Platelets have significant role in modulating clot formation. Additionally, emerging data indicates that platelets have considerable roles in inflammation and immune response. Platelets gather at the damaged cite and adhere to white blood cells. Subsequently, they release cytokines and chemokines which are chemotactic for neutrophils and monocytes. Therefore, platelets are necessary for targeting lymphocytes, neutrophils and monocytes to inflammation site. Those interactions enhance inflammation. Moreover, platelets serve as an immune cell by engulfing microbes. Presence of platelets affect prognosis in some bacterial or viral infection and several other diseases.
Platelets are anucleated discoid shape hematopoietic cells which have considerable roles in modulating hemostasis. Recent studies indicate that platelets are also involved in inflammation, infection, host response and even cancer. Platelets express and secrete adhesion molecules to accumulate in damaged sites. Adhesion molecules favor adhesion of platelets to leukocytes and granulocytes. Furthermore, platelets secrete immune modulators which are chemotactic for neutrophils, monocytes and lymphocytes. Those interaction results in formation of platelet-granulocyte or platelet-leukocyte aggregates which triggers further inflammation.1–3 Platelets are also involved in natural immunity because they can capture and engulf microbes. In addition; they prevent dissemination of bacteria by clot formation.3,4
GranulesPlatelets are small fragments of megakaryocytes. There are 150–400.000 platelets per microliter of blood. Each of them contain three types of granules: alpha [chemokines such as: CXCL7, CXCL4 (PF4), CXCL1 (GROa), CXCL5, CCL5 (RANTES), CCL3 (MIP1a), coagulation factors, Platelet-derived growth factor receptors (PDGF), Transforming growth factor beta (TGF-b), P-selectin, fibrinogen, vWF, fibronectin,], dense (calcium, magnesium, nucleotides (ADP,ATP), serotonin, histamine) and lysosomal [gylcohydrolase, proteases (cathepsin, asid phospatase,colagenase, elastase)] granules.5 P-selectin is an α-granule derived mediator which facilitates rolling and tethering of leukocyte and adhesion of leukocytes to endothelium following to activation of platelets. Dense bodies induce vasoconstriction, production of pro-inflammatory cytokines and modulation of inflammation.6 Dense granules contains high amount of serotonin. Recent research reveals that recruitment of neutrophils is promoted by platelet derived serotonin in acute inflammation.7 Ions such as Ca and Mg probably effects signal transduction during all those interactions. Some enzymes (e.g. cathepsin) of lysosomal granules nonspecifically breakdown microbe so they are classified as first line defenders of immunity.3
Pro-inflammatory cytokines are released in inflammation and accepted as one of the key regulatory of inflammation. Those cytokines can be secreted from different cell types, have different targets and activate different pathways. Interleukin-1 (IL-1) is an important cytokine secreted mostly by monocytes and macrophages that stimulate acute phase reactants, fever and adhesion molecules. Evidence indicates that platelets secrete IL-1 as well.8 Activated platelets induce dendritic cells (DC) to release immunoregulatory cytokine IL-10.9 In addition; platelets can stimulate monocytes which in turn secrete Interleukin-8.10
ChemokinesGranules of thrombocytes contain chemokines produced by megakaryocytes. RANTES (regulated on activation, normal T cell expressed and secreted, also known as CCL5) is a CC chemokine family member. Monocytes tether to endothelium via P-selectin and this induce platelet derived RANTES release. Thereby, recruitment of further monocytes from circulation is triggered.10,11 Platelet derived RANTES has also immune-modulator affect. RANTES enhance cytotoxic ability of CD8 T-helper cells and cytokine production in CD8 T-cells.12 Furthermore, RANTES mRNA expression increases after platelet interacts with B-cells and IgG synthesis from differentiated B-cells is promoted13 CXC chemokines such as IL-8, neutrophil-activating peptide-2 (NAP-2) coordinates recruitment and activation of neutrophils.14,15 Beta-thromboglobulin (β-TG), platelet factor 4 (PF4) are also CXC class chemokines that are chemotactic for neutrophils. PF4 induces differentiation of monocyte to macrophages and augment monocyte survival.16 PF4 can directly kill intra erythrocytic parasites after contact with parasitized cells.17 Thus, platelets might be accepted as natural anti-parasitic. Moreover, platelet derived RANTES and PF4 augment surface monocyte arrest. As a summary, platelets are necessary for targeting lymphocytes, neutrophils and monocytes to inflammation site.A chemokine derived peptide; “Thrombocidin” which is antibacterial and antifungal is stored in α-granules Thus, platelets has an impact on innate immune system.18,19
