Pulmonary hyalinizing granuloma (PHG) was first described by Engleman et al. in 1977, it is a benign fibro-sclerosing pulmonary condition consisting of concentric hyaline lamellae that could present as multiple, bilateral, or mildly symptomatic lesions, showing characteristics of amyloid or atypical birefringence patterns. It can present at any age, typically with non-specific symptoms and usually has an excellent prognosis with no malignant potential.1,2 Its etiology and pathogenesis remain unclear, and a definitive diagnosis is provided by histopathological study of the lesions describing hyalinized lamellar collagen bundles surrounded by plasma cells, lymphocytes, and histiocytes.3 Clinical manifestations with this disease can vary from non-specific such as cough, chest pain, breathlessness and fever, it is present incidentally in 25% of patients.
Where there are multiple randomly distributed pulmonary nodules, the differential diagnoses include: infection (tuberculosis, histioplasmosis, septic emboli and fungal infections), autoimmune causes (rheumatoid arthritis, granulomatosis with polyangiitis), granulomatous disease (sarcoidosis, lymphatoid granulomatosis and plasma cell granuloma) and more importantly, it can mimic a primary lung cancer or metastatic disease warranting due investigation and management.4
We present the case of a 76-year-old female patient, a non-smoker, with a relevant medical history, including arterial hypertension, hypercholesterolemia, and Sjögren's syndrome. She sought evaluation in the clinic due to the appearance of a polylobulated lesion in the upper right lobe. During the initial assessment, the patient reported sporadic coughing without expectoration and dyspnea classified as grade 2 on the Medical Research Council (mMRC) scale. There were no reports of hemoptysis or constitutional symptoms.
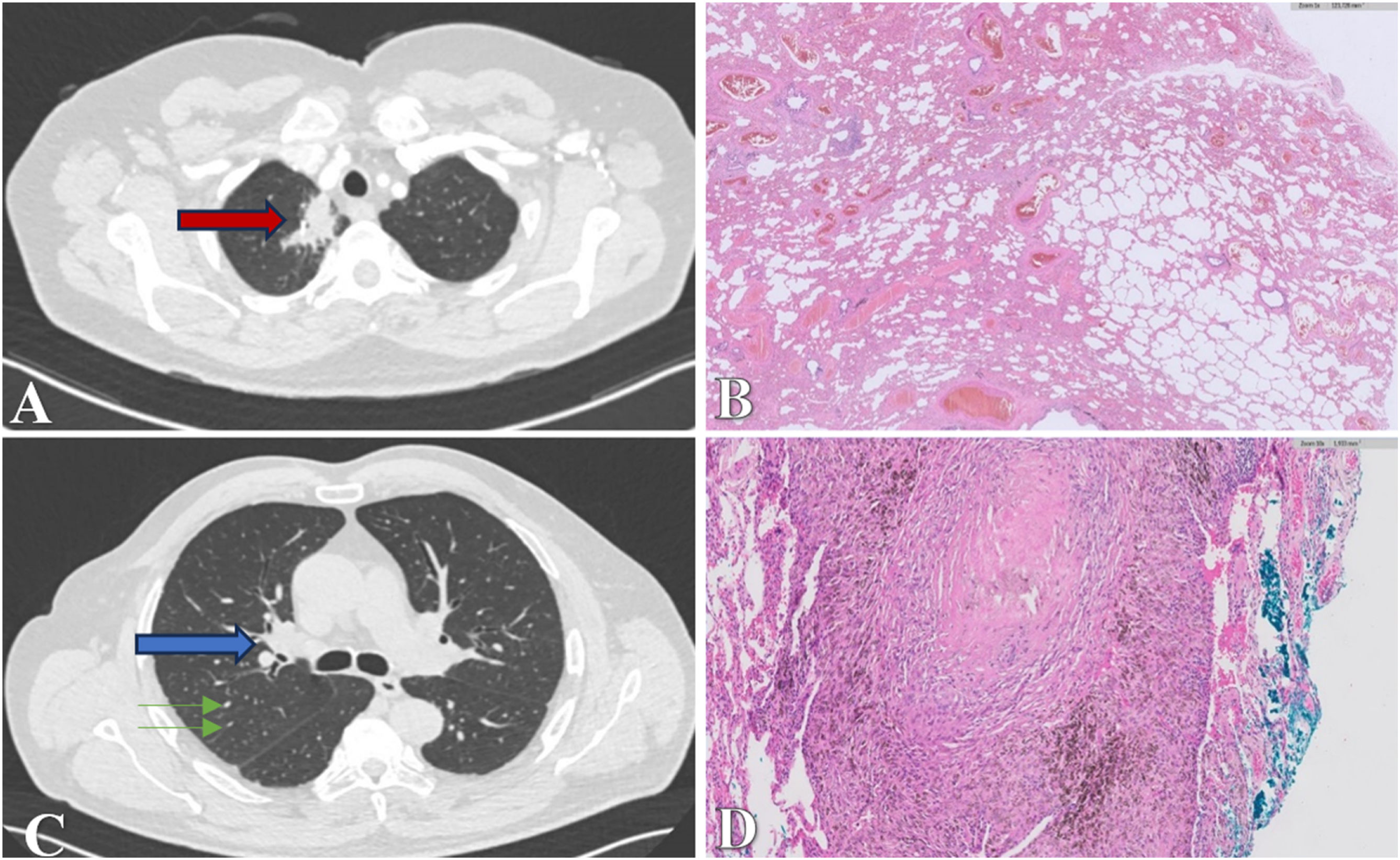
To characterize the nature of the lesion, a thoracoabdominal positron emission tomography-computed tomography (PET-CT) (Siemens Biograph mCT) scan was performed, revealing an apical mass within the medial region of the upper right lobe, measuring 17.7mm×34.4mm×33mm. This mass demonstrated a pronounced association with the pleural and mediastinal surfaces, yet no signs of infiltration were observed. Notably, the lesion showed elevated metabolic activity (maximum standardized uptake value, SUV max 2.92), raising suspicions of malignancy. For definitive characterization, a core needle biopsy (CNB) was conducted, yielding pleural fragments displaying a lymphoplasmacytic inflammatory infiltrate, alongside sparse fragmented alveolar spaces and an absence of cytological atypia.
