Maternal folic acid (FA) supplementation is one of the most popular nutritional interventions during pregnancy for its protective effect against neural tube defects (NTDs).
The purposes of this review are: (a) to gather the current evidence regarding supplementation of maternal diet with FA and (b) to problematize the available literature in terms of dosages, critical temporal windows, and its potential benefits and risks.
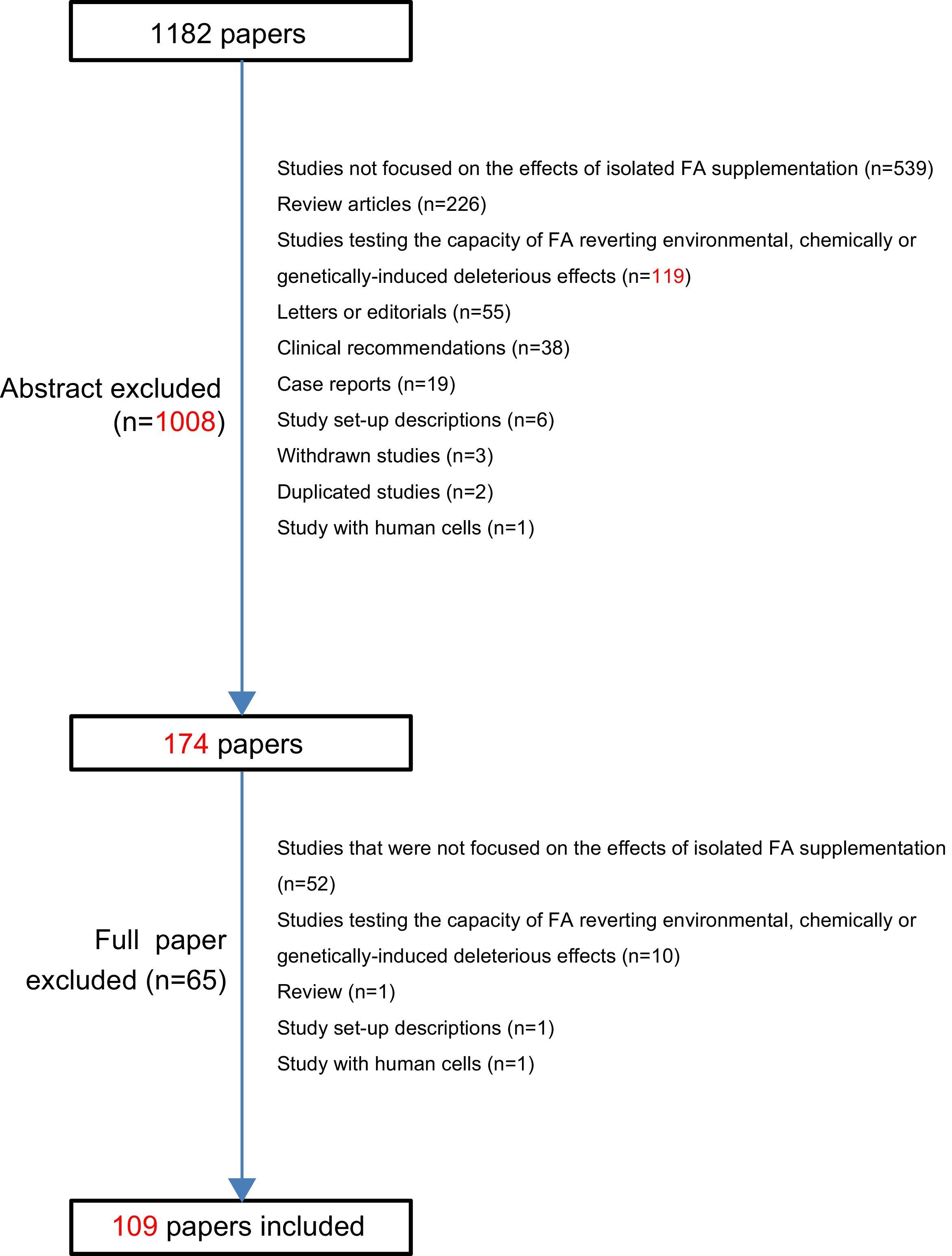
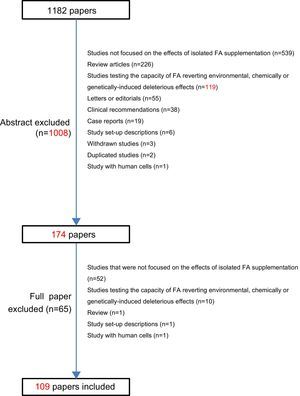
The expression (pregnancy OR fetus OR offspring OR mother) AND (“folic acid” AND supplementation) was searched on PubMed database, filtering for articles published from 2005 to 2014. Publications referring to FA supplementation during the periconceptional period or pregnancy in which there was a conclusion about the effects of isolated FA supplementation on pregnant woman, pregnancy or offspring were included. Of the initial 1182 papers, 109 fulfilled the inclusion criteria.
The majority of the publications reported FA supplementation outcomes on offspring's health, with emphasis in NTDs, allergy/respiratory problems, cancer and behaviour problems. Some inconsistency is observed on the impact of FA supplementation on different outcomes, except for NTDs. It is also visible an increased concern about the impact of excessive supplementation, either in terms of doses or exposure's duration.
In conclusion, there is a growing interest in FA supplementation issues. The protective effect of FA supplementation over NTDs has been confirmed, being the periconceptional period a critical window, and it is frequently suggested that allergy/respiratory outcomes arise from (excessive) FA supplementation particularly later in pregnancy. Further research on critical doses and time of exposure should be conducted.
Folate is a term that refers to a group of water-soluble vitamins of the complex B which are naturally found in foods such as leafy green vegetables, citrus fruits and liver. Folic acid (FA), the synthetic and completely oxidized form of folate, is used in vitamin supplements and in fortified cereal products.
Within the cells, folates act as cofactors in reactions which are determinant in cell division and cell maintenance, as well as in the regulation of gene expression through epigenetic mechanisms.1 Indeed folates are a source of s-adenosylmethionine, the main cellular methyl donor that modulates genome-wide methylation thus regulating the expression of genes. This is believed to be the process by which folates affect foetal programming. In addition, folates have a determinant role in the re-methylation of plasma homocysteine to methionine,2 for which blood folate levels correlate negatively with blood homocysteine levels.
Maternal FA supplementation is one of the most popular nutritional interventions during pregnancy and it has been extensively studied for its effect on neural tube formation and foetal growth3 as folate deficiency at conception and during pregnancy may lead to abnormal development.4
Particularly since the 1990s, when evidence emerged about the protective effect of FA supplementation against the recurrence and occurrence of neural tube defects (NTDs),5,6 there has been increasing concern about the role of periconceptional dietary supplementation and fortification strategies, as NTDs represents an important cause of morbidity and mortality.7
Many countries worldwide practice mandatory fortification of cereal grain products with FA, as a public health policy, and several EU countries maintain a voluntary fortification of different foodstuffs.8 In addition, the WHO preconizes the supplementation with FA, in the periconceptional period and pregnancy, with 0.4mg FA/day (together with 30–60mg iron), increasing up to 5mg/day for women at high risk of birth defects.9 Notwithstanding, the WHO also pointed out that the protection against NTDs only happens when the supplementation occurs until the fourth gestational week, while supplementation in other time periods is likely to be beneficial in relation to other issues of maternal and foetal health, such as foetal growth and preterm birth. However, the evidence regarding these other outcomes is not consistent.
It is known that at 0.4mg/day, all the oxidized form of FA is converted into biologically active metabolites during absorption, and so, its consumption is generally considered safe. Intakes above the recommended levels are likely to lead to the appearance of unmetabolized FA in foetal and maternal circulation, which has been demonstrated in countries with food fortification.10 Although the impact of this is not completely understood, it is suggested that unmetabolized FA in maternal and foetal blood may act as methyl donor for the regulation of gene expression, with consequences in foetal programming.11
In Portugal, although the recommendations follow the WHO guidelines,12 and there are multivitamin-mineral supplements containing 0.4mg FA or similar (which are sold without a medical prescription), the available reimbursed pills containing isolated FA have a total dose of 5mg. This dose corresponds to approximately 12.5 times the WHO recommended dose for low risk pregnancy. Furthermore, the lack of systematized health practices in many countries, being Portugal part of the problem, may lead to excessive FA supplementation resulting from the simultaneous use of FA pills and the consumption of FA-enriched foods or FA containing multivitamin-mineral supplements, sometimes, throughout all pregnancy.13 In addition, there is also a big portion of non-planned pregnancies all over the world14 and several studies suggest that the compliance of women to periconceptional FA supplementation varies widely.15
Both folate restriction and excessive supplementation lead to epigenetic marks, raising a potential concern about its role in inducing unforeseen changes in the methyloma and thus in the phenotype of the mother and the offspring1 at a short or a long-term. Accordingly, a remarkable number of studies have reported the outcomes of maternal FA supplementation on the offspring's health, addressing interactions such as in children's asthma, cancer, insulin resistance,1 autism16 and behaviour/inattention problems.17
All these facts contribute to a renewed interest in the implications of maternal nutrition, before and during pregnancy, and its influences in pre and post-natal development, raising questions related to the magnitude, timing, type and duration of exposure to FA.18
This review has two main purposes: first, to gather evidence published worldwide regarding supplementation of maternal diet with FA during pregnancy and the main outcomes of this supplementation either in pregnant women or in the offspring, in a short-to-long term; second, with the pulled information, to problematize this supplementation in terms of FA dosages, critical temporal windows, as well as its potentially benefits and risks, to the mothers and offspring, regarding the current state of the art.