Pattern recognition receptorMembrane of a platelet is covered by a great number of receptors such as transmembrane, pattern recognition and FcR receptors that are stimulated by paracrine, exocrine and autocrine signaling. Toll like Receptors (TLR) are members of pattern recognition receptor family. Recent researches confirm that there is expression of TLR1, TLR2, TLR4, TLR6, TLR9, TLR7 and TLR9on platelets. They are activated upon interaction with a stimulator like viruses, microbes or other hematopoietic cells.20–24 Encephalomyocarditis virus (EMCV) activates the platelet-TLR7 receptor. Subsequently, thrombocytopenia is observed because platelet interacts with leukocytes and forms aggregates following to internalization of neutrophils. Therefore, platelets-TLR7 is important in host survival.20 TLR2 recognizes bacteria. Stimulation of TLR2 amplifies P-selectin expression, enhances pro-inflammatory response of platelet and increases formation of platelet-neutrophil aggregation.24 TLR4 enhances bacterial trapping by stimulating formation of neutrophil extracellular trapping (NETs).23 TLR4 activation by bacterial LPS causes formation of platelet-granulocyte aggregates.25 Platelets also present bacteria to neurophils via toll TLR.2
CLEC-2 (C-type lectin-like receptor) is a kind of ITAM receptor (also known as tyrosine kinase-dependent platelet activation receptor) which is expressed on T cells, platelets, DC and probably other hematopoietic cells. It is also expressed on platelets. Podoplanin (also known as Gp38) is potent ligand for celec-2 receptor and found in lymphatic endothelial cells. CLEC2-podoplanin interaction is important in separation of blood-lymphatic during embryogenesis, proliferation of lymphatic cells, development of lymph nodes, recruitment of lymphocyte in long-term. Briefly it influences development of tissues of immune system and immune response.26,27 On the other hand, CLEC-2 receptors of platelets bind podolomin expressing tumor cells which is considered as one of the metastasis mechanism.28
CD40L (also known as CD154 a member of TNF (Tumor Necrosis Factor) family) is found in platelets and its receptor is CD40 which is described on many cell types including B-cells and antigen presenting cells. CD40-CD40L interaction contributes to binding of platelets to monocytes, macrophages, DCs and lymphocytes. That interaction induce several immune and inflammatory response such as production of superoxide and reactive oxygen species (ROS) in neutrophils, production of antigen specific IgG, activation of B cells, switching of B cell isotype, priming of T cell, formation of germinal center, activation of macrophages, maturation of DC and enhancing cytotoxic T–cell response.1,13,29–32
Complement system induces platelets and vice versa. It has been known for a long time that complement system promotes thrombus formation by activating platelets. However, emerging studies indicates platelets also activate the complement system through P-selectin–C3B interaction.33 Platelets have also receptors for C1Q which has a role in classic pathway.34
Membrane glyocoproteins function as receptors so are involved in adhesion. Leukocyte recruitment after an injury depends on interaction of A mb2 (Mac-1) integrin of leukocytes and glycoprotein (GP) Ib-a of platelets.35
Platelets express high affinity IgE receptor (FcepsilonRI) on their cell membrane. Stimulation of FcepsilonRI receptor triggers release of serotonin and RANTES and subsequently promotes IgE mediated allergic reaction.36
Adhesion moleculesAdhesion molecules are secreted from α-granules of platelets and they are quite important in thrombus formation. As it is mentioned before, P-selectin is a type of adhesion molecule is secreted from α-granules. Main function of P-selectin is mediating rolling and tethering through adhesion. However, recent researches reveal the key stone role of P-selectin in platelet mediated inflamation. P selectin interacts with those expressing PSGL1 (P selectin glycol protein) such as neutrophils, monocytes, DC, endothelial cells and other activated platelets. P-selectin-PSGL1 interaction results in production of superoxide anion radicals in macrophages and monocytes, stimulation of neutrophil rolling, transendothelial migration and leukocytes integrin activation. Furthermore, cell adhesion via P-selectin regulates gene expression in leukocytes. Generation of dentritic like cells from monocytes by P-selectin stimulation is seen experimentally. As a result, DC might present antigens captured by platelets due to DC-platelet interaction through P-selectin. Ultimately, disturbing P-selectin-PSGL1 interaction reduces inflammation.2,37–43
Experimentally, activation of TLR7 agonist enhance p-selectin expression and stimulate platelets-white blood cells (WBC) interaction.20 Thus, we can conclude that adhesive molecules of platelets might be necessary to attract granulocytes to injured sites. Integrins are transmembrane receptors which have significant impact on platelet adhesion. Different subtypes of integrins are discovered in different cell types including hematopoetic cells (such as leukocyte and platelet), collagen and endothelial fibroblasts. β1, β2 and β3 integrins are expressed on platelets and mostly involved in adhesion of platelets to extracellular matrix and fibrinogen.44,45
ICAM-2 (intercellular adhesion molecule 2) is a member of Ig superfamily and the only ligand of β2 integrin presents on platelets. ICAM-2 of platelets contribute to adhesion and tethering of T cells via binding of leukocyte integrin; LFA1 (Leukocyte function antigen1).46 In short, leukocyte-platelet interaction requires integrins.
