According to the GLOBOCAN, gastric cancer is the fifth most common cause of cancer and the third most frequent cause of cancer-related death, in both sexes, all over the world. It often presents late in life, bearing a poor overall survival. Mass screening programs are not cost-effective in most countries and therefore primary prevention and personalized treatment are regarded as the best options to reduce gastric cancer mortality. Immune inhibitory checkpoints, such as Programmed cell Death-1 (PD-1):Programmed cell Death-Ligand 1 (PD-L1), allow the tumor to evade immune destruction — a potential new hallmark of cancer, through innate and adaptive immune resistance mechanisms. PD-1 monoclonal antibodies, nivolumab and pembrolizumab, are already approved therapies for advanced stage melanoma. This review addresses PD-L1 significance in Helicobacter pylori infection persistence and gastric cancer development, providing rationale for PD-L1 targeted therapies.
During the 20th century, the incidence of Gastric Cancer (GC) dropped by over 85% in the United States and a similar decline has been reported in other Western countries.1 However, in 2012 GC accounted worldwide for 951.594 newly diagnosed cancer patients (6.8% of all cancer new cases) and for 723.073 cancer-related deaths (8.8% of all cancer-related deaths), being the fifth most common cause of cancer and the third most frequent cause of cancer-related deaths, in both sexes.2
Despite advances in the recognition of new risk factors and prevention, diagnosis and treatment, GC remains a global health problem and carries a poor overall survival.3 TNM staging (Tumor, Node, Metastasis) remains the most powerful prognostic factor for GC and, whenever possible, surgical resection is the cornerstone approach of GC treatment. If GC is recognized at early stages, e.g. when they are confined to the mucosa or submucosa (so-called “Early GC”), the 5-year survival rate after gastrectomy can exceed 90%, irrespective of lymph-node metastasis status. Nevertheless, most GCs present at an advanced stage, with a 5-year survival rate after surgery remaining below 20%.4
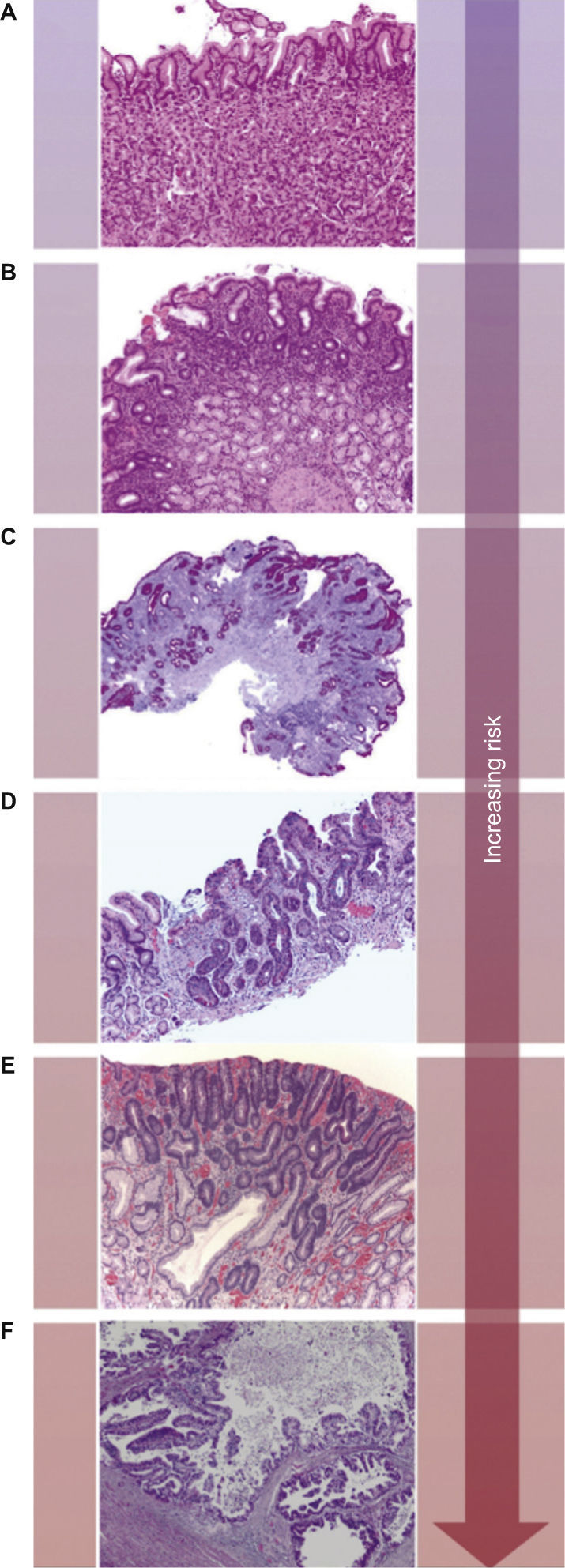
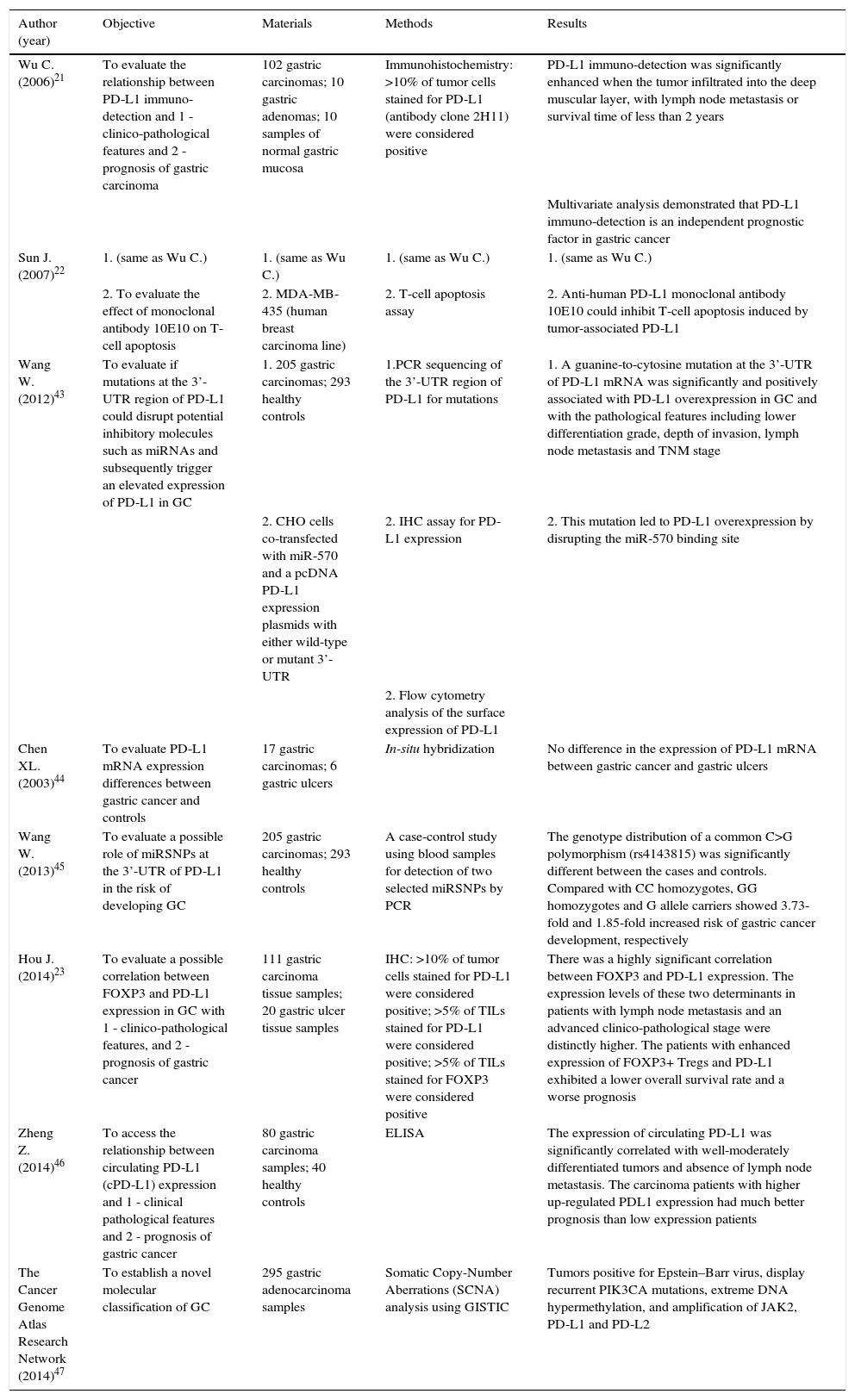