MethodsTo conduct this systematic review, the authors searched PubMed database and included the MeSH terms “pregnancy”, “fetus”, “offspring”, “mother”, “folic acid” and “supplementation” combined in the following expression: (pregnancy OR fetus OR offspring OR mother) AND (“folic acid” AND supplementation). The search was performed in December 2014 with the following filters: all articles available published from 2005 to 2014, written in English, Portuguese, Spanish or French. The search retrieved a total of 1182 publications.
Based on our objectives, the three authors established a first list of exclusion criteria. During the process of abstract reading, some criteria were added to this first list. Abstracts were reviewed by two of the authors separately – each of them evaluated 650 abstracts, with an overlap of approximately 100 abstracts, in order to calibrate the agreement in the abstracts’ revision. The third researcher participated in the meeting where the concordance between the other two were discussed and participated in the decision of discordant classifications, as well as in the definition of new exclusion criteria throughout all the process. The agreement between both researchers was very high. Whenever doubts were raised they were shared by the three researchers and the third element was critical for the final decision.
The inclusion criteria was: all original papers referring to isolated FA supplementation during periconceptional period or pregnancy in which there was a conclusion about the effects of FA supplementation on the pregnant woman, pregnancy or offspring. Whenever the abstract was not available or if it did not clearly revealed any kind of association between the FA supplementation and the outcomes, the authors accessed the full text article to decide about inclusion or exclusion.
The exclusion criteria (and the number of excluded articles after abstract reading) were: review articles (n=226), case reports (n=19), letters to the editor or editorials (n=55), clinical recommendations (n=38), study set-up descriptions (n=6), withdrawn studies (n=3), duplicated studies (n=2), studies with human cells (n=1), studies that were not focused on the effects of isolated FA supplementation (n=539), studies testing the capacity of FA reverting environmental, chemically or genetically-induced deleterious effects (n=119).
So, the first abstracts’ assessment allowed the exclusion of a total of 1008 of the 1182 abstracts retrieved. At this point, the remaining 174 full-text articles were read and analyzed and this resulted in the exclusion of 65 further publications: reviews (n=1), study set-up descriptions (n=1), studies with human cells (n=1), studies testing the capacity of FA reverting environmental, chemically or genetically-induced deleterious effects (n=10), studies that were not focused on the effects of isolated FA supplementation (n=52). Afterwards, from the initial 1182 retrieved, 1073 were excluded and 109 publications completely fulfilled the inclusion criteria of this review (see Fig. 1).
In order to systematize the findings, three tables were created containing the main features of each paper: author(s) and year of publication, country, studied population, sample size, type of study, intervention (timing and dosages of FA supplementation) and results and main conclusions. The first table describes included animal studies (Table 1), the second one, the included human studies reporting effects of FA supplementation over the offspring (Table 2) and the last one the included human studies reporting effects of FA supplementation over the mother and over pregnancy outcomes (Table 3).
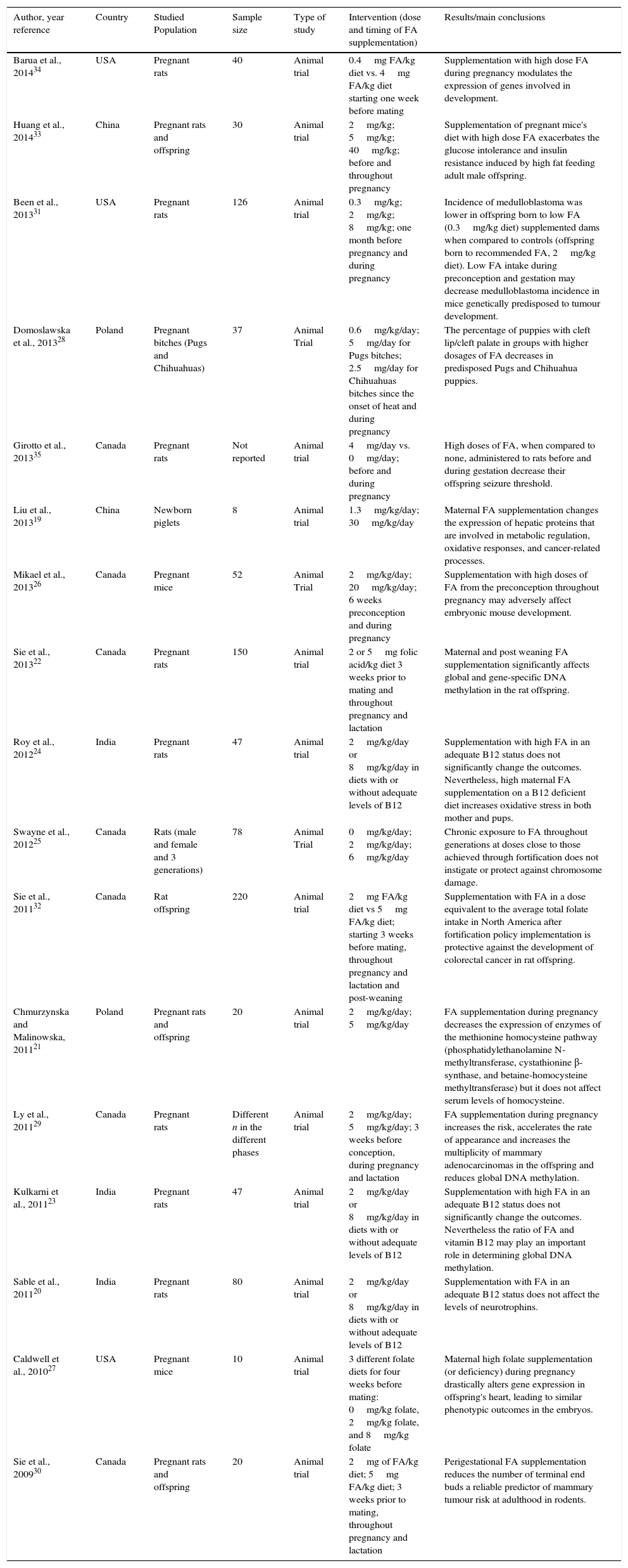
Description of the included animal studies (n=17).
| Author, year reference | Country | Studied Population | Sample size | Type of study | Intervention (dose and timing of FA supplementation) | Results/main conclusions |
|---|---|---|---|---|---|---|
| Barua et al., 201434 | USA | Pregnant rats | 40 | Animal trial | 0.4mg FA/kg diet vs. 4mg FA/kg diet starting one week before mating | Supplementation with high dose FA during pregnancy modulates the expression of genes involved in development. |
| Huang et al., 201433 | China | Pregnant rats and offspring | 30 | Animal trial | 2mg/kg; 5mg/kg; 40mg/kg; before and throughout pregnancy | Supplementation of pregnant mice's diet with high dose FA exacerbates the glucose intolerance and insulin resistance induced by high fat feeding adult male offspring. |
| Been et al., 201331 | USA | Pregnant rats | 126 | Animal trial | 0.3mg/kg; 2mg/kg; 8mg/kg; one month before pregnancy and during pregnancy | Incidence of medulloblastoma was lower in offspring born to low FA (0.3mg/kg diet) supplemented dams when compared to controls (offspring born to recommended FA, 2mg/kg diet). Low FA intake during preconception and gestation may decrease medulloblastoma incidence in mice genetically predisposed to tumour development. |
| Domoslawska et al., 201328 | Poland | Pregnant bitches (Pugs and Chihuahuas) | 37 | Animal Trial | 0.6mg/kg/day; 5mg/day for Pugs bitches; 2.5mg/day for Chihuahuas bitches since the onset of heat and during pregnancy | The percentage of puppies with cleft lip/cleft palate in groups with higher dosages of FA decreases in predisposed Pugs and Chihuahua puppies. |
| Girotto et al., 201335 | Canada | Pregnant rats | Not reported | Animal trial | 4mg/day vs. 0mg/day; before and during pregnancy | High doses of FA, when compared to none, administered to rats before and during gestation decrease their offspring seizure threshold. |
| Liu et al., 201319 | China | Newborn piglets | 8 | Animal trial | 1.3mg/kg/day; 30mg/kg/day | Maternal FA supplementation changes the expression of hepatic proteins that are involved in metabolic regulation, oxidative responses, and cancer-related processes. |
| Mikael et al., 201326 | Canada | Pregnant mice | 52 | Animal Trial | 2mg/kg/day; 20mg/kg/day; 6 weeks preconception and during pregnancy | Supplementation with high doses of FA from the preconception throughout pregnancy may adversely affect embryonic mouse development. |
| Sie et al., 201322 | Canada | Pregnant rats | 150 | Animal trial | 2 or 5mg folic acid/kg diet 3 weeks prior to mating and throughout pregnancy and lactation | Maternal and post weaning FA supplementation significantly affects global and gene-specific DNA methylation in the rat offspring. |
| Roy et al., 201224 | India | Pregnant rats | 47 | Animal trial | 2mg/kg/day or 8mg/kg/day in diets with or without adequate levels of B12 | Supplementation with high FA in an adequate B12 status does not significantly change the outcomes. Nevertheless, high maternal FA supplementation on a B12 deficient diet increases oxidative stress in both mother and pups. |
| Swayne et al., 201225 | Canada | Rats (male and female and 3 generations) | 78 | Animal Trial | 0mg/kg/day; 2mg/kg/day; 6mg/kg/day | Chronic exposure to FA throughout generations at doses close to those achieved through fortification does not instigate or protect against chromosome damage. |
| Sie et al., 201132 | Canada | Rat offspring | 220 | Animal trial | 2mg FA/kg diet vs 5mg FA/kg diet; starting 3 weeks before mating, throughout pregnancy and lactation and post-weaning | Supplementation with FA in a dose equivalent to the average total folate intake in North America after fortification policy implementation is protective against the development of colorectal cancer in rat offspring. |
| Chmurzynska and Malinowska, 201121 | Poland | Pregnant rats and offspring | 20 | Animal trial | 2mg/kg/day; 5mg/kg/day | FA supplementation during pregnancy decreases the expression of enzymes of the methionine homocysteine pathway (phosphatidylethanolamine N-methyltransferase, cystathionine β-synthase, and betaine-homocysteine methyltransferase) but it does not affect serum levels of homocysteine. |
| Ly et al., 201129 | Canada | Pregnant rats | Different n in the different phases | Animal trial | 2mg/kg/day; 5mg/kg/day; 3 weeks before conception, during pregnancy and lactation | FA supplementation during pregnancy increases the risk, accelerates the rate of appearance and increases the multiplicity of mammary adenocarcinomas in the offspring and reduces global DNA methylation. |
| Kulkarni et al., 201123 | India | Pregnant rats | 47 | Animal trial | 2mg/kg/day or 8mg/kg/day in diets with or without adequate levels of B12 | Supplementation with high FA in an adequate B12 status does not significantly change the outcomes. Nevertheless the ratio of FA and vitamin B12 may play an important role in determining global DNA methylation. |
| Sable et al., 201120 | India | Pregnant rats | 80 | Animal trial | 2mg/kg/day or 8mg/kg/day in diets with or without adequate levels of B12 | Supplementation with FA in an adequate B12 status does not affect the levels of neurotrophins. |
| Caldwell et al., 201027 | USA | Pregnant mice | 10 | Animal trial | 3 different folate diets for four weeks before mating: 0mg/kg folate, 2mg/kg folate, and 8mg/kg folate | Maternal high folate supplementation (or deficiency) during pregnancy drastically alters gene expression in offspring's heart, leading to similar phenotypic outcomes in the embryos. |
| Sie et al., 200930 | Canada | Pregnant rats and offspring | 20 | Animal trial | 2mg of FA/kg diet; 5mg FA/kg diet; 3 weeks prior to mating, throughout pregnancy and lactation | Perigestational FA supplementation reduces the number of terminal end buds a reliable predictor of mammary tumour risk at adulthood in rodents. |
USA – United States of America; FA – folic acid.