Subsequent radiological assessments, conducted one month later during the lesion's follow-up, revealed intensified pathological fluorodeoxyglucose uptake (FDG) (SUVmax 4.7) (Fig. 1A), heightening suspicions of malignancy. Additionally, an increase in FDG uptake was detected in the right hilar lymph node (measuring 5mm with an SUVmax of 2.9), a finding not present in the previous study. In response to these findings, an endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) was performed. However, no suitable samples were obtained, as the visualized adenopathy in all accessible regions measured less than 4mm and displayed benign ultrasonographic characteristics. Additionally, a repeat CNB of the lesion resulted in pulmonary parenchyma samples characterized by fibrosis and a predominantly lymphomacrophagic inflammatory infiltrate.
Case one. (A) CT scan showing an apical mass within the medial region of the upper right lobe (red arrow). (B) Pathological analysis revealing extensive dense collagenous material accompanied by lymphoplasmacytic inflammatory cellularity, vascular space dilation without atypia, and hyalinized areas, consistent with pulmonary hyalinizing granuloma. Adjacent lung tissue displays distorted architecture due to a fibroinflammatory process, patchy distribution, and heterogeneous temporal appearance, consistent with usual interstitial pneumonia. Case two. (C) CT scan showing right hilar lymphadenopathies with partial calcification up to 15mm (blue arrow) and diffuse multilobar micronodularity (green arrows). (D) Pathological examination revealing subpleural hyalinized nodules surrounded by anthracotic pigment-laden macrophages without evidence of necrosis, consistent with pulmonary hyalinizing granuloma.
In light of the discrepancy between radiological progression and histopathological findings, persistent uncertainty regarding malignancy, and the growing distress of the patient, it was decided to proceed with the surgical resection of the pulmonary lesion through a right upper lobectomy performed with robot-assisted thoracic surgery (RATS). The pathological analysis of the resected specimen confirmed findings consistent with pulmonary hyalinizing granuloma, with adjacent lung tissue displaying characteristics in line with usual interstitial pneumonia (Fig. 1B).