Adenocarcinoma is the most common malignancy of the stomach, comprising over 90% of all GCs. The Lauren classification is often used to classify GC into three broad categories, namely intestinal type, diffuse type and a remaining group of GC that cannot be placed in one of these two categories (indeterminate/unclassified type). Intestinal type GC is composed of tumor cells with glandular, tubular or papillary growth pattern with various degrees of differentiation.5 This subtype occurs in elder patients and are often preceded by a precancerous process, frequently initiated by Helicobacter pylori (H. pylori) infection, defined as the Correa's cascade (arising in a background of chronic gastritis and progressing to chronic atrophic gastritis, intestinal metaplasia, dysplasia and eventually carcinoma) (Fig. 1).3
The Correa's cascade of multistep gastric carcinogenesis. The model describes a sequence of precursor conditions with increasing risk for development of intestinal-type gastric carcinoma. (A) Normal mucosa. (B) Chronic gastritis. (C) Chronic atrophic gastritis (Periodic-acid-Schiff-Alcian blue stain). (D) Intestinal metaplasia. (E) Dysplasia. (F) Intestinal-type gastric carcinoma. Adapted from: Hartgrink et al.3.
Inhibitory checkpoints, involving Programmed cell Death-Ligand 1 (PD-L1; B7-H1 or CD274), are a group of molecular mechanisms able to down-regulate immune responses and, consequently, are thought to play an important role in the persistence of chronic infections and tumors. This review addresses the possible role of PD-L1 expression in the persistence of H. pylori infection and the development of GC, and aims at providing rationale for new therapeutic approaches (“targeted therapies”). To better introduce and approach our main topic of discussion we divided the article in six major sections, addressing the following issues: 1 - how the immune system may facilitate tumor progression (“Tumor microenvironment and cancer immunoediting”); 2 - the potential application of tumor immune infiltrate characterization into the clinical setting (“Tumor immune infiltrates and clinical translation”); 3 - the mechanisms of PD-L1 expression and its effect on T-cells (“The PD-1:PD-L1 T-cells inhibitory checkpoint”); 4 - clinico-pathological correlation and prognostic value of PD-L1 expression in tumors (PD-L1 expression and significance in tumors); and, 5 and 6 -, the role of PD-L1 in H. pylori infection and GC (PD-L1 in Helicobacter pylori infection; PD-L1 in gastric cancer).
Regarding the search strategy and selection criteria of the last two sections (5 and 6), we searched Pubmed, using MeSH terms, with the entries (“Antigens, CD274” AND “Stomach Neoplasms”) and (“Antigens, CD274” AND “Helicobacter pylori”), for articles in English published until January 2015. All articles were included. We generated the final reference list on the basis of significance and originality for a more comprehensive review.