Included human studies describing health outcomes of the offspring (n=75).
| Reference | Country | Studied population | Sample size | Type of study | Intervention (dose and timing of FA supplementation) | Results/main conclusions |
|---|---|---|---|---|---|---|
| Yang et al., 201575 | China | Pregnant women and offspring | 150 cases; 212 controls | Case-control | FA supplementation during pregnancy (with quantification) | Supplementation of women with a high dose of FA during pregnancy is associated with increased risk of infant asthma. Supplementation of women with low dose FA during pregnancy is associated with decreased risk of infant asthma. |
| Ajrouche et al., 201470 | France | Cases of childhood leukaemia | 747 cases; 1421 controls | Case-control | FA supplementation during periconception (with timing) | Slight inverse association is observed between FA supplementation starting in the 3 months preconception and the risk of childhood leukaemia. |
| Csaky-Szunyogh et al., 201451 | Hungary | Cases of congenital heart defects | 302 cases; 469 controls | Case-control | FA supplementation during pregnancy (yes or no) | High doses FA are protective against left-ventricular outflow-tract, particularly coarctation of the aorta. |
| Greenop et al., 201466 | Australia | Cases of childhood brain tumours | 293 cases; 726 controls | Case-control | FA supplementation during pregnancy (with timing) | Reduced risk of brain tumours in children of mothers that took FA corroborating folate's important contribution to genomic integrity and DNA methylation. |
| Mashuda et al., 201463 | Tanzania | Infants with congenital anomalies | 445 | Cross-sectional | FA supplementation during periconception (yes or no) | No use of FA in the periconceptional period is significantly associated with congenital anomalies. |
| Valera-Gran et al., 201487 | Spain | Pregnant women and offspring | 2213 | Cohort | FA supplementation during pregnancy (with quantification) | Poorer psychomotor development observed in children born from mothers who were supplemented with high dose of FA (> 5mg/day) during pregnancy when compared to children born to mothers using the recommended dose of FA (0.4–1mg/day) during pregnancy. |
| Veeranki et al., 201473 | USA | Pregnant women and offspring | 167,333 | Cohort | FA supplementation during pregnancy (with timing; majority: 1mg/day) | Children of women who had FA supplementation only in the first trimester of pregnancy have higher probability to have a diagnosis of bronchiolitis and greater severity of bronchiolitis, when compared to children of women that did not have FA supplementation during pregnancy. |
| Agopian et al., 201339 | USA | Cases of neural tube defects | 1239 cases; 8494 controls | Case-control | FA supplementation in the periconception and first trimester of pregnancy | Lack of FA supplementation (after fortification establishment) is not a major determinant of spina bifida or anencephaly. |
| Boeke et al., 201385 | USA | Pregnant women and offspring | 895 | Cohort | FA supplementation early in pregnancy (with quantification) | Supplementation with FA early in pregnancy is not associated with child memory at 7 years. |
| Esmaeili et al., 201341 | Iran | Cases of lipomyelomeningocele (LipoMMC) | 35 cases; 70 controls | Case-control | FA supplementation during periconception and first trimester of pregnancy | Periconceptional FA supplementation is significantly lower in cases when compared to controls. FA periconceptional supplementation is an independent protective factor against LipoMMC. |
| Haggarty et al., 2013101 | UK | Pregnant women | 913 | Cohort | FA supplementation during pregnancy (with quantification) | FA use after the 12th week of pregnancy affects DNA methylation in the offspring, particularly in repeat elements and in IGF2 gene. |
| Hollis et al., 201362 | USA | Cases of trisomy 21 | 907 cases; 983 controls | Case-control | FA supplementation in the preconception | FA supplementation in the preconception is associated specifically with meiosis II errors in older mothers. |
| Li X et al., 201355 | China | Cases of congenital heart defects | 358 cases; 422 controls | Case-control | FA supplementation during periconception and pregnancy (with timing) | Supplementation with FA is associated with reduced risk of congenital heart defects. The earlier FA supplementation begins before pregnancy and the longer supplementation lasts, the lower the risk of CHDs is. |
| Rozendaal et al., 201350 | The Netherlands | Cases of clefts | 367 cases; 2945 controls | Case-control | FA supplementation during pregnancy (with timing and quantification) | FA supplement use during 0–12 weeks postconception is associated with increased risk of lip/alveolus cleft. |
| Suren et al., 201391 | Norway | Pregnant women and offspring | 85,176 | Cohort | 0.4mg/day in the month before pregnancy and during the first trimester | FA supplementation in the preconception and in the first trimester of pregnancy is associated with a decreased risk of autistic disorder in the offspring. |
| van den Hil et al., 2013104 | The Netherlands | Pregnant women and offspring | 2863 | Cohort | FA supplementation (no use, use when pregnancy was known, using during the periconception) | FA supplementation during pregnancy is not associated with childhood systolic or diastolic blood pressure. |
| Vereczkey et al., 201352 | Hungary | Cases of atrioventricular canal defects | 77 cases; 38,151 controls | Case-control | FA supplementation during pregnancy (with timing) | High doses of FA early in pregnancy are associated with a reduced rate of atrioventricular canal defects. |
| Wehby et al., 201348 | Brazil | Women intending to get pregnant | 273 | Human trial | 0.4mg/day or 4mg/day before pregnancy and during the first trimester | High dose of FA supplementation in the periconceptional period does not change recurrence rates of oral cleft. High dose of FA also does not impair foetal growth. |
| Wang et al., 201340 | China | Cases of neural tube defects | 459 cases; 459 controls | Case-control | FA supplementation in the preconception and during the first trimester (with timing) | FA supplementation during the periconceptional period decreases the risk of neural tube defects. |
| Bekkers et al., 201281 | The Netherlands | Pregnant women and offspring | 3786 | Cohort | FA supplementation between 30 and 36 gestational weeks (with quantification) | Prenatal FA supplementation is not associated with adverse respiratory or allergic outcomes in children (1–8 years of age), apart from a slight increased risk of wheeze at 1 year old. |
| Chandler et al., 201237 | USA | Cases of anencephalia and spina bifida | 954 cases; 6268 controls | Case-control | FA supplementation in the 3 months previous conception and during all pregnancy (with quantification) | FA intake, as well as other micronutrients, including thiamin, betaine, riboflavin, vitamin B6, vitamin C, vitamin E, niacin, iron, retinol, and vitamin A, may be protective against anencephaly. However, folate intake does not affect the risk for spina bifida. |
| Chatzi et al., 201283 | Greece | Pregnant women and offspring | 553 | Cohort | FA supplementation early in pregnancy (with quantification) | Daily supplementation with high dose FA (5mg/day) in early pregnancy is associated with improved vocabulary development, communicational skills and verbal comprehension at 18 months of age. Higher doses of FA do not show any association with additional improvements in the neurodevelopment scales. |
| Dunstan et al., 201278 | Australia | Pregnant women and offspring | 628 | Cohort | Folate intake and FA supplementation in the third trimester of pregnancy (with quantification) | FA supplementation is higher in children with subsequent eczema, but it was not associated with other allergic outcomes. |
| Forns et al., 201288 | Spain | Adolescents | 393 | Cohort | FA supplementation during pregnancy | Supplementation of the maternal diet with FA may influence inattentive and hyperactive/impulsive symptomatology during pre-adolescence. |