The case was deliberated upon in a multidisciplinary clinical session, correlating the described pattern of usual interstitial pneumonia with tumoral involvement rather than diffuse interstitial pathology. The absence of radiological anomalies in the surrounding lung parenchyma, coupled with observed clinical and functional stability, suggests a low likelihood of diffuse interstitial lung disease as an underlying pathology.
During subsequent consultations following lobectomy, the patient maintained a consistent degree of dyspnea, reporting a grade 2 level on mMRC Scale, with no emergence of new symptoms or limitations in her usual activities.
The second case presented involves a 65-year-old male with occupational asbestos exposure, having worked in construction for 35 years. The patient reports being asymptomatic from a respiratory standpoint, with no weight loss or loss of appetite. The physical examination is unremarkable.
Functional tests, including spirometry, plethysmography, and diffusion, were conducted and showed normal results. A chest CT (Siemens SOMATOM Definition AS 128) scan revealed mediastinal and right hilar adenopathies, with diameters of up to 15mm, some displaying partial calcifications (Fig. 1C). Additionally, diffuse micronodules and ill-defined lingular pleuroparenchymal thickening were observed.
Subsequently, a PET-CT scan was performed, showing uptake in the hiliomediastinal adenopathies with SUVmax up to 5.9, suggestive of an active granulomatous process. There was also uptake with SUVmax 3.0 in a paravertebral pleuroparenchymal thickening in the lower right lobe.
Bronchoscopy with bronchoalveolar lavage in the middle lobe revealed no endobronchial findings. EBUS TBNA of lymph nodes 11L and 7 was performed. Bacilloscopy in bronchoalveolar lavage was negative, with no germ isolation. Notably, there was a lymphocytic cellularity with 34.87% lymphocytes, 64.8% macrophages, 0.3% neutrophils, and <0.01% eosinophils. The CD4/CD8 ratio was 3.31. The fine needle aspiration FNA of lymph nodes 11L and 7 was diagnostically satisfactory, negative for malignancy, with no visualization of opportunistic germs.
Considering the possibility of pleural malignancy, a biopsy was requested. Samples were obtained from the right lung, diaphragmatic implant, posterobasal, and parietobasal pleura. The pulmonary parenchyma exhibited extensive emphysematous changes, with subpleural fibrosis areas containing hyalinized nodules surrounded by anthracotic pigment-laden macrophages, and no evidence of necrosis. Additionally, an intense lymphoplasmacytic inflammatory infiltrate was observed (Fig. 1D).
Throughout the clinical progression, corticosteroid therapy was not administered to the patient. Notably, he remained asymptomatic from a clinical perspective, and there were no discernible changes in the postoperative imaging evaluations.
In conclusion, PHG represents a rare fibrosclerosing pulmonary condition that presents diagnostic challenges due to its resemblance to other nodular pulmonary disorders, including neoplastic and granulomatous processes. The cases presented underscore the importance of considering PHG within the differential diagnosis of pulmonary nodules or masses, particularly in individuals with atypical clinical presentations or radiological findings.5 As evidenced by the diagnostic journey of the patients described, the complexity of PHG necessitates a multidisciplinary approach involving pulmonologists, thoracic surgeons, and pathologists. Furthermore, these cases underscore the significance of a comprehensive literature review and collective clinical experience in managing such complex cases, underscoring the critical role of an accurate diagnosis in guiding treatment decisions and ultimately impacting patient prognosis. Continued research and collaboration are essential to further clarify the clinical characteristics, diagnostic modalities, and optimal management strategies for PHG.
Informed consentInformed consent was obtained from the patient for the publication of his clinical data and the use of diagnostic images.
FundingNo funding was received for this study.
Authors’ contributionsM. Carrasco Sánchez: Contributed to the design and implementation of the research, to the analysis of the results and to the writing of the manuscript.
J. Pavón Guede: Conceived of the presented idea and supervised the work.
B.A. Paz Fernández: Performed the anatomopathological study.
M. García-Salmones Martín: Performed bronchoscopy and supervised the work.
Conflicts of interestThe authors have no conflict of interest to declare.