Tumor microenvironment and cancer immunoeditingIn the multistep process that leads to the formation of a new malignant tissue, transformed cells progressively acquire a series of biological capabilities allowing them to grow, survive, invade and metastasize. Such capabilities, denominated the “hallmarks of cancer”, include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis and activating invasion and metastasis.6
Carcinogenesis does not merely involve neoplastic cells: increasing evidence establishes that tumor-associated stromal cells, forming the so-called tumor microenvironment, that includes extracellular matrix, cancer-associated fibroblasts, tumor vasculature and immune cells, interact with tumor cells contributing to the acquisition of hallmark traits.7 Regarding immune cells, one might think that this ever-alert system serves as a barrier against carcinogenesis and cancer progression, which is not always the case. Actually, data support the concept of “cancer immunoediting”, a process in which immunity has not only a host-protective function, by recognizing and protecting tissues from nascent tumour cells (“cancer immunosurveillance”), but also a tumor-promoting action.8,9
The interplay between immune system and cancer has three main sequential phases: elimination, equilibrium and escape.8,9 Tumor genomic alterations are prone to generate foreign antigens that can trigger the immune response and activate the “cancer immunosurveillance” mechanism.10 Tumor cells are mainly eliminated by this response; however, tumor cell variants may be maintained in a state of dormancy, with the immune system controlling its outgrowth and editing its immunogenicity, but not eliminating them.11 When this equilibrium is broken, tumor cells successfully progress to the escape phase during which an edited and poorly immunogenic tumor evolves.8,9
Infiltrating immune cells supply multiple bioactive substances, such as mitogenic growth mediators, proteolytic enzymes or angiogenic cytokines, to the tumor microenvironment and to neoplastic cells, contributing to the acquisition of hallmark traits.7 Pro-tumorigenic infiltrates are characterised by: 1 - the presence of macrophages of the M2 subtype, Myeloid Derived Supressor Cells (MDSCs), neutrophils, FoxP3+ T regulatory cells (Tregs) and Th17 cells; 2 - a peritumoral distribution of immune cells and 3 - a Th2 and Th17 cytokine profile.12 On the other hand, anti-tumor immune infiltrates are characterized by: 1 - the presence of dendritic cells (DCs), macrophages of the M1 subtype, cytotoxic T lymphocytes (CTLs) and Natural Killer cells (NKCs); 2 - an intratumoral distribution of immune cells and 3 - a Th1 cytokine profile.12 The presence of immunosuppressive cells in the tumor microenvironment, such as MDSCs and Tregs, and immunosuppressive molecules, such as PD-L1, are all mechanisms by which tumor cells are able to evade immune destruction, a concept that, according to Hananhan et al. (2011), emerges as a potential new hallmark of cancer.6
Tumor immune infiltrates and clinical translationCharacterization of tumor immune infiltrates has generated a great interest, since it has been shown to be an independent prognostic factor, as well as a predictive factor of tumor response to therapy.12,13
Regarding the prognostic value of tumor immune infiltrates, Pages F. et al. (2010), analysed data from large cohorts of human tumors and concluded that “infiltration of the primary tumor by memory T-cells, particularly of the Th1 and cytotoxic phenotypes, is the strongest favourable prognostic factor in terms of freedom from disease and overall survival at all stages of clinical disease”.13
Concerning the predictive value of tumor immune infiltrates, Fridman W.H. and colleagues (2011) suggested that the degree of pre-therapeutic immune response can influence the efficacy of conventional chemotherapies and, moreover, that chemotherapy can stimulate anti-tumoral immune responses, correlating positively with tumor mass reduction and longer survival of the patients.12
These evidences underline the importance of the immune system as an active part of the carcinogenesis process and its clinical significance and provide a scientific basis for the therapeutic potential of cancer immunotherapy, such as those based on inhibitory checkpoint blockade.
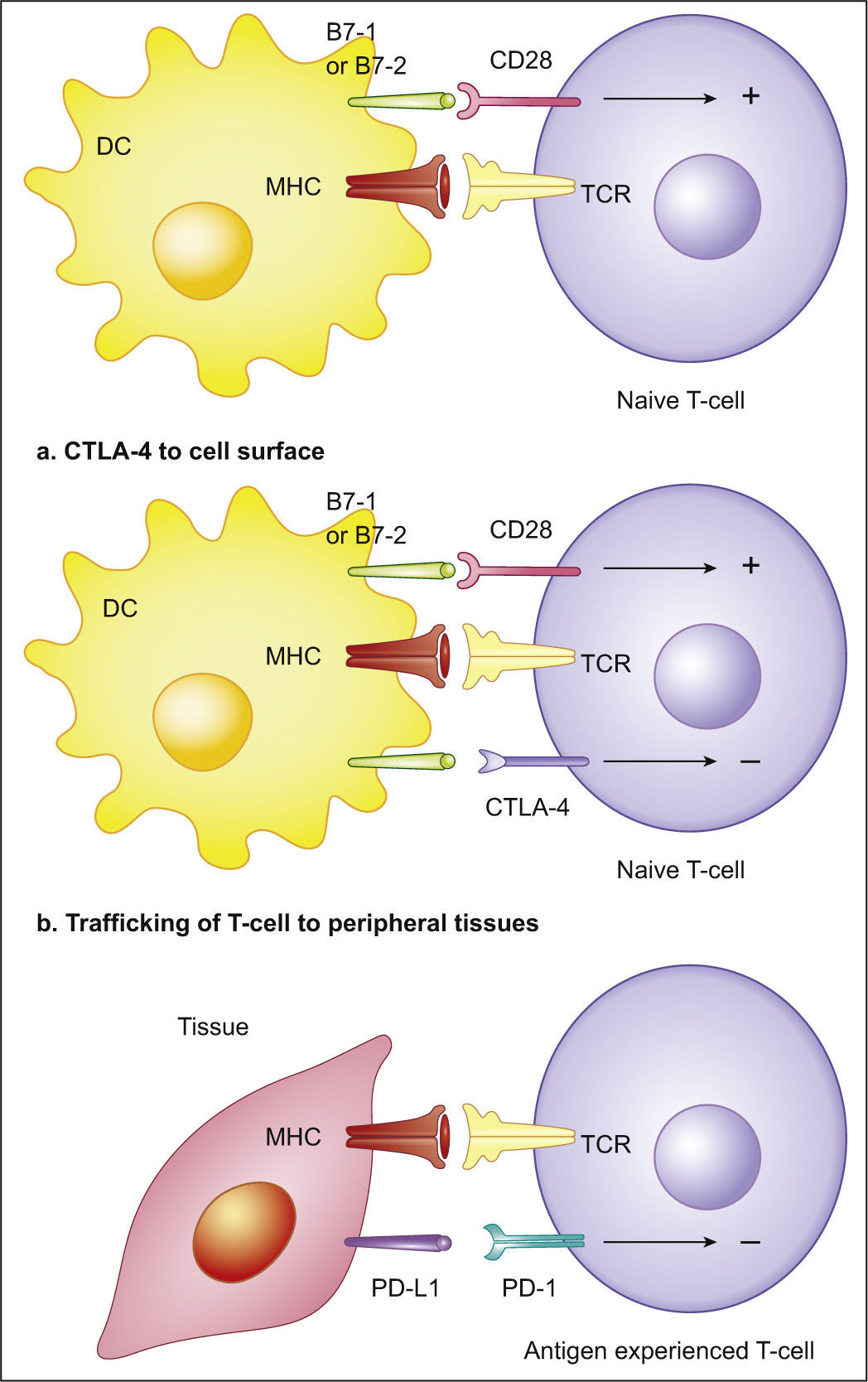
