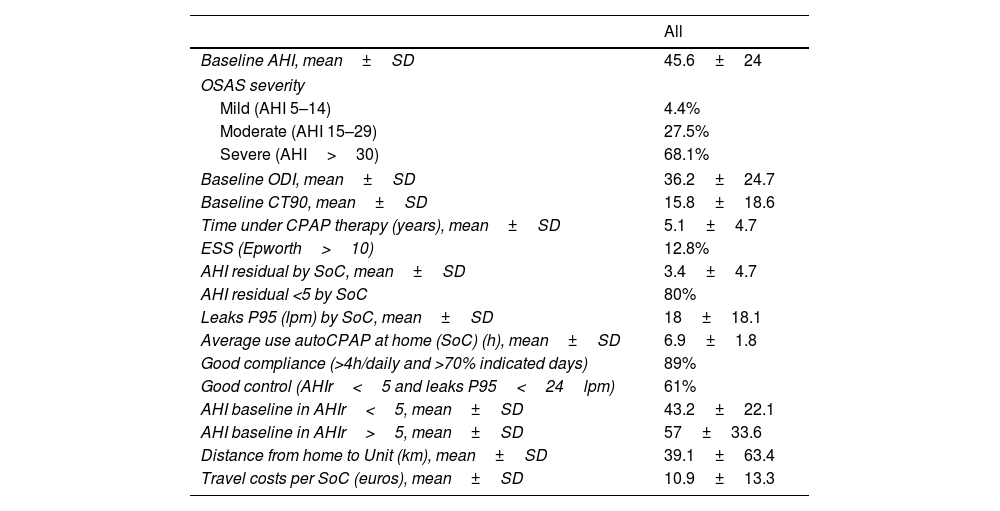
Patients’ characteristics (II).
| All | |
|---|---|
| Baseline AHI, mean±SD | 45.6±24 |
| OSAS severity | |
| Mild (AHI 5–14) | 4.4% |
| Moderate (AHI 15–29) | 27.5% |
| Severe (AHI>30) | 68.1% |
| Baseline ODI, mean±SD | 36.2±24.7 |
| Baseline CT90, mean±SD | 15.8±18.6 |
| Time under CPAP therapy (years), mean±SD | 5.1±4.7 |
| ESS (Epworth>10) | 12.8% |
| AHI residual by SoC, mean±SD | 3.4±4.7 |
| AHI residual <5 by SoC | 80% |
| Leaks P95 (lpm) by SoC, mean±SD | 18±18.1 |
| Average use autoCPAP at home (SoC) (h), mean±SD | 6.9±1.8 |
| Good compliance (>4h/daily and >70% indicated days) | 89% |
| Good control (AHIr<5 and leaks P95<24lpm) | 61% |
| AHI baseline in AHIr<5, mean±SD | 43.2±22.1 |
| AHI baseline in AHIr>5, mean±SD | 57±33.6 |
| Distance from home to Unit (km), mean±SD | 39.1±63.4 |
| Travel costs per SoC (euros), mean±SD | 10.9±13.3 |
SoC: standard of care; AHI: apnoea hypopnea index; ODI: oxygen desaturation index; ESS: Epworth Sleepiness Scale; lpm: litres per minute.
Significant differences were observed between gender such as the mean age (59.2 years for men vs 67.8 years for women; p<0.009), mean baseline AHI (40.9 in men vs 67.6; p<0.001), mean baseline ODI (32.2 in men vs 55.5; p=0.018) and mean BMI (28.7 for men vs 31.6; p 0.012). No other gender differences were significant. No significant differences were observed regarding leakage (19.1 vs 12.6lpm; p=0.169), home/hospital distance (38.7 vs 40.9km; p=0.891) and cost of travel (10.9 vs 10.7; p=0.961). To note that the type of interface (nasal or oronasal) was not detailed in this study as the leak limit is the same for all ResMed interfaces used.
Treatment compliance and therapy control by SoCAt the start of the study, under the SoC pathway, 89% of the patients presented good compliance (use >4h/daily and >70% of the days) with a daily average of 6.9±1.8h (range: 0.4–11). The mean residual AHI was 3.4±4.7 (range: 0–22.4) and the mean P95 leak was 18±18.1 litres per minute (range: 0–104), demonstrating an adequate therapeutic control with the SoC.
Patients with AHIr <5 were found to present lower baseline AHI compared to those with uncontrolled residual AHI (AHIr>5) (43.2 vs 57; 95% CI 0.5–27.2; p 0.042) mainly within the men (Table 2). 61% of the patients demonstrated a good therapeutic control (i.e. AHIr<5 and leak P95<24 litres per minute). Residual sleepiness score, evaluated using the ESS >10, was observed in 12.8% of the patients.