| Kiefte-de Jong et al., 201277 | The Netherlands | Pregnant women and offspring | 8742 | Cohort | FA supplementation during periconception of after, but before the 10th gestational week (0.4-0.5mg/day) | Periconceptional FA supplementation and supplementation within the first 10 weeks of pregnancy are not associated with higher prevalence wheezing, shortness of breath or atopic dermatitis during childhood. High folate and vitamin B-12 concentrations during pregnancy is associated with higher prevalence of atopic dermatitis in the offspring. |
| Martinussen et al., 201276 | USA | Pregnant women and offspring | 1499 | Cohort | FA supplementation during pregnancy (with quantification) | FA supplementation in pregnancy is not associated with the risk of asthma in children at 6 years of age. |
| Milne et al., 201267 | Australia | Cases of childhood brain tumours | 327 cases; 867 controls | Case-control | FA supplementation in the month previous pregnancy and in each trimester | Supplementation with FA before and likely during pregnancy may be protective against childhood brain tumours. |
| Paranjothy et al., 201261 | UK | Cases of foetus with gastroschisis | 124 cases; 217 controls | Case-control | FA supplementation in the first trimester of pregnancy | Supplementation with FA for a minimum of 6 weeks during the first trimester of pregnancy is protective against foetal gastroschisis. |
| Schmidt et al., 201290 | USA | Cases of autism spectrum disorders | 559 cases; 278 controls | Case-control | FA supplementation 3 months before pregnancy and in each month of pregnancy (with quantification) | Periconceptional FA may be protective against autism spectrum disorder particularly for mothers and children with inefficient folate metabolism. |
| Wang et al., 201295 | China | Pregnant women and offspring | 1388 | Human trial | FA supplements; Iron-FA supplements; multivitamin supplements during pregnancy | Results do not support a greater advantage of the effect of maternal multi-micronutrient supplementation on child growth over iron-FA or FA only during the first 30 months. |
| Ahrens et al., 201142 | USA | Cases of spina bifida | 205 cases; 6357 controls | Case-control | Dietary folate intake, including fortified foods+FA supplementation in the 2 months before and 2 months after conception | Over a setting of FA fortification, FA supplementation in a regular basis (≥4 days per week) around the conception moment or initiated in early pregnancy is not associated with spina bifida. |
| Bean et al., 201153 | Georgia | Cases of Down syndrome | 1011 | Case-control | FA supplementation before conception, and during pregnancy (with timing) | Lack of maternal FA supplementation is associated with septal defects in infants with Down syndrome. |
| Binkley et al., 201180 | Canada | Cases of children with peanut allergy | 1300 cases; 113 controls | Case-control | FA supplementation in the periconception | FA supplementation before or after conception is not associated with the risk peanut allergy in childhood. |
| Campoy et al., 201186 | Spain | Pregnant women and offspring | 161 | Human trial | After 20 weeks of pregnancy: 0.4mg of 5-MTHF; 0.4mg of 5-MTHF+DHA; only DHA | FA and/or DHA supplementation are not significantly associated with a better cognitive function of children. |
| De Marco et al., 201136 | Italy | Cases of neural tube defects | 133 cases; 273 controls | Case-control | FA supplementation in the 3 months previous conception and 3 months after conception | Lack of folate periconceptional supplementation increases the risk of spina bifida. |
| Hossein-nezhad et al., 201197 | Japan | Pregnant women and offspring | 113 | Cohort | FA supplementation during pregnancy (with timing and quantification; 1mg/day) | Daily supplementation with FA during the whole pregnancy has a positive impact on the bone turnover markers in mothers and their newborns, when compared with FA supplementation only until the end of second trimester. |
| Hoyo et al., 201199 | USA | Pregnant women | 438 | Cross-sectional | FA supplementation in the preconception and during pregnancy (with quantification) | Methylation levels at the H19 DMR decrease (in a more pronounced fashion in male infants) with increasing maternal FA intake. |
| Jacobsen et al., 2011102 | Denmark | Singleton sons of mothers enrolled in a cohort | 347 | Cohort | FA supplementation during pregnancy | Semen characteristics are similar among sons of mothers supplemented with FA during pregnancy when compared with sons of mothers not supplemented with FA (or with unknown FA supplementation). However FA supplemented group has higher levels of FSH and LH. |
| Jia et al., 201144 | China | Cases of non-syndromic cleft lip with/without cleft palate | 537 cases; 221 controls | Case-control | FA supplementation early in pregnancy | Maternal FA supplementation during early pregnancy has a protective effect against cleft lip and cleft palate. |
| Magdelijns et al., 201179 | The Netherlands | Pregnant women and offspring | 2834 | Cohort | FA supplementation in the preconception and during pregnancy | FA supplementation of maternal diet is not associated with increased risk of atopic outcomes including wheezing and asthma in the offspring. |
| Mohammadzadeh et al., 201156 | Iran | Cases of hypospadias | 25 cases; 6124 controls | Case-control | FA supplementation in the 3 months before conception and during first trimester of pregnancy | Iron and FA supplementation may have preventive effect in hypospadias. |
| Pastor-Valero et al., 201192 | Spain | Pregnant women | 786 | Cohort | FA supplementation during pregnancy: non-users, moderate users (0.2-0.9mg/day) and high users (2.5-10.5mg/day) | Periconceptional supplementation with FA in doses over 1mg/d is associated with decreased birth length and may increase the risk of low birth weight. |
| Reutter et al., 201158 | Germany | Families of cases bladder exstrophy-epispadias complex | 441 | Cross-sectional | FA supplements in the periconception or in the first 10 weeks of gestation | FA supplementation in the periconceptional period seems to prevent against the development of the severe phenotype of bladder exstrophy-epispadias complex (associations are not statistically significant). |
| Roth et al., 201184 | Norway | Pregnant women and offspring | 38,954 | Cohort | FA supplementation 1 month before conception and during pregnancy, until the 17th gestational week (with quantification) | FA supplementation in early pregnancy (either FA alone or in combination with other supplements) is associated with a reduced risk of severe language delay in children at 3 years of age. |
| Wu et al., 201147 | China | Cases of nonsyndromic orofacial clefts | 211 cases; 188 controls | Case-control | FA supplementation during the first trimester of pregnancy | Maternal folic acid supplementation during the first trimester of pregnancy shows a protective effect on the aetiology of nonsyndromic orofacial clefts. |
| Carmichael et al., 2010a43 | USA | Cases of craniosynostosis | 815 cases; 6789 controls | Case-control | FA supplementation during pregnancy | FA supplementation during pregnancy is not associated with craniosynostosis occurrence. |
| Carmichael et al., 2010b122 | USA | Cases of anencephaly and spina bifida | 330 cases; 625 controls | Case-control | FA supplementation during pregnancy | FA supplementation during pregnancy is only modestly associated with a reduced risk of NTDs. |
| Glaser et al., 2010106 | UK | Pregnant women and offspring | 5344 | Cohort | FA supplementation during pregnancy+dietary folate intake | FA supplementation during pregnancy does not contribute to aetiological pathways that are shared between schizophrenia and non-clinical psychotic symptoms in adolescents. |
| Gong et al., 201038 | China | Cases of neural tube defects (<2 years) | 349 cases; 349 controls | Case-control | FA supplementation in the preconception | FA supplementation in the preconception prevents against NTDs. |
| Ma et al., 201060 | USA | Cases of microtia | 420 cases; 6789 controls | Case-control | FA supplementation one month before conception and/or during pregnancy (with timing) | Periconceptional FA supplementation reduces the risk for microtia, only among non-obese women. |
| Milne et al., 201071 | Australia | Cases of acute lymphoblastic leukaemia | 416 cases; 1361 controls | Case-control | FA supplementation in the preconception and during pregnancy (with timing and quantification) | Maternal folate supplementation during the preconception, the first trimester or the final 6 months of pregnancy is not associated with the risk of childhood acute lymphoblastic leukaemia. |