The Programmed cell Death-1:Programmed cell Death-Ligand 1T-cells activationT-cells, in order to be completely activated, require two mechanisms: one, that gives specificity to the immune response, occurs between the T-cells receptor (TCR) and the antigen-MHC complex, on the Antigen Presenting Cell (APC); the other modulates antigen-specific lymphocytes’ response through a balance between co-stimulatory and inhibitory signals, regulating T-cell clonal expansion, cytokine secretion and effector functions.14 The B7 family consists of co-stimulatory and inhibitory molecules that are crucial for an adequate T-cells activity. PD-L1 is an inhibitory molecule of this family. Under physiological conditions, the inhibitory signals are critical for establishing peripheral tolerance and avoiding auto-immune episodes, or for preventing excessive damage to tissues during the immune response against pathogens.14 Therefore, a balance between stimulatory and inhibitory signals is crucial to a finely tuned T-cells activity that is capable both to provide protection against pathogens and emerging tumors and to prevent auto-immunity and tissue damage.14
Programmed cell Death-1Programmed cell Death-1 (PD-1) is the receptor for PD-L1 (Fig. 2) and PD-L2 (B7-DC or CD273) and is expressed on T and B lymphocytes, NKCs, DCs and activated monocytes.14 With regard to T-cells, PD-1 expression does not occur on resting T-cells: the expression is induced after T-cells activation and ligation of PD-L1 must occur close to the TCR in order to accomplish its inhibitory action. Moreover, during T-cells-APC interaction, PD-1 redistributes from the uniform cell surface expression to the intercellular synapse.14 PD-1 mediated inhibitory effects are greater at low levels of TCR stimulation and can be overcome by CD28 costimulation and interleukin-2 (IL-2) activity. PD-1 activation leads to the inhibition of PI3K-Akt signaling pathway, resulting in 1 - inhibition of T-cells proliferation and effector functions (citotoxicity and cytokine secretion), 2 - induction of T-cells apoptosis (by inhibition of Bcl-x survival factor induction) and 3 - promotion of the differentiation of CD4+ naive T-cells into Treg.14–18
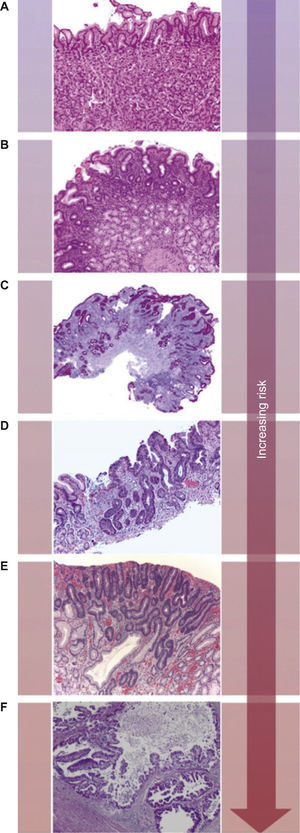
Co-stimulatory and inhibitory signals for T-cells. PD-L1 mediated inhibition of T-cells occurs via ligation to PD-1 and B7-1 on T-cells (full arrows) and through reverse signaling inhibition via ligation to B7-1 on APCs (full arrow). Also depicted are co-stimulatory and inhibitory signals mediated by CD28 and CTLA-4 on T-cells, respectively, through ligation to both B7-1 and B7-2 on APCs (dashed arrows). Adapted from: Keir et al.14.
By contrast, PD-L1 is more ubiquitous, being expressed on T and B lymphocytes, DCs, macrophages, mesenchymal stem cells, bone marrow-derived mast cells and also on non-hematopoietic cells (e.g. lung cells, liver non-parenchymal cells, placental syncytiotrophoblasts and keratinocytes). PD-L1 expression is under the influence of type I and II interferon (IFN): IFN regulatory factor 1 (IRF-1) binding sites on PD-L1 promoter are responsible for both constitutional and inducible PD-L1 expression. MyD88, TRAF6, MEK and JAK2 are involved in PD-L1 induction and loss or inhibition of PTEN (Phosphatase and Tensin Homolog), by activating PI3K-Akt signaling, results in PD-L1 overexpression.14
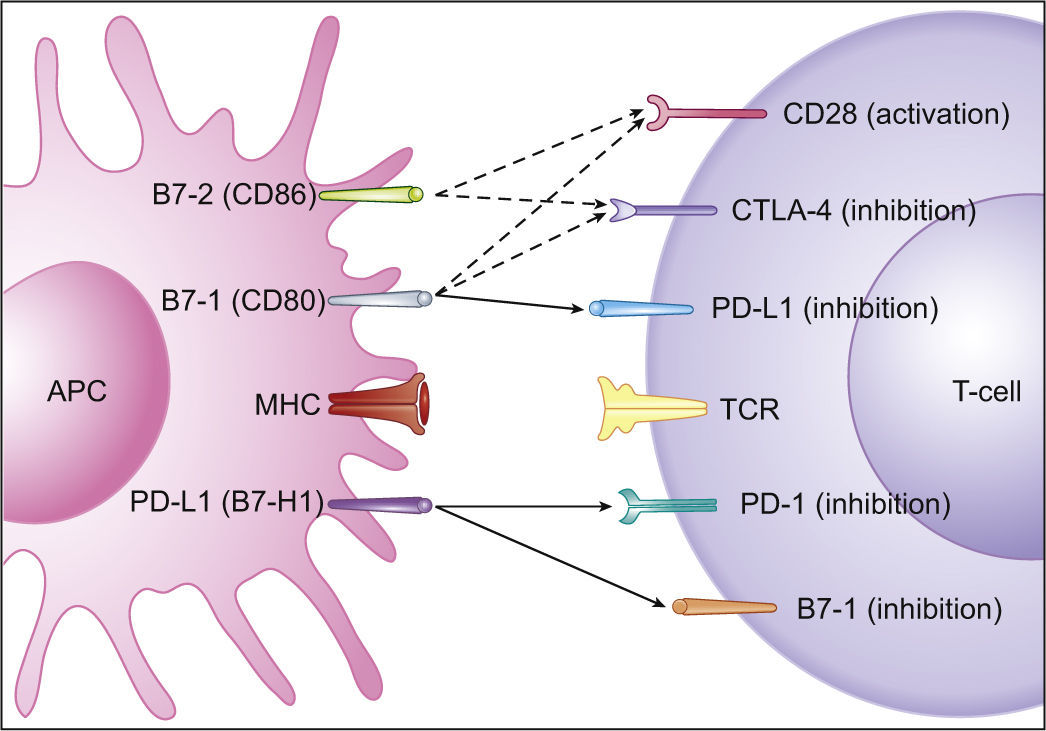
Increasing evidences have identified B7-1 as a secondary receptor of PD-L1, aside from PD-1, that can lead to T-cells inhibition.14,19 Moreover, as PD-L1 is also expressed on T lymphocytes, T-cells response can be inhibited through reverse signaling, as a bidirectional interaction between B7-1 and PD-L1 occurs (Fig. 2).14
PD-L2 expression is much more restricted than PD-L1 expression, being inducibly expressed on DCs, macrophages, and bone marrow-derived mast cells.14
Programmed cell Death-Ligand 1 expression and significance in tumorsA mechanism that tumors exploit to control anti-tumoral immune activity is through continuous ligation to inhibitory checkpoint receptors in lymphocytes, such as PD-1.19 PD-1 acts as a T-cells inhibitor mainly by limiting T-cells activity within neoplastic tissues and its ligand, PD-L1, is often overexpressed on tumor cells (Fig. 3).14,19–23 On the contrary, the other primary ligand of PD-1, i.e. PD-L2, is not usually overexpressed in solid tumors and has not great importance in tumor immune response. When PD-1 ligation occurs it results in decreased lymphocyte proliferation and cytokine production14-16 and increased lymphocyte apoptosis.14,22,24 Although the major role of the PD-1 pathway is to limit activated T-cells effector functions within tissues (T-cell-tumor cell interaction), it can also down-regulate T-cells activation (T-cell-APC interaction).14,19,25
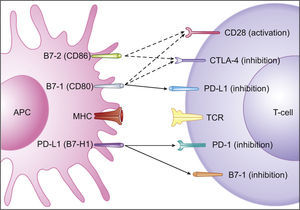