Role of platelet in certain diseasesEven the exact reason is still uncertain; in many viral infections non-immune thrombocytopenia is observed. Thrombocytopenia may be a sign of infection, due to the fact that platelets are recruited to the site of inflammation and adhere to WBC to enhance their affect and form aggregates so the number of circulating thrombocytes decreases. Platelets could be related to prognosis of viral infection. Furthermore, platelet is a SOFA (Sequential Organ Failure Assessment) parameter which is used in sepsis evaluation.47 Coxsackie virus B is a picornoviridia family member and it carries linear positive sense ssRNA. It is the most common cause of myocarditis. If platelets are depleted, the risk of myocarditis development increases.48 Dengue virus is positive-stranded RNA virus of the flaviviridae family and transmitted through mosquitoes. It causes dengue fever. Thrombocytopenia is seen due to mitochondrial dysfunction and apoptotic caspase stimulation in case of Dengue virus infection.49 EMCV infection may cause TLR-7 mediated thrombocytopenia, probably due to formation of platelet-leukocyte aggregates and more importantly presence of platelet TLR7 increases survival during infection in mices.20 Human immunodeficiency virus (HIV) is retrovirus which may causes acquired immunodeficiency syndrome (AIDS). It is demonstrated that platelets can engulf HIV virus.4 However, consequences of this engulfment are contradictory. Virus engulfment might hide virus from further immune reactions or it may prevent dissemination of virus. Experimental autoimmune encephalomyelitis is mouse model of Multiple Sclerosis (MS). Myelin sheath destruction, inflammation and lesion formation are main characteristics. In a study on experimental autoimmune encephalomyelitis, platelets were shown to promote CNS inflammation.50
Platelets are engaged in bacterial infection as well. Platelet can directly engulf bacteria namely Staphylococcus aureus which is responsible from mild to life threatening infections including skin infections, pneumonia, endocarditis and osteomyelitis.4 Bacterial engulfment by platelets might be accepted as first line defense mechanism of the immune system. Bacterial lipopolysaccharides (LPS) are outer surface membrane of gram-negative bacteria and affect immune response of human to bacteria. LPS stimulates secretion of α and dense granules and expression of P-selectin on platelets through TLR4 signaling pathway. TREM1 is a member of Ig superfamily expressed by myeloid cells. A ligand for TREM-1(triggering receptor expressed on myeloid cells) has recently found in platelets. TREM-1 ligand activates neutrophils in presence of LPS.25,51 On the other hand, platelets may cause dissemination of bacteria for example; Streptococcus pyogenes binds to the platelets and so bacteria spread.52
Neurophil extracellular trap formation (NET)Neutrophils play a role in resistance to pathogens mainly via three mechanisms: antimicrobial cytokine secretion, engulfment and NET formation.53 Following to administration of virus such as Poxvirus or bacteria, platelets adhere to surface of neutrophils and extracellular fiber matrix called NET is released.23,54 NET formation is induced by activation of platelet TLR4 in severely septic human plasma.23 In another study; inhibition of activated platelets reduces NET formation in vivo experiment of transfusion-related acute lung injury (TRALI).55
Aggregate formationChronic or continued interaction of platelets with endothelial cells or WBCs can cause excessive immune stimulation or complex accumulation.3 Excessive platelet-monocyte complex accumulation might be a finding of vascular diseases, for instance: Elevated level of monocyte-platelet aggregates are accepted as early hallmark of MI acute myocardial infarction (AMI).51,56 Furthermore, circulating platelet–leukocyte aggregates (PLAs) might be a marker of sepsis.57 Additionally, aggregate formation may lead to atherosclerosis and platelets may facilitate it because α-granules contain pro-angiogenic proteins.5,58
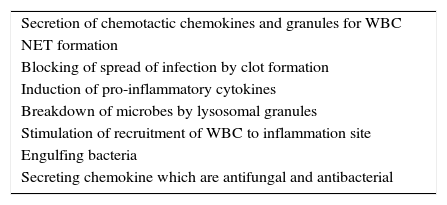
SummaryTo conclude, platelets are involved in immune response by direct and indirect mechanisms and have many significant roles in fighting infection (Table 1). Multiple interactions between platelets and other immune cells, expressing immune receptors, secreting immune mediator indicates the roles of platelets in immune system and inflammation. Furthermore, functional platelet activation might increase therapy response in situation of inflammation or infection. However, if platelets are activated continuously or chronically, this may cause different uncontrolled diseases notably vascular diseases especially atherosclerosis.
Roles of platelets in immune system.
| Secretion of chemotactic chemokines and granules for WBC |
| NET formation |
| Blocking of spread of infection by clot formation |
| Induction of pro-inflammatory cytokines |
| Breakdown of microbes by lysosomal granules |
| Stimulation of recruitment of WBC to inflammation site |
| Engulfing bacteria |
| Secreting chemokine which are antifungal and antibacterial |
The authors declare no conflicts of interest.