| Nilsen et al., 201094 | Norway | Pregnant women and offspring | 2934 | Cohort | FA supplementation during pregnancy (with quantification)+dietary folate intake | No evidence that low dietary folate intake or low plasma folate concentration during the second trimester is associated with infant birth size. |
| Ortega-Garcia et al., 201069 | Spain | Incident cases of nervous system tumours, aged <15 years | 67 cases; 155 controls | Case-control | FA supplementation during pregnancy (>=0.4mg/day) | FA supplementation in pregnancy is protective against nervous system tumour (NST), especially central NST. |
| Roza et al., 201017 | The Netherlands | Pregnant women and offspring | 4214 | Cohort | FA supplementation in the preconception and during the first trimester of pregnancy (0.4mg/day if FA supplements as single; no dosage specified in FA in multivitamins) | Inadequate FA supplementation during early pregnancy may be associated with a higher risk of behavioural problems in the offspring. |
| Schlotz et al., 201089 | UK | Pregnant women and offspring | 100 | Cohort | FA supplementation+dietary folate intake during pregnancy (with timing and quantification) | Low maternal folate status (red blood cell folate and dietary intake) is associated with high hyperactivity scores in the offspring. |
| Stalberg et al., 201068 | Sweden | Cases of brain tumours, aged <15 years | 515 cases; 525 controls | Case-control | FA supplementation in the preconception and during pregnancy | Prenatal FA supplementation shows a tendency to protection against brain tumour in the offspring. |
| van Beynum et al., 201054 | The Netherlands | Cases of congenital heart defects | 611 cases; 5744 controls | Case-control | FA supplementation during periconception (0.4mg/day) | Periconceptional FA supplementation is related to 20% reduction in the prevalence of any congenital heart defect. |
| Timmermans et al., 200993 | The Netherlands | Pregnant women and offspring | 6353 | Cohort | FA supplementation in the periconception (0.4-0.5mg) | Periconceptional FA supplementation is associated with increased foetal growth resulting in higher placental and birth weight, and decreased risks of low birth weight and SGA. |
| Fryer et al., 2009100 | UK | Pregnant women (at delivery) | 24 | Cross-sectional | FA supplementation during pregnancy, considering daily dosage or total amount during pregnancy | Folate indices during pregnancy are inversely associated with foetal LINE-1 methylation. |
| Haberg et al., 200974 | Norway | Pregnant women and offspring | 32,077 | Cohort | FA supplementation during pregnancy (with timing) | FA supplementation (in the first trimester of pregnancy and later) is associated with increased risk of wheeze and lower respiratory tract infections in the offspring up to 18 months of age. |
| Julvez et al., 200982 | Spain | Pregnant women and offspring | 420 | Cohort | FA supplementation during the first trimester of pregnancy | FA supplementation during pregnancy is positively associated with neurodevelopment (verbal, motor, verbal-executive function, social competence and attention) of the offspring at 4 years of age. |
| Lewis et al., 2009103 | UK | Pregnant women and offspring | 5783 | Cohort | FA supplementation during pregnancy+dietary folate intake | FA supplementation at 18th or 32nd weeks of pregnancy is not associated with changes in body composition of the offspring in childhood (9 years of age). |
| Ormond et al., 200957 | UK | Cases of hypospadias | 471 cases; 490 controls | Case-control | FA supplementation during pregnancy | Folate supplementation during the first trimester of pregnancy associated with a reduced risk of hypospadias. |
| Stewart et al., 2009105 | Nepal | Pregnant women and offspring | 4926 women and 3524 live-born infants | Human trial | Women given daily supplements from the time of enrolment until 3 mo postpartum: 1) vitamin A alone as the control; 2) FA (400 mcg); 3) FA with iron (60 mcg); 4) FA with iron and zinc (30mg); or 5) a multiple micronutrient supplement containing FA | FA supplementation during pregnancy presents a beneficial effect on both offspring kidney function and risk of metabolic syndrome. |
| Whitrow et al., 200911 | Australia | Pregnant women and offspring | 557 | Cohort | FA supplementation during preconception and during pregnancy (with timing and quantification) | FA supplementation late in pregnancy increases the risk of asthma in child at 3.5 years and persistent asthma at 3.5 years and 5.5 years. |
| Gambhir et al., 200859 | Germany | Families of cases bladder exstrophy-epispadias complex | 214 | Cross-sectional | FA supplementation in the preconception and during the first trimester of pregnancy | Paradoxically, mothers of children with cloacal exstrophy CE, the most severe defect of exstrophy-epispadias complex, are more compliant to FA supplementation starting before the 10th week of pregnancy than mothers of the combined group of isolated epispadias/classic exstrophy of the bladder (E/CBE), the least severe manifestations. This may be due to the fact that the prevention of the related NTDs and omphalocele are most responsive to FA supplementation started in the preconception. |
| Little et al., 200849 | Canada | Cases of orofacial clefts | 190 cases; 248 controls | Case-control | FA supplementation 3 months before and 3 months after conception+dietary folate intake | Total folate intake or dietary folate intake during pregnancy are not associated with cleft-lip palate or cleft palate. |
| Michels et al., 200864 | The Netherlands | Cases of position plagiocephaly | 18 cases; 7625 controls | Nested case-control | FA supplementation during periconception (with quantification, based on the type of FA supplements) | Periconceptional FA supplementation in high dosage may cause more position plagiocephaly. |
| van Eijsden et al., 200896 | The Netherlands | Pregnant women and offspring | 3153 | Cohort | FA supplementation during pregnancy (with timing) | Folate depletion apparently contributes to the excess risk of foetal growth restriction that is associated with short interpregnancy intervals. |
| Wehby and Murray, 20084 | USA | Pregnant women and offspring | 6774 | Cohort | FA supplementation in the three months previous to be aware of pregnancy and three months after | FA supplementation in the specified time frame in pregnancy may improve development of offspring at 3 years of age. |
| Bille et al., 200745 | Denmark | Cases of acute lymphoblastic leukaemia | 192 cases; 828 controls | Case-control | FA supplementation during the first trimester of pregnancy | Supplementation with 0.4mg/day in the first 12 weeks of pregnancy had a protective effect for oral clefts. |
| Dockerty et al., 200772 | Canada | Cases of acute lymphoblastic leukaemia | 97 cases; 303 controls | Case-control | FA supplementation during pregnancy (with or without other micronutrients) | No association was found between reported FA intake during pregnancy and childhood acute lymphoblastic leukaemia. |
| Wilcox et al., 200746 | Norway | Cases of cleft lip | 573 cases; 763 controls | Case-control | FA supplementation early in pregnancy (with quantification: no FA; 0.1 to 0.39mg/day; ≥0.4mg/day) | FA supplementation during early pregnancy was associated with 33% decreased risk of isolated cleft lip (with or without cleft palate) but not associated with reduced risk of cleft palate alone. |
| Bower et al., 200665 | Australia | Cases of NTDs | 475 cases; 578 controls | Case-control | FA supplementation in the month previous conception and during pregnancy (with quantification: ≤0.2mg/day or >0.2mg/day) | Folate supplementation was not preventive against birth defects other than NTDs (e.g. orofacial clefts, congenital heart defects, urinary tract defects) |
| Tobias et al., 200598 | UK | Pregnant women and offspring | 4588 | Cohort | FA intake during pregnancy | Maternal folate intake during pregnancy was significantly associated with spinal bone mineral content. |
FA – folic acid; 5-MTHF – 5-methyltetrahydrofolate; DHA – docosahexaenoic acid; NTDs – neural tube defects.
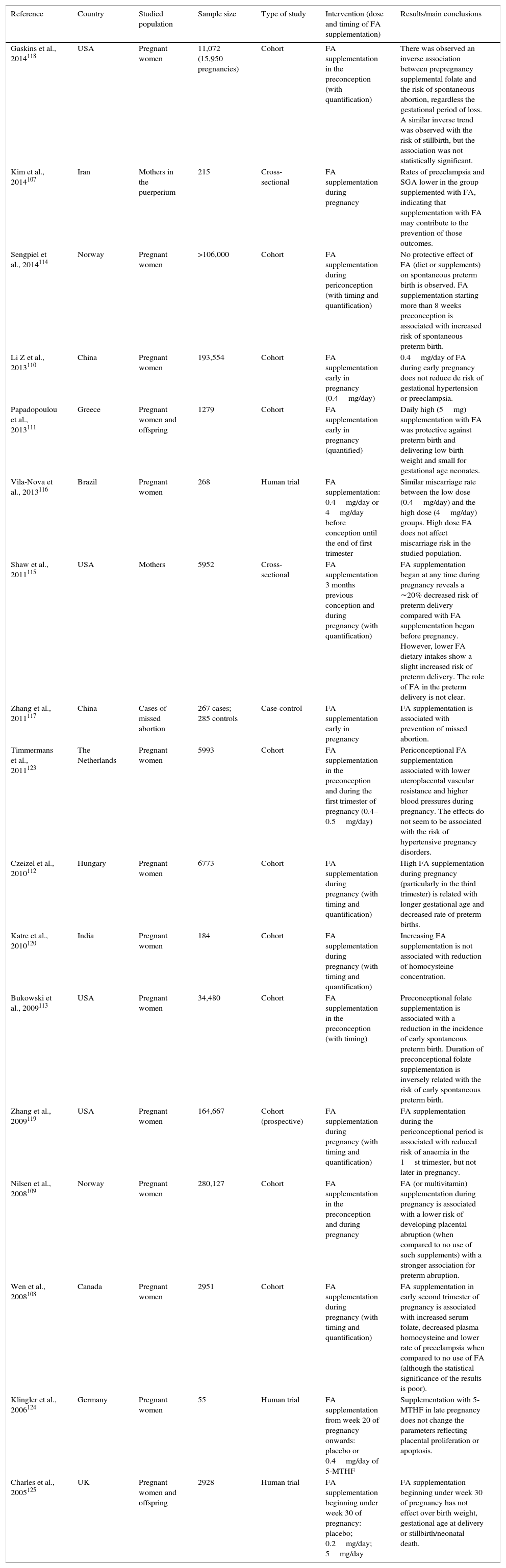
Included human studies describing health outcomes of the mother and the pregnancy (n=17).