Immune inhibitory checkpoints on T-cells (PD-1 and CTLA-4). T-cells, in order to be completely activated, require two mechanisms: one gives specificity to the immune response and occurs between the T-cells receptor (TCR) and the antigen-MHC complex, on the Antigen Presenting Cell (APC); the other modulates antigen-specific lymphocytes’ response through a balance between co-stimulatory and inhibitory signals, regulating T-cell clonal expansion, cytokine secretion and effector functions. Co-stimulatory signals are mediated by CD28 interaction with B7-1 (CD80) and B7-2 (CD86). Co-inhibitory signals are mediated by (a) CTLA-4 interaction with B7-1 (CD80) or B7-2 (CD86) during antigen presentation, resulting in a lower amplitude activity of T-cells, and (b) PD-1 interaction with PD-L1 expressing tissues, resulting in a limited T-cells activity. DC (dendritic cell); TCR (T-cells receptor); MHC (Major Histocompatibility Complex); CTLA4 (Cytotoxic T Lymphocyte-associated Antigen-4); PD-1 (Programmed cell Death protein-1); PD-L1 (Programmed cell Death-Ligand 1). Adapted from: Pardoll.19
Cytotoxic T Lymphocyte-associated Antigen-4 (CTLA-4) is another immune inhibitory checkpoint, but its ligands, B7-1 and B7-2, are not usually overexpressed on tumor cells.19 Its functions on limiting anti-tumoral immune activity are referable to the regulation of the amplitude of the early stages of T-cells activation, down-modulating Th cells activity (Fig. 3).19 Curiously, both PD-1 and CTLA-4 contribute to an enhanced Treg activity, which can further favor the progression of cancer.14,19 PD-1 also refrains NKCs lytic activity and PD-1 blockade may consequently enhance NKCs anti-tumoral activity.19
PD-L1 expression enables tumor cells to inhibit the anti-tumoral immunological activity and occurs both in a constitutive and an inducible status. Constitutive or innate immune resistance develop through genomic mutations in tumor cells, (e.g. loss of PTEN),26 determining a constitutive PD-L1 overexpression; on the other hand, inducible or adaptive immune resistance arise through inflammatory molecules production by CTLs (e.g. IFN-γ), with the property of inducing PD-L1 overexpression.19
PD-L1 expression has been reported in a wide variety of solid tumors, including lung cancer, hepatocellular carcinoma and intra-hepatic cholangiocarcinoma, gastric, colorectal, pancreatic, ovarian, breast, cervical and oral cancer, head and neck squamous cell carcinomas, nasopharyngeal, esophageal, urothelial and renal cell cancer, nephroblastoma, melanoma and gliomas.14,19–23,27 Moreover, PD-L1 overexpression has been associated to higher tumor pathological stage and identified as a prognostic biomarker of poor survival in lung cancer, intra-hepatic cholangiocarcinoma, gastric, colorectal, pancreatic, ovarian, breast, urothelial and renal cell cancer and melanoma.20,21,27
PD-L1 is the primary ligand of PD-1 and available data indicate an enhanced anti-tumor immunity when blocking this interaction.19,22,28 Currently, three categories of PD-L1 antibodies (Abs) have been identified: 1 - Abs that exclusively block PD-1 interaction 2 - Abs that exclusively block B7-1 interaction and 3 - Abs that block both PD-1 and B7-1 interactions. Differences in Abs specificities may help to explain distinct functional outcomes, namely that monoclonal Abs exclusively against PD-1:PD-L1 or B7-1:PD-L1 pathways should have reduced effects on T-cells inhibition as compared with the dual-specificity Abs.14
Two anti-PD-1 monoclonal antibodies (mAbs), pembrolizumab and nivolumab, have been approved for the treatment of unresectable or metastatic melanoma.29 Recently, Tumeh et al (2014) have found that tumor regression achieved by pembrolizumab on metastatic melanoma patients was associated with the presence of CD8+ T-cells, PD-1 and PD-L1 positive cells on pre-treatment samples, both at the invasive margin and inside the tumor, and with a T-cell population with less-diverse antigen specificity. The most powerful response predictor was the presence of CD8+ T-cells at the invasive margin. Moreover, responders showed significantly more proliferation of CD8+ T-cells compared to the progression group.30
As stated above, specifically targeting PD-L1 with a mAb can have positive effects on anti-tumoral immunity, not only by inhibiting PD-1:PD-L1 interaction, but also by blocking B7-1 ligation and reverse signaling. In 2012, a phase 1 clinical trial demonstrated that the Ab-mediated blockade of PD-L1 activity in patients with advanced cancers, including non-small-cell lung cancer (NSCLC), melanoma, and renal cell cancer, induced durable tumor regression (objective response rate of 6 to 17%) and prolonged stabilization of disease (rates of 12 to 41% at 24 weeks).31 Grade 3 or 4 adverse reactions occurred in 9% of patients.31 Although not compared head to head, toxic effects of anti-PD-L1 mAb appear milder than ones provoked by Ipilimumab (anti-CTLA-4 mAb), the first approved mAb targeting an immune inhibitory checkpoint for the treatment of unresectable or metastatic melanoma. However, no predictive biomarkers of PD-L1 inhibition were evaluated and no immunohistochemical evaluation was performed on tumor specimens, two shortcomings that preclude the adequate selection of the patients who would benefit from this target therapy.