Patients’ monitoring preference and costs pertained to the patientsThe mean level of satisfaction (VAS) of patients performing the SoC was high (9.73±1.28 points at the device collection) (data not shown). However, only 20% of the patients described the autoSet S9® device (SoC) as more comfortable than their actual device (AirSense 10®).
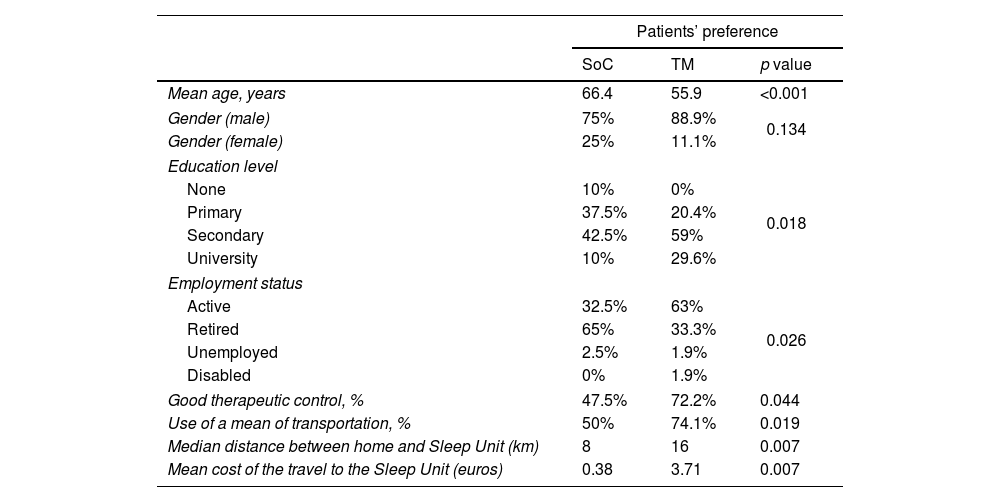
Regarding the monitoring and follow-up pathway, 54% of the patients preferred TM. Table 3 presents the distribution of the patient's preference between SoC and TM. 79.6% of patients preferring TM had a secondary-university education (p=0.018) and 63% were active workers while 65% of those preferring the SoC option were retired (p=0.026). The reason that the patients evoked to justify their preferences are presented in Table 4. About reasons for which patients preferred TM over SoC, we found that those wanting to avoid transfers were younger than those who did not (56.9±63.2 years; p=0.017). The patients that find TM more comfortable than SoC were older (61.7±52.4 year's old; p=0.029). Patients willing to avoid time off work performing SoC were younger (52.1 vs 61.7 years; p=0.024). Within the patients who preferred TM to avoid expenses, there was a majority of retirees (67%, p<0.001) and people who have to involve a family member to go to the hospital (62%) (p=0.002). Patients who preferred TM to avoid expenses, spent on travel to perform SoC a median of 30€, p=0.043; data not shown).
Patients’ preference distribution between SoC and TM.
| Patients’ preference | |||
|---|---|---|---|
| SoC | TM | p value | |
| Mean age, years | 66.4 | 55.9 | <0.001 |
| Gender (male) | 75% | 88.9% | 0.134 |
| Gender (female) | 25% | 11.1% | |
| Education level | |||
| None | 10% | 0% | 0.018 |
| Primary | 37.5% | 20.4% | |
| Secondary | 42.5% | 59% | |
| University | 10% | 29.6% | |
| Employment status | |||
| Active | 32.5% | 63% | 0.026 |
| Retired | 65% | 33.3% | |
| Unemployed | 2.5% | 1.9% | |
| Disabled | 0% | 1.9% | |
| Good therapeutic control, % | 47.5% | 72.2% | 0.044 |
| Use of a mean of transportation, % | 50% | 74.1% | 0.019 |
| Median distance between home and Sleep Unit (km) | 8 | 16 | 0.007 |
| Mean cost of the travel to the Sleep Unit (euros) | 0.38 | 3.71 | 0.007 |
SoC: standard of care; TM: telemonitoring; km: kilometres.