| Reference | Country | Studied population | Sample size | Type of study | Intervention (dose and timing of FA supplementation) | Results/main conclusions |
|---|---|---|---|---|---|---|
| Gaskins et al., 2014118 | USA | Pregnant women | 11,072 (15,950 pregnancies) | Cohort | FA supplementation in the preconception (with quantification) | There was observed an inverse association between prepregnancy supplemental folate and the risk of spontaneous abortion, regardless the gestational period of loss. A similar inverse trend was observed with the risk of stillbirth, but the association was not statistically significant. |
| Kim et al., 2014107 | Iran | Mothers in the puerperium | 215 | Cross-sectional | FA supplementation during pregnancy | Rates of preeclampsia and SGA lower in the group supplemented with FA, indicating that supplementation with FA may contribute to the prevention of those outcomes. |
| Sengpiel et al., 2014114 | Norway | Pregnant women | >106,000 | Cohort | FA supplementation during periconception (with timing and quantification) | No protective effect of FA (diet or supplements) on spontaneous preterm birth is observed. FA supplementation starting more than 8 weeks preconception is associated with increased risk of spontaneous preterm birth. |
| Li Z et al., 2013110 | China | Pregnant women | 193,554 | Cohort | FA supplementation early in pregnancy (0.4mg/day) | 0.4mg/day of FA during early pregnancy does not reduce de risk of gestational hypertension or preeclampsia. |
| Papadopoulou et al., 2013111 | Greece | Pregnant women and offspring | 1279 | Cohort | FA supplementation early in pregnancy (quantified) | Daily high (5mg) supplementation with FA was protective against preterm birth and delivering low birth weight and small for gestational age neonates. |
| Vila-Nova et al., 2013116 | Brazil | Pregnant women | 268 | Human trial | FA supplementation: 0.4mg/day or 4mg/day before conception until the end of first trimester | Similar miscarriage rate between the low dose (0.4mg/day) and the high dose (4mg/day) groups. High dose FA does not affect miscarriage risk in the studied population. |
| Shaw et al., 2011115 | USA | Mothers | 5952 | Cross-sectional | FA supplementation 3 months previous conception and during pregnancy (with quantification) | FA supplementation began at any time during pregnancy reveals a ∼20% decreased risk of preterm delivery compared with FA supplementation began before pregnancy. However, lower FA dietary intakes show a slight increased risk of preterm delivery. The role of FA in the preterm delivery is not clear. |
| Zhang et al., 2011117 | China | Cases of missed abortion | 267 cases; 285 controls | Case-control | FA supplementation early in pregnancy | FA supplementation is associated with prevention of missed abortion. |
| Timmermans et al., 2011123 | The Netherlands | Pregnant women | 5993 | Cohort | FA supplementation in the preconception and during the first trimester of pregnancy (0.4–0.5mg/day) | Periconceptional FA supplementation associated with lower uteroplacental vascular resistance and higher blood pressures during pregnancy. The effects do not seem to be associated with the risk of hypertensive pregnancy disorders. |
| Czeizel et al., 2010112 | Hungary | Pregnant women | 6773 | Cohort | FA supplementation during pregnancy (with timing and quantification) | High FA supplementation during pregnancy (particularly in the third trimester) is related with longer gestational age and decreased rate of preterm births. |
| Katre et al., 2010120 | India | Pregnant women | 184 | Cohort | FA supplementation during pregnancy (with timing and quantification) | Increasing FA supplementation is not associated with reduction of homocysteine concentration. |
| Bukowski et al., 2009113 | USA | Pregnant women | 34,480 | Cohort | FA supplementation in the preconception (with timing) | Preconceptional folate supplementation is associated with a reduction in the incidence of early spontaneous preterm birth. Duration of preconceptional folate supplementation is inversely related with the risk of early spontaneous preterm birth. |
| Zhang et al., 2009119 | USA | Pregnant women | 164,667 | Cohort (prospective) | FA supplementation during pregnancy (with timing and quantification) | FA supplementation during the periconceptional period is associated with reduced risk of anaemia in the 1st trimester, but not later in pregnancy. |
| Nilsen et al., 2008109 | Norway | Pregnant women | 280,127 | Cohort | FA supplementation in the preconception and during pregnancy | FA (or multivitamin) supplementation during pregnancy is associated with a lower risk of developing placental abruption (when compared to no use of such supplements) with a stronger association for preterm abruption. |
| Wen et al., 2008108 | Canada | Pregnant women | 2951 | Cohort | FA supplementation during pregnancy (with timing and quantification) | FA supplementation in early second trimester of pregnancy is associated with increased serum folate, decreased plasma homocysteine and lower rate of preeclampsia when compared to no use of FA (although the statistical significance of the results is poor). |
| Klingler et al., 2006124 | Germany | Pregnant women | 55 | Human trial | FA supplementation from week 20 of pregnancy onwards: placebo or 0.4mg/day of 5-MTHF | Supplementation with 5-MTHF in late pregnancy does not change the parameters reflecting placental proliferation or apoptosis. |
| Charles et al., 2005125 | UK | Pregnant women and offspring | 2928 | Human trial | FA supplementation beginning under week 30 of pregnancy: placebo; 0.2mg/day; 5mg/day | FA supplementation beginning under week 30 of pregnancy has not effect over birth weight, gestational age at delivery or stillbirth/neonatal death. |
FA – folic acid; 5-MTHF – 5-methyltetrahydrofolate.
Of the 109 included papers, the majority of them (n=92) were performed in humans and a total of 17 corresponded to animal studies. Considering the type of study, of the 109, most of them (n=85, 78%) were observational in nature (whether case–control studies (n=38), cohort studies (n=40), or cross-sectional studies (n=7)) and only 24 (22%) were intervention trials, a great proportion of which conducted with laboratory animals (n=17; 70.8%).
In terms of chronology, significantly more publications were found in the last five years (n=84; 77%) comparing to the first five years of research (2005–2009).
When the papers were identified by country, there is a vast dispersion around the world. However, the most represented ones were the USA (n=20), The Netherlands (n=11), China (n=11), Canada (n=11) and UK (n=9). The papers selected were divided in 2 main categories: animal trials and human studies. In order to further systematize the analysis, human studies were divided on those focused on health outcomes of the offspring and those on health outcomes of the mother or pregnancy Results of each category are shown separately.
Animal trialsIn the animal trials category (n=17, Table 1), all the publications were focused on health outcomes in the offspring, and a preponderance of those related to the role of FA supplementation towards gene expression (n=4), gene methylation (n=2) and its consequences in embryogenesis (n=4) was found. Other outcomes were carcinogenesis (n=4), including mammary tumours in the offspring (n=2), colorectal cancer (n=1) and medulloblastoma (n=1); metabolic syndrome (n=1); behavioural changes (n=1) and seizure (n=1).
Gene methylation and gene expressionSix of the 17 animal trials focused their attention on gene expression or methylation and globally folate supplementation during pregnancy is shown to regulate gene expression.19–21 Many of the animal studies also corroborate the impact of FA supplementation on gene methylation. Namely, Sie et al. demonstrated that maternal FA supplementation modulates global and gene-specific DNA methylation in the rat offspring22 and, for instance, Kulkarni et al. showed that there was a reduction in DNA methylation levels of placental tissues occurring after exposure of pregnant dams to excess of folates.23 In what concerns critical doses of supplementation, it seems that excessive supplementation (8mg/kg instead of the recommended dose of 2mg/kg) has a determinant role both in gene expression and gene methylation.24 However, another study concluded that chronic exposure to FA throughout generations at doses close to those achieved through fortification does not instigate or protect against chromosome damage.25
EmbryogenesisOf the four trials studying embryogenesis outcomes, one suggests that high levels of FA supplementation may adversely affect foetal mouse embryonic development26 and that the excessive maternal FA supplementation on a deficient B12 diet, although not affecting neurodevelopment, may increase oxidative stress in mothers and pups.25
In addition, folate intake during pregnancy is suggested to have a dual effect on cardiogenesis, depending on the presence of environmental toxins.27
In all these cases, there is a common trend suggesting that high doses of FA supplementation, and also longer exposures, may indeed induce harmful outcomes in the offspring. The exception is the protective effect of high doses of FA regarding orofacial clefts in two specific predisposed breeds of dogs.28
Tumours, behavioural changes, metabolic syndrome and seizureRegarding mammary tumours, trials describing opposite outcomes were found: exposure to high levels of FA (5mg/kg diet) during pregnancy and lactation may increase the risk of mammary tumours in rat offspring29 and the same dose of FA supplementation is suggested to lower the risk of this same outcome.30 Although the doses and the experimental models used were the same, the first paper investigates the effect of FA supplementation on chemically-induced mammary tumours, while the second paper explores the effect of this supplementation on spontaneous mammary tumours. It is likely that a high maternal FA environment may be protective against the development of spontaneous preneoplastic mammary lesions,29 but, on the other hand, it may increase the susceptibility to chemically-induced breast carcinogenesis in the offspring.
In the case of medulloblastoma, the only trial concluded that low maternal periconceptional FA levels, when compared to the recommended levels, may decrease the incidence of these tumours in mice genetically predisposed to tumour development.31
Still regarding cancer, Sie et al.32 suggest that maternal, but not post-weaning, supplementation with FA in a dose equivalent to the average total folate intake in North America after fortification policy implementation is protective against the development of colorectal cancer in rat offspring. They also suggest that this protective effect may result from an increase in global DNA methylation and a decrease in epithelial proliferation in the colorectum.
In what concerns metabolic syndrome, one study showed that high FA supplementation during pregnancy may have an adverse effect by exacerbating the detrimental effect of high fat diet on glucose intolerance and insulin resistance in male offspring.33
In addition, it was suggested that unregulated high FA supplementation during pregnancy may lead to alterations in brain development resulting in changes in behaviour.34 And finally, 4mg FA/day before and during gestation, seems to decrease by 42% the offspring's seizure threshold.35
Human studies: health outcomes of the offspringAlmost 2/3 (n=74) of the included papers were focused on studying, in humans, the role of maternal dietary FA supplementation over the foetal and child's health (Table 2).
Of the 74 papers, a recurrent outcome studied was embryo's defects (n=29), including: NTDs (n=8), orofacial clefts (n=7), heart defects (n=5), bladder exstrophy/epispadias/hypospadias (n=4) and other congenital abnormalities (n=5).
Seven of the included papers focused on offspring's tumours (brain tumours (n=4) and leukaemia (n=3)) and 4 of the included papers concluded about offspring's asthma (n=4), low tract infections (n=2) or allergy/atopy (n=4).
Also we can observe a major concern regarding infant foetal growth (n=5), behaviour problems (n=3) or autistic disorder (n=2) and neurodevelopment/memory (n=5). Other studied outcomes were offspring's bone turnover/development (n=2), DNA methylation (n=3), semen characteristics (n=1), body composition (n=1), blood pressure (n=1), metabolic syndrome (n=1) and, at last, schizophrenia/psychotic symptoms (n=1) and psychomotor development (n=2).
NTDsFocusing our attention on NTDs, of the 8 papers, 6 suggest a protective effect of FA supplementation in the occurrence of such defects. Importantly these papers highlight the importance of the pre/periconceptional period.36–41 The remaining 2 articles conclude that, in a background of FA fortification, FA supplementation or its absence are not associated with the risk of NTDs.42,43 Notably, all the included studies focusing on NTDs were of the case–control type, with a large sample size, except one41 that included about one hundred participants among cases and controls. So, it seems reasonable that pre/periconceptional FA supplementation indeed plays a role in the prevention of NTDs.