More recently, Herbst et al (2014) conducted another study to evaluate the safety, activity and predictive biomarkers of PD-L1 inhibition, using an engineered humanized anti-PD-L1 mAb (MPDL3280A) on patients with advanced incurable cancer.32 Responses (partial or complete remission), that appeared to be rapid and durable, were obtained in 32 of 175 (18%), 11 of 53 (21%), 11 of 43 (26%), 7 of 56 (13%) and 3 of 23 (13%) of patients with all tumor types, non-small cell lung cancer (NSCLC), melanoma, renal cell carcinoma and other tumors (including colorectal cancer, GC, and head and neck squamous cell carcinoma), respectively. The most commonly reported adverse event was fatigue, which commonly occurred with a low grade fever. Grade 3 or 4 adverse reactions occurred in 13% of patients. The authors detected a statistically significant association between clinical response, a denser immune infiltrate and high levels of PD-L1 expression in pre-treatment samples, especially on tumor-infiltrating immune cells while no statistically significant difference was found between clinical activity and PD-L1 expression on tumor cells.
Another phase 1 clinical trial, enrolling patients with metastatic bladder cancer, enlarged the list of tumors susceptible to anti-PD-L1 mAb treatment (MPDL3280A) and further supported the concept that better response rates are reached when higher PD-L1 expression on tumor-immune infiltrating cells, but not on tumor cells per se, was found in pre-treatment samples.33
Altogether, the above mentioned studies support the notion that PD-1 signaling blockade whether through PD-1 or PD-L1 mAbs is effective when an established immune infiltrate targeting tumor specific mutant antigens (TSMAs), composed of CD8+ T-cells and expressing PD-L1, and whose activity is being suppressed, is already lying in the tumoral tissue and becomes “re-activated”.
A recent report using pre-clinical models found that TSMAs are important targets of T-cells, “re-activated” by checkpoint blockade cancer immunotherapy (specifically, anti-CTLA-4 and anti-PD-1 mAbs), and that TSMAs can be used to generate vaccines that are as effective as anti-CTLA-4 and anti-PD-1 mAbs in inducing tumor rejection.34
Programmed cell Death-Ligand 1 in Helicobacter pylori infectionH. pylori is one of the most common pathogens amongst the world's population and a major cause of peptic ulcer disease, chronic gastritis and GC.35
H. pylori colonization results in a local infiltration of neutrophils and macrophages, as well as T and B cells, some of which are specific for H. pylori antigen.35 However, the infection normally persists, suggesting that H. pylori may alter the normal host immune response. Several studies, addressing H. pylori effects on immune infiltrates and specifically on T-cells, demonstrated that: 1 - T-cells exposed to H. pylori incur in an impaired ability to proliferate36; 2 - H. pylori may cause apoptosis in Fas-bearing T-cells by inducing the expression of Fas ligand37; 3 - VacA H. pylori toxin impairs class II Major Histocompatibility Complex (MHC)-antigen presentation38 and 4 - blocks T-cells proliferation by inducing a G1-S cell cycle arrest.39
Gastric epithelial cells (GECs) may behave as secondary APCs due to their constitutive expression of class II MHC molecules and, interestingly, such expression may be increased during H. pylori infection.40 Moreover, B7-1 (CD80) and B7-2 (CD86) are expressed in gastric epithelium and some studies demonstrated B7-2 up-regulation following H. pylori infection.40
Das S. et al. (2006) first reported that PD-L1 expression on GECs, in vitro, but not PD-L2 or B7-H3, was significantly induced following pylori infection.41 The same results, regarding PD-L1 expression, were reproduced by Wu Y.Y. and colleagues in 2010.42 Furthermore, Das S. et al. (2006) determined that 1 - PD-L1 induced expression was not H. pylori strain specific; 2 - PD-L1 expression was still induced when direct contact between H. pylori and GECs was prevented, suggesting that molecules secreted by H. pylori might be implicated, even though the presence of known virulence factors, such as CagA, VacA and urease B, were not implicated; and 3 - CD4+ T-cells proliferation, cytokine production and surface expression of the activation marker CD69 was impaired by PD-L1.41 On this GECs-T-cells crosstalk, the in vitro studies performed by Wu Y.Y. et al. (2010) added that 1 - PD-L1 expression on GECs is induced by activated T-cells and this can happen without direct contact; 2 - PD-L1 expression on GECs is induced by the Th1 cytokines IFN-γ and TNF-α; and 3 - PD-L1 expression on the surface of GECs mediates T-cells apoptosis.42 Furthermore, it was noted that H. pylori-positive human gastric biopsies showed higher PDL1 expression than H. pylori-negative gastric biopsies. Finally, Beswick et al. (2007) demonstrated, in vitro, that H. pylori-induced PD-L1 expression on GECs can turn naïve T-cells into CD4+CD25+FoxP3+ regulatory T-cells and that these cells can inhibit activated T-cells proliferation.18
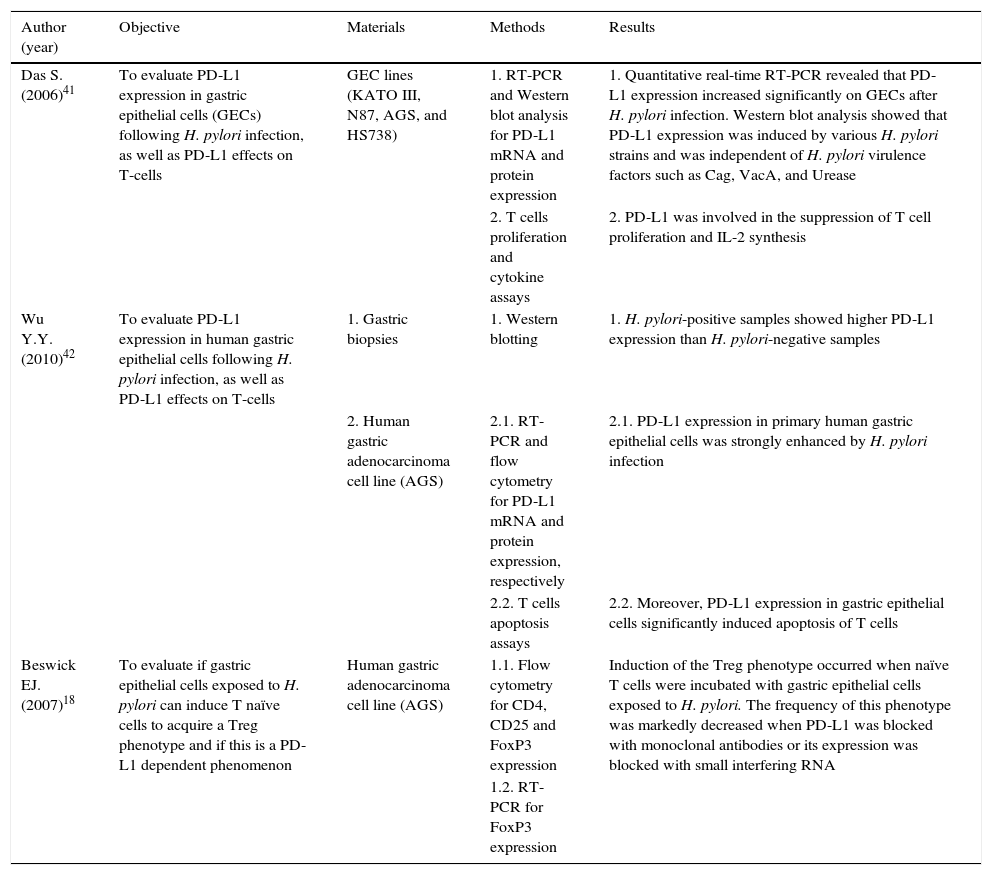
Altogether, these observations support that PD-L1 might concur to the observed T-cells hypo-responsiveness and chronicity of H. pylori infection. Interestingly, this mechanism could also down-regulate immune surveillance mechanisms needed to clear transformed neoplastic cells that may arise within infected gastric tissue, thereby creating a favorable environment for GC development. A summary of the studies describing the role of PD-L1 in H. pylori infection is depicted in Table 1.