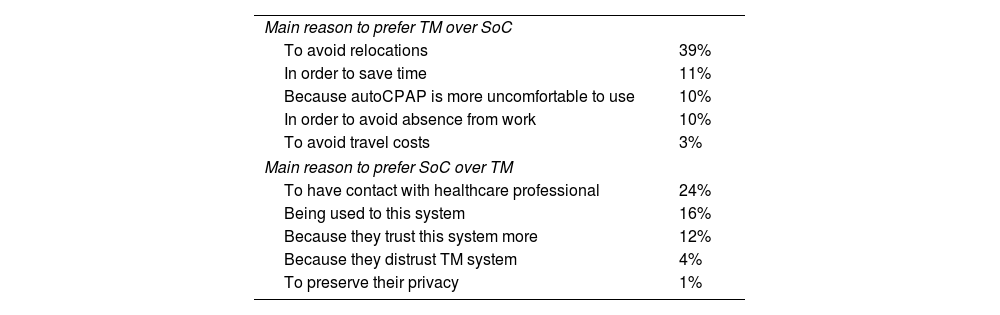
Patients’ main reason for choosing TM or SoC.
| Main reason to prefer TM over SoC | |
| To avoid relocations | 39% |
| In order to save time | 11% |
| Because autoCPAP is more uncomfortable to use | 10% |
| In order to avoid absence from work | 10% |
| To avoid travel costs | 3% |
| Main reason to prefer SoC over TM | |
| To have contact with healthcare professional | 24% |
| Being used to this system | 16% |
| Because they trust this system more | 12% |
| Because they distrust TM system | 4% |
| To preserve their privacy | 1% |
SoC: standard of care; TM: telemonitoring.
Patient's costs are only observed in the SoC procedure. They are related to the distance, time, amount spent, and absenteeism from work, to be able to attend the SoC visit to the hospital (Table 5). The patient himself came to pick up and return the autoCPAP device in 70% of the SoC performed. Among those, 47% used private transport, 12% public transport, 4% both private and public transport and 37% no transport. Among the 50% of the active patients, 29% had to be absent from work.
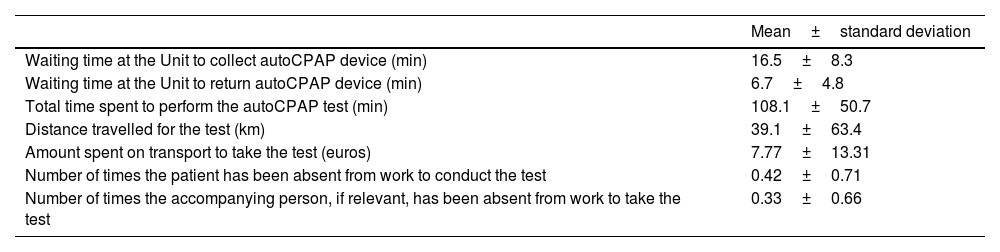
Average values of the patient's costs of performing SoC.
| Mean±standard deviation | |
|---|---|
| Waiting time at the Unit to collect autoCPAP device (min) | 16.5±8.3 |
| Waiting time at the Unit to return autoCPAP device (min) | 6.7±4.8 |
| Total time spent to perform the autoCPAP test (min) | 108.1±50.7 |
| Distance travelled for the test (km) | 39.1±63.4 |
| Amount spent on transport to take the test (euros) | 7.77±13.31 |
| Number of times the patient has been absent from work to conduct the test | 0.42±0.71 |
| Number of times the accompanying person, if relevant, has been absent from work to take the test | 0.33±0.66 |
SoC: standard of care.
The costs for the Sleep Unit derived from the consumption of healthcare resources of the SoC and those derived from the TM evaluation are detailed in Table 6.
The paired cost analysis performed found a significant difference between SoC and TM Unit's costs (3.09 vs 0.47 euros; p<0.001).
DiscussionThis study sample shows that females are older, more obese and severe OSAS than males, this is unusual and may be explained by a sample selection bias. Regarding the reliability of the survey responses, only 4% of the patients were uneducated and they have spent a mean of 5.1±4.7 years under CPAP therapy before the start of this study so it can be deemed that the patients have the necessary experience to answer the questions reliably.
Looking at SoC, the vast majority of the patients (89%) were good compliers, with an average daily use of 6.9±1.8h, which can be explained by the improvement of not only sleep symptoms but also quality of life of the patients, therefore increasing compliance to therapy.15 Interestingly, patients with the good compliance were those that presented significantly lower baseline AHI and ODI compared to patients with poor compliance. This finding is not consistent with previous studies on compliance that demonstrated that generally the best compliance is observed in patients with the most severe symptoms as they experience greater improvement.3
Only 12.8% of the patients presented residual sleepiness (ESS>10), which is lower than the scores observed in previous studies who had significant improvement with ESS scores between 11 and 14 points.16,17 This finding can be explained by the good therapy control showed in our sample.