Oral cleftsLooking towards FA supplementation and the occurrence of orofacial clefts, 4 papers highlighted a possible protective effect in early pregnancy.44–47 Other two publications discussed the possible protective effects of FA supplementation on this congenital defect but they did not show any association regardless the use of 0.4mg or 4mg/day of FA by mothers.48,49 Just one publication reported an increased risk of orofacial clefts when there was a consistent supplement use during the aetiologically relevant period (weeks 0–12 post conception)50 and these results led their authors to decisively emphasize the idea that it would be advisable to restrict FA supplementation to the period recommended for NTDs until more information is available. Indeed, when the exposure to FA is restricted to “early pregnancy” period (first weeks/first trimester post conception), among five studies only one demonstrates an adverse effect, while four of them consistently showed a beneficial protective effect against orofacial clefts. Importantly no extra effects were reported on higher doses of supplemented FA (4mg/day comparing to 0.4mg/day).
Heart defectsOffspring's heart defects were covered by five of the included papers, four of them reporting a reduction in the risk of heart defects among offspring of mothers supplemented with FA,51–54 regardless of the time and dose of exposure. In fact, the authors observed beneficial effects even for high dose of FA51,52 (5 and 6mg/day) and others show the same effect with only 0.4mg FA/day.54 In addition, one of the studies revealed that FA supplementation can be related to a reduced risk of congenital heart defects, the preconceptional period as a critical window for this protective effect.55
Other general congenital defectsFive studies reported the effects of maternal FA supplementation on bladder defects (epispadias, hypospadias, bladder exstrophy), most of them concluding on the possible protective effect of FA supplementation in the periconceptional period56,57 or suggesting that this supplementation could potentially alleviate phenotypic severity of these defects.58,59 Other findings regarding congenital defects also revealed a protective effect of FA supplementation on the risk of microtia60 or gastroschisis,61 while the lack of FA supplementation seems to be associated with observed meiosis II nondisjunction errors in older mothers.62 Another study support the protective effect of FA against several congenital defects.63 In contrast to the beneficial outcomes, it seems that higher doses of FA, although with low statistic correlation, might have an adverse effect by causing more plagiocephaly cases.64 An Australian case–control study reported no evidence of folate (either FA supplements or dietary folate intake) as an important factor in the prevention of birth defects other than NTDs.65 With the obtained results, we cannot fundament a hard statement on timings and doses but, in the case of bladder defects, a protective effect of FA supplementation was unanimously reported, when started before conception. In the other cases, no statements can be drawn because there are few cases of each reported outcome.
Offspring's tumoursSeveral papers (n=7) were focused on the relationship between FA supplementation in pregnancy and the risk of offspring's tumours. Regarding brain/nervous system tumours, all the selected studies indicate a possible protective role of supplementation with FA during pregnancy in childhood brain tumours.66–69 No critical temporal windows of the maximum benefit of this supplementation are reported but, in three of the papers, preconceptional period and the first trimester of pregnancy seem to be determinant.
On the other hand, findings regarding the risk of offspring's leukaemia are less expressive, as FA supplementation is reported as having an inverse borderline association if supplementation is started three months before conception,70 or no association with the risk of childhood acute lymphoblastic leukaemia.71,72
Respiratory and allergic outcomesThe interest on this area of knowledge is covered by the publications (n=10) regarding childhood asthma, low respiratory infections or atopy/allergy. One study suggests that offspring of mothers that were supplemented with FA during the first trimester of pregnancy, had higher relative odds of being diagnosed with bronchiolitis compared with the group of no supplementation73 and other study reports that FA supplementation in pregnancy is associated with slightly increased risk of wheeze and lower respiratory tract infections up to 18 months of age, consistent with epigenetic mechanisms.74 Other study suggests a higher general risk of asthma in the offspring of supplemented mothers and, furthermore, if this supplementation is performed late in pregnancy, there might be a significantly increased risk of asthma.11 Higher risks were documented with higher doses of FA supplementation (above 72mg during the whole pregnancy, corresponding to 180 days of supplementation with 0.4mg/day).75 Only one study did not show any relation with FA supplementation and higher risk of asthma at the age of six years.76
Focusing on allergic outcomes, it is suggested that high maternal plasma folate and serum vitamin B-12 concentrations can be associated with the development of atopic dermatitis, but not with wheezing and shortness of breath in childhood.77 A dose-dependent effect was reported in the third trimester: infants exposed to ≥0.5mg FA/day were more likely to develop eczema than those taking <0.2mg FA/day.78 In contrast, one of the publications found no association between FA supplementation during pregnancy and atopic diseases in the offspring.79 Lastly, it seems that specific risk of childhood peanut allergy is not modified by maternal FA supplementation.80
Another study also did not find association between prenatal FA supplementation and adverse respiratory or allergic outcomes in children of 1–8 years of age.81
So, of the 10 articles that cover respiratory and allergic outcomes of the offspring, seven report an adverse effect of FA supplementation, three of them highlighting the adverse effect of supplementation in late pregnancy. However adverse effects are also seen for periconceptional period exposure or for reported high FA intake. Of the 10 articles, only three report no association between FA supplementation during pregnancy or the periconceptional period over these same outcomes. Thus, it seems plausible that FA supplementation, particularly in late pregnancy, will increase the risk of respiratory or allergic outcomes in the offspring.
Behaviour problems, neurodevelopment and psychomotor disordersThese are also some of the most addressed outcomes in what concerns FA supplementation (n=10). The included evidence suggests that FA supplements in pregnancy can be positively associated with children's neurodevelopment at the age of four years,82 being corroborated by other studies showing a possible effect of FA supplementation on enhanced vocabulary development, communication skills and verbal comprehension at 18 months of age (but not doses ≥5mg/day)83 as well as a reduced risk of severe language delay in children at the age of three.84 Nonetheless, evidence also demonstrates no association with child memory at seven years old85 and no significant effect of supplementation on the cognitive function of children.86 Contradictory findings about psychomotor development were established as some evidence points towards a higher risk of delay within the use of FA supplements, at doses above 5mg/day,87 while other authors suggest that prenatal FA supplementation may improve development at the age of three, reporting also a significant poorer performance in the personal-social domain.4 Facing these results, no conclusion can be extrapolated as data seem to be in discordance. In terms of behaviour problems, higher risk of hyperactivity and inattention has been attributed to an inadequate use of FA during the first trimester of pregnancy,17 also estimating that omission errors seem to be lower in children whose mothers took dietary supplementation with FA during pregnancy.88 Additionally, although in a small association and with possible confounding, lower folate status in early pregnancy might impair foetal brain development and induce hyperactivity and inattention problems in childhood.89 Though, still in what concerns behaviour problems, it seems that there is no association between lower folate status during pregnancy and the appearance of such kind of outcomes.
Autistic disorders are also one of the possible outcomes for the offspring and it seems that a lower intake (less than 0.6mg/day) increases the risk of having children with autism spectrum disorders, and this risk was strongest for mothers and children with inefficient folate metabolism (MTHFR 677C>T variant genotypes).90 Also, a more recent cohort study suggests that prenatal FA supplements around the time of conception (dose of 0.4mg/day) seem to be positively associated with lower risk of this type of disorders.91
Foetal growth and other outcomesA high FA dose (4mg/day) in the periconception seems not to compromise foetal growth, when compared with a recommended dose of 0.4mg/day.48 Other study reported that the periconceptional use of FA supplementation greater than 1mg/day can be associated with decreased birth weight.92 However, periconceptional FA supplementation in recommended doses (0.4–0.5mg/day) can be associated with increased foetal growth, and higher placental weight, resulting in higher birth weight and decreased risk of low birth weight or small for gestational age (SGA).93 On the other hand, low dietary folate intake or low plasma folate concentrations during the second trimester of pregnancy seem not to affect birth size.94 In addition, a trial comparing maternal multi-micronutrient with iron-FA or FA only did not support a greater advantage of the first on child growth during the first 30 months.95 Notwithstanding, foetal growth restriction commonly observed in situations of short interpregnancy intervals, was more frequent when folate depletion occurred.96 Once again, inconsistency regarding the effects of FA supplementation in the periconceptional period is observed. Regarding the impact of a low folate status, it seems relevant for foetal growth impairment only when it results from short interpregnancy intervals.
Other observed effects of FA supplementation during pregnancy were bone turnover/development with the suggestion that daily FA supplementation of 1mg during pregnancy could have a positive impact in bone turnover both in mothers and newborns, thus concluding that both pregnant mothers and their foetuses could benefit, in this particular parameter, from FA supplementation during the whole pregnancy.97
Another older publication refers to an association between maternal folate intake (cohort study with mean FA supplementation of 0.257mg/day) and bone development in childhood, observed as volumetric bone mineral density of the spine regardless of height and weight.98 Both studies lead to the expectation that FA supplementation may be beneficial in terms of bone health later in life.