Summary of the studies describing the role of PD-L1 in H. pylori infection.
| Author (year) | Objective | Materials | Methods | Results |
|---|---|---|---|---|
| Das S. (2006)41 | To evaluate PD-L1 expression in gastric epithelial cells (GECs) following H. pylori infection, as well as PD-L1 effects on T-cells | GEC lines (KATO III, N87, AGS, and HS738) | 1. RT-PCR and Western blot analysis for PD-L1 mRNA and protein expression | 1. Quantitative real-time RT-PCR revealed that PD-L1 expression increased significantly on GECs after H. pylori infection. Western blot analysis showed that PD-L1 expression was induced by various H. pylori strains and was independent of H. pylori virulence factors such as Cag, VacA, and Urease |
| 2. T cells proliferation and cytokine assays | 2. PD-L1 was involved in the suppression of T cell proliferation and IL-2 synthesis | |||
| Wu Y.Y. (2010)42 | To evaluate PD-L1 expression in human gastric epithelial cells following H. pylori infection, as well as PD-L1 effects on T-cells | 1. Gastric biopsies | 1. Western blotting | 1. H. pylori-positive samples showed higher PD-L1 expression than H. pylori-negative samples |
| 2. Human gastric adenocarcinoma cell line (AGS) | 2.1. RT-PCR and flow cytometry for PD-L1 mRNA and protein expression, respectively | 2.1. PD-L1 expression in primary human gastric epithelial cells was strongly enhanced by H. pylori infection | ||
| 2.2. T cells apoptosis assays | 2.2. Moreover, PD-L1 expression in gastric epithelial cells significantly induced apoptosis of T cells | |||
| Beswick EJ. (2007)18 | To evaluate if gastric epithelial cells exposed to H. pylori can induce T naïve cells to acquire a Treg phenotype and if this is a PD-L1 dependent phenomenon | Human gastric adenocarcinoma cell line (AGS) | 1.1. Flow cytometry for CD4, CD25 and FoxP3 expression | Induction of the Treg phenotype occurred when naïve T cells were incubated with gastric epithelial cells exposed to H. pylori. The frequency of this phenotype was markedly decreased when PD-L1 was blocked with monoclonal antibodies or its expression was blocked with small interfering RNA |
| 1.2. RT-PCR for FoxP3 expression |
In 2006, Wu C. et al. showed that immunohistochemical PD-L1 expression was strongly positive in 42.2% of 102 human gastric carcinomas, weakly positive in adenoma samples and totally negative in normal gastric tissue.21 Moreover, PD-L1 expression was significantly correlated to a higher number of lymph node metastasis, larger tumor size, increased depth of invasion and poorer overall survival (a statistically significant difference was met by comparing the 5-year survival rate observed in patients with or without PD-L1 positive tumors - 30.2% vs 64.5%, respectively). Moreover, in this study, multivariate analysis identified PD-L1 expression as an independent prognostic factor for gastric carcinomas. Same results were presented in 2007 by Sun J. et al., adding that one of the anti-PD-L1 mAbs developed during the investigation significantly reduced the T-cells apoptosis induced by PD-L1 overexpression.22
Later, in 2012, Wang W. et al. observed that PD-L1 expression by immunohistochemistry was found in 42.9% of 205 GC specimens with no expression in normal tissues.43 With regard to PD-L1 mRNA levels within normal and GC specimens, no statistically significant differences were found, as previously verified by Chen X.L. et al.44 These results led Wang W. et al. (2012) to identify a novel regulatory mechanism for the expression of PD-L1 in GC, involving a guanine-to-cytosine somatic mutation at the 3’-UTR of PD-L1 gene, which disrupts post-transcriptional and translational controls mediated by miR-570 leading to PD-L1 overexpression.43 The occurrence of this mutation was significantly associated with a lower differentiation grade, increased depth of tumor invasion, higher number of lymph node metastasis and advanced TNM stage.43 When accessing a possible relationship between PD-L1 mutation and the risk factors linked to GC the authors found a positive correlation with age, smoking and drinking, but not with sex and H. pylori. Wang W. et al. also observed that a single nucleotid polymorphism at the PD-L1 miR-570 binding site was associated with an increased risk for the development of GC, further supporting the hypothesis that PD-L1 may play an important role in GC carcinogenesis.45
Hou J. et al. (2014) found that an increase in the Treg cell population was positively correlated with PD-L1 overexpression in GC and that both variables were associated with a higher number of lymph node metastasis, an advanced clinico-pathological stage and a lower overall survival rate.23 Moreover, PD-L1 was expressed both on GC cells (42.0±3.6%) and tumor infiltrating lymphocytes (17.21.6%).