Our study showed that half of the patients (54%) preferred TM over SoC to monitor their therapy control, mostly to avoid travel (39%), and save time (11%). Previous studies showed similar results, with a majority of patients inclining towards TM while almost 40% considering this method as intrusive and less trustworthy than the standard monitoring method.18
Age seems to be a determining factor in the choice of TM over SoC. Most of the patients in this situation were mostly younger, active workers, educated people, persons that need means of transportation, travellers from a greater distance (16km on average) and people that spent a mean of 3.71€ per visit. Those patients also demonstrated to have a good therapeutic control of their OSAS. Telemonitoring would save patients time, money and avoid to have time off work. The patients that preferred SoC were older (mainly retirees) and did not encounter the same disadvantages as younger people to go to the Sleep Unit to perform tests. They travelled less distance (mean=8km) and they supported less expenses (mean=0.38€). It is clear that SoC pathway have more impact on young active patients.
To conduct the SoC procedure, more than two thirds of the patients went to the Sleep Unit by their own means to collect and return their devices, using a means of transport. Indeed, patients financed their travel themselves, with an average of 2h of transport for a mean cost of 8€, corresponding to 0.19€ spent per km. The Regional Management of Healthcare Government Authority may in some cases fund a mean price of 0.98€ per km, which is higher than the price observed in this study but overall, this expense is passed on to the patient.19 We found a high absenteeism from work, reaching 29%, which have to be considered as a cost even if no studies were found referring to this. Therefore, performing SoC for therapy control implies not only a waste of time but also expenses, either personal or economic (e.g. absenteeism from work), which can explain why certain patients have preferred TM for their follow-up.
One limitation of our study is that the patients are used to their own CPAP device (AirSense 10, ResMed®) while the device used for the autoCPAP test (SoC) is different (AirSense 9, ResMed®), which may be assumed as a cause of bias but it is not expected to have a real impact on the results as there is no significant differences on the features of these two devices, except the fact that the AirSense 10 enable telemonitoring.
Our findings on the differences between the direct material costs of SoC vs TM to monitor therapy control are in line with the results presented in the Turino et al. study,10 i.e. a total cost per patient 28% lower in the TM group compared to the SoC group. The main saving was made on travel costs and absenteeism as follow-up visits became useless with telemonitoring. Additionally, TM enabled the Sleep Unit staff time without negative impact on the treatment effectiveness or compliance.13 Finally, the running of a therapy control through SoC procedure test was more time consuming than therapy control through TM (45 vs 10min) highlighting that the implementation of TM would increase by four the potential number of patients assessed in the same time. This is particularly relevant in the Sleep Unit in which TM could be used to reduce the workload pressure and optimize the care activity. If patients had been cared by telemonitoring, based on their residual AHI, only 20% of them would have needed to undergo SoC. This study provides keys to the Sleep Unit to improve their cost-effectiveness.
Finally, in the last context of pandemic, the Spanish Society of Pneumology and Thoracic Surgery (SEPAR) conjointly with the Spanish Sleep Society (SES) published a consensus document on the Sleep Units activity COVID-19 and prioritizing telemedicine whenever possible, reinforcing the use of TM to manage OSAS patients.20
In conclusion, more than half population of patients in this study was in favour of TM, notably the younger individuals. The SoC as therapy follow-up has an economic impact on patients and even affects the work absenteeism. Telemonitoring enables to avoid both. Sleep Unit activity would be optimized by the use of TM for patient's follow-up saving direct costs in materials and work time. Additionally, TM allow patient's follow-up without exposing either patients or HCPs to possible sources of contamination, for example COVID-19.
FundingThis study has not received funding. It has been conducted with the own resources of the Respiratory Sleep Disorders Unit. ResMed provided medical writing support for this article. ResMed is a funding organization willing to bear the APC costs associated with the publication process, if necessary.
Authors’ contributions- •
Santiago Antonio Juarros Martínez has contributed as principal investigator in the original idea, in the design of the study, in the statistical data analysis and in the writing of the manuscript.
- •
María Del Pilar Andrés Porrase has contributed as a collaborating researcher in the fieldwork for data collection.
- •
Milagros Del Olmo Chiches has contributed as a collaborating researcher in the fieldwork for data collection.
- •
María Isabel Muñoz Diez has contributed as a collaborating researcher in the fieldwork for data collection.
The authors declare that they have no conflicts of interest in relation to this work.
Thanks to Ana Mayoral Aguilera (Medical Director of Oxigen Salud SL) for supporting data collection from telemonitored therapy devices, Silvia Arribas Santos (Nurse of Oxygen Salud SL) for support on patient recruitment, and Sara Correia (Business and Market Access Developer for Spain and Portugal, ResMed) for supporting this manuscript.
Thank you also to ICSCYL (Fundación Instituto de Estudios de Ciencias de la Salud de Castilla y León. Spain) for their institutional collaboration.