In addition, FA intake during foetal development was causally related to subtle and persistent DNA methylation (IGF2/H19) in the offspring of FA supplemented mothers and such results may lead to a further understanding of the foetal origin of adult diseases,99 also corroborated by other authors.100,101 Other observed isolated outcomes were, for instance, semen characteristics of the offspring, with no differences found between those whose mothers did not take FA or with unknown supplementation, in relation to the supplemented ones.102 No evidence was found to support the hypothesis that maternal dietary folate intake and intra-uterine exposure to folate is related to body composition of the offspring at the age of nine years,103 as well as no association on offspring's blood pressure was documented.104 An experimental study in Nepal revealed that FA supplementation during pregnancy appeared to have the greatest beneficial effect on both offspring's kidney function and lower risk of metabolic syndrome among the offspring.105 Finally, there was no support on the idea that maternal FA intake during pregnancy contributes to common aetiological pathways that are shared between schizophrenia and non-clinic psychotic symptoms in adolescents.106
Human studies: health outcomes of pregnancy or the motherLooking into the casuistry of the outcomes of FA supplementation in pregnancy or maternal health, 18 papers were identified, roughly divided in pregnancy complications or delivery outcomes (n=13) (Table 3). Seven of these papers cover placental abruption and preeclampsia, six are associated to preterm birth or gestational age, three focus on abortion and other two on haematological status/homocysteinemia (n=2).
Pregnancy complications, abortion and delivery outcomesNo concordance was found on the protective effect of FA supplementation regarding preeclampsia and gestational hypertension (GHT). Some studies found that this intervention may help to prevent preeclampsia and associated SGA107,108 as well as placental abruption.109 Meanwhile, other research found that the dose of 0.4mg/day FA alone in the early pregnancy cannot prevent GHT and preeclampsia.110
Regarding preterm delivery we found four studies where a protective effect of preconceptional and/or prenatal FA supplementation has a protective effect109,111–113 and another study114 that reported no protective effects or even an increase of the risk if the supplementation starting more than 8 gestational weeks. Shaw et al.115 concluded in their study that the role of FA on preterm delivery is no clear.
Concerning abortion, results were totally different among the publications, as some defend that high dose FA (4mg/day versus 0.4mg/day) does not increase miscarriage risk116 while others conclude that FA supplementation was protective on missed abortion.117,118 So, it is difficult to conclude about the real outcomes of FA supplementation, when regarding these areas, even considering studies involving large sample sizes.
Haematological status of pregnant womenOther studies analyzed haematological status of pregnant women and reported reduced risk of anaemia in the first trimester for 0.4mg/day FA supplementation, but not latter in pregnancy119 and that higher doses of FA are not associated with the lowering of homocysteine concentration.120 With two totally different outcomes in terms of haematological status, no conclusions can be drawn.
DiscussionA period of ten years retrieve more than 1000 papers dedicated to the thematic of FA supplementation in the context of pregnancy which were conducted all over the world. After thorough filtration for inclusion/exclusion criteria even the remaining papers corresponded to widespread contributions from the different continents reflecting the growing and widespread scientific interest in this particular area of knowledge.
Although reviews were excluded from the study, they indeed represented a huge part of the total papers retrieved in the search (n=227 of the 1182) and this also shows the growing interest and need of systematization of the huge amount of gathered information on the use of FA supplements during pregnancy.
Animal trials focused essentially on gene expression and methylation, and they found evidence to support that gene expression and methylation suffer the influence of high doses and longer periods of FA supplementation.22,23,34
In what concerns human studies, from this review we can extract several general findings: first, we found studies covering associations of FA supplementation with a wide range of health outcomes over the offspring or over the mother/pregnancy. Second, the observed associations are beneficial effects in the majority of the studies, but there are also many studies reporting either no effects of (high) FA supplementation during pregnancy or, importantly, adverse health effects. In addition, regarding the increasing concerns about the potential health effects of excessive exposure to FA during in utero development, there are just a small number of studies with the ability to conclude about this issue. Indeed, more human studies, and specifically human trials, are needed to ascertain about the safety of exposure to excessive amounts of FA during pregnancy. However we should be aware of the ethical issues that arise in this kind of studies. Throughout the review, we found few human trials and, as they focused on the effect of FA associated with other vitamins and/or nutrients, almost all of them were excluded as it was not possible to attribute the observed effects to FA supplementation per se.
Although, numerous outcomes associated with exposure to FA have been studied and some findings appear to be clear and consistent throughout the literature, others are not. There are several factors that may contribute to this difficulty in establishing clear associations: firstly, the difficulty in establishing the timing of supplementation with accuracy (more feasible in cohort studies, but very difficult in case–control studies, widely used for the study of rare outcomes, such as congenital defects or tumours), the dosage supplemented, as well as the combination of these two pieces of information – the timing and dosage – analyzing the data. Moreover, it cannot be forgotten that the studied outcomes are not entirely justified by FA supplementation but that in addition many of the determinants of taking folic acid – e.g. socio-economic factors, planning and vigilance of pregnancy – may also determine the studied outcomes – e.g. preterm delivery, size at birth. Also, many of the included publications describe studies that were performed in countries where fortification is mandatory, that may be a confounder weakening the magnitude of the association between the supplementation and outcomes.
In general lines, the main outcomes of the publications included in this review are in offspring's health, namely in relation to congenital defects, allergy/respiratory problems, cancer and psychomotor and neurodevelopment outcomes. As a great variety of studies were focused on diverse outcomes, and established the role of FA supplementation throughout different dosages and timings of exposure, and even in different populations (e.g. with mandatory fortification), it is hard to conclude about the real implications of such intervention. Regarding NTDs, the protective role of FA supplementation seems to be unquestionable. It seems consensual that a supplementation of 0.4mg FA in the periconceptional period allows the protection against NTDs, with no associated harmful effects. The harmful outcomes, including epigenetic changes, that have been subject of much attention, are associated with the supplementation that occurs at higher doses and/or extended throughout pregnancy11,22,23,101 and not just in the periconceptional period.
Regarding animal studies, many of them use the recommended dose of FA for pregnancy in the rat (2mg/kg of diet) but also higher doses of FA (corresponding up to as much as 20 times the recommended dosage, with 8mg/kg of diet as the most commonly used). This allows us to observe clearly the health impact of excessive doses of FA, much more than in the human observational studies. Although they have obvious limitations in extrapolating the results to human reality, these studies are very important because they allow freedom for interventions that may give clues to the effects which cannot be obtained in human trials.
It is somehow a priority to inform all health professionals who work with pregnant women that despite FA is a water soluble vitamin which is therefore easily excreted whenever it is in excess, in cases of FA supplementation (with the synthetic form) this excess may not be as harmless as it is thought to be and the real impact in later life is still not totally known.11 So, it is important to raise the awareness on the importance of respecting the right doses at the appropriate intervals, clarifying its safety.
As referred above, there is an urgent need to establish the critical dosages, and also importantly the critical timings to achieve the best outcome, avoiding possible harmful endpoints. Also, we find that it would be interesting to know more about what the professionals that daily work with pregnant women know about this issue, which will represent perhaps the most important key of intervention.121 We suggest that research should be performed in order to understand exactly how they prescribe on the field, their knowledge, their doubts and also, possible confounding factors that may interfere with the prescription of this type of supplementation, namely if the pregnant women's opinion/knowledge is important or not in their decision.
Limitations of this review should be considered. First of all, we only included a period of ten years, allowing the potential omission of the information published mainly before the time period considered. However, considering the huge amount of papers published in this period, we believe that the current state of the art is herein suitably represented. We are also aware that our inclusion/exclusion criteria may influence our findings. But even considering only healthy pregnancies, without any adverse exposure, authors obtained contradictory results. So, the inclusion of such additional studies would certainly tangle the revision and possibly impair the extraction of clear conclusions. Another point that may weaken this review is the fact that not all the material obtained was, in fact, read by all of the three authors. Although this may lead to possible gaps and confounders, it was the best methodological strategy that enabled the achievement of our objectives. Importantly, the meetings, and the discussion and establishment of inclusion/exclusion criteria, contributed to the homogenization of the global work. Lastly, we found it difficult to discuss/conclude on the real and critical temporal windows of FA supplementation, either in terms of timings or doses. This task was hard because, for the same kind of outcome we obtained different type of studies, sample sizes and interventions. In view of this, we consider that any conclusions extracted may represent very useful information.
We also find many strengths in our work. First, it is a summary of the evidence of the different works all around the world, regarding FA supplementation and its possible beneficial and harmful outcomes, not only in terms of pregnancy itself, but also in mothers and offspring's future health. So, this review may be an important tool for the identification of questions for future research, as well as in terms of health practices, as it intends to highlight the global knowledge in the last years. Also, as we only included outcomes directly related to isolated FA supplementation, focusing on the central matter, the gathered data may be a start point for future works, namely regarding the definition of critical temporal windows and safety doses of exposure, an emergent contributor for Public Health policies.
ConclusionsThere is an unequivocal growing interest around FA supplementation in pregnancy, reflected by the number and sort of studies that we found when performing this review, and also the diversity of specific outcomes they focused.
The majority of the publications reported FA supplementation effects on the offspring's health, with emphasis in areas such as NTDs, allergy/respiratory problems, cancer and behaviour problems. In terms of each general outcome, we frequently observed some heterogeneity, except for the case of NTDs. In spite of the possible protective effect of the FA supplementation in many outcomes (e.g. NTDs, heart defects, other general congenital malformations, autistic disorders, birth size, bone turnover, abortion, as others), we also denote a rising concern on the excessive effects of supplementation, whether in terms of doses or times of exposure.
Animal trials, even though representing a small part of this review and with obvious difficulties in extrapolation for human reality, also contribute towards the understanding of the molecular and particularly epigenetic pathways that may be the base of offspring's (and also maternal) persistent modifications liable to cause disease in the future generations.
In terms of real and perceived critical temporal windows of supplementation, we can see that the protective effect of FA supplementation against NTDs is very prevalent in the periconceptional period, while the adverse effect on allergy/respiratory problems is much associated with exposure in later periods of pregnancy, mainly if this exposure lasts the whole pregnancy period. Regarding the effective dose, it seems that a daily dose of 0.4mg in the periconceptional period causes no damage.
Critical doses and time of exposure should be the focus of future research in this area.
Conflicts of interestThe authors declare no conflicts of interest.