Zheng Z. et al. (2014) established that circulating PD-L1 (cPD-L1) expression in advanced GC patients was higher than in normal controls and found a positive correlation between higher cPD-L1 expression, well-moderately differentiated tumors and absence of lymph node metastasis. Curiously, amongst advanced GC patients with high cPD-L1 expression, those with higher up-regulated cPD-L1 had a better 5-year survival rate than those with lower up-regulated cPD-L1 expression (65.6% vs 44.7%, respectively).46 This apparently contradictory results may be explained by the different method (ELISA) used to ascertain PD-L1 expression as compared to other studies. Another interesting explanation is that cPD-L1 acts as an antagonist of its receptors, behaving as an endogenous inhibitor with prognostic impact.
Recently, The Cancer Genome Atlas group attempted to develop a novel molecular classification of GC, recognizing gastric tumors positive for EBV as one of four proposed subgroups. EBV-positive tumors showed amplification of PD-L1/2 genes and a strong immune cells presence, providing a rationale for testing immune checkpoint blockade of the PD-1 signaling pathway in this molecular subtype.47 A summary of the studies describing the role of PD-L1 in H. pylori infection is depicted in Table 2.
Summary of the studies describing the role of PD-L1 in gastric cancer.
| Author (year) | Objective | Materials | Methods | Results |
|---|---|---|---|---|
| Wu C. (2006)21 | To evaluate the relationship between PD-L1 immuno-detection and 1 - clinico-pathological features and 2 - prognosis of gastric carcinoma | 102 gastric carcinomas; 10 gastric adenomas; 10 samples of normal gastric mucosa | Immunohistochemistry: >10% of tumor cells stained for PD-L1 (antibody clone 2H11) were considered positive | PD-L1 immuno-detection was significantly enhanced when the tumor infiltrated into the deep muscular layer, with lymph node metastasis or survival time of less than 2 years |
| Multivariate analysis demonstrated that PD-L1 immuno-detection is an independent prognostic factor in gastric cancer | ||||
| Sun J. (2007)22 | 1. (same as Wu C.) | 1. (same as Wu C.) | 1. (same as Wu C.) | 1. (same as Wu C.) |
| 2. To evaluate the effect of monoclonal antibody 10E10 on T-cell apoptosis | 2. MDA-MB-435 (human breast carcinoma line) | 2. T-cell apoptosis assay | 2. Anti-human PD-L1 monoclonal antibody 10E10 could inhibit T-cell apoptosis induced by tumor-associated PD-L1 | |
| Wang W. (2012)43 | To evaluate if mutations at the 3’-UTR region of PD-L1 could disrupt potential inhibitory molecules such as miRNAs and subsequently trigger an elevated expression of PD-L1 in GC | 1. 205 gastric carcinomas; 293 healthy controls | 1.PCR sequencing of the 3’-UTR region of PD-L1 for mutations | 1. A guanine-to-cytosine mutation at the 3’-UTR of PD-L1 mRNA was significantly and positively associated with PD-L1 overexpression in GC and with the pathological features including lower differentiation grade, depth of invasion, lymph node metastasis and TNM stage |
| 2. CHO cells co-transfected with miR-570 and a pcDNA PD-L1 expression plasmids with either wild-type or mutant 3’-UTR | 2. IHC assay for PD-L1 expression | 2. This mutation led to PD-L1 overexpression by disrupting the miR-570 binding site | ||
| 2. Flow cytometry analysis of the surface expression of PD-L1 | ||||
| Chen XL. (2003)44 | To evaluate PD-L1 mRNA expression differences between gastric cancer and controls | 17 gastric carcinomas; 6 gastric ulcers | In-situ hybridization | No difference in the expression of PD-L1 mRNA between gastric cancer and gastric ulcers |
| Wang W. (2013)45 | To evaluate a possible role of miRSNPs at the 3’-UTR of PD-L1 in the risk of developing GC | 205 gastric carcinomas; 293 healthy controls | A case-control study using blood samples for detection of two selected miRSNPs by PCR | The genotype distribution of a common C>G polymorphism (rs4143815) was significantly different between the cases and controls. Compared with CC homozygotes, GG homozygotes and G allele carriers showed 3.73-fold and 1.85-fold increased risk of gastric cancer development, respectively |
| Hou J. (2014)23 | To evaluate a possible correlation between FOXP3 and PD-L1 expression in GC with 1 - clinico-pathological features, and 2 - prognosis of gastric cancer | 111 gastric carcinoma tissue samples; 20 gastric ulcer tissue samples | IHC: >10% of tumor cells stained for PD-L1 were considered positive; >5% of TILs stained for PD-L1 were considered positive; >5% of TILs stained for FOXP3 were considered positive | There was a highly significant correlation between FOXP3 and PD-L1 expression. The expression levels of these two determinants in patients with lymph node metastasis and an advanced clinico-pathological stage were distinctly higher. The patients with enhanced expression of FOXP3+ Tregs and PD-L1 exhibited a lower overall survival rate and a worse prognosis |
| Zheng Z. (2014)46 | To access the relationship between circulating PD-L1 (cPD-L1) expression and 1 - clinical pathological features and 2 - prognosis of gastric cancer | 80 gastric carcinoma samples; 40 healthy controls | ELISA | The expression of circulating PD-L1 was significantly correlated with well-moderately differentiated tumors and absence of lymph node metastasis. The carcinoma patients with higher up-regulated PDL1 expression had much better prognosis than low expression patients |
| The Cancer Genome Atlas Research Network (2014)47 | To establish a novel molecular classification of GC | 295 gastric adenocarcinoma samples | Somatic Copy-Number Aberrations (SCNA) analysis using GISTIC | Tumors positive for Epstein–Barr virus, display recurrent PIK3CA mutations, extreme DNA hypermethylation, and amplification of JAK2, PD-L1 and PD-L2 |
GC is one of the most common malignancies worldwide. Although early recognition of the disease can achieve high success rates by endoscopic or surgical resection, it often presents late in its natural course leading to a poor overall survival rate. Mass screening programs are not cost-effective in most countries and therefore primary prevention and personalized treatment are regarded as the best options to reduce advanced stage GC mortality rates.
PD-L1 is a ligand of PD-1, an immune inhibitory checkpoint expressed on T-cells. Activation of PD-1 signaling pathway by PD-L1 has been shown to dampen T-cells activity, an event that, when balanced with co-stimulatory signals, is crucial to maintain peripheral tolerance and prevent excessive damage to tissues when clearing an infection. In some cases, PD-L1 protein expression is up-regulated, which appears to be a mechanism favoring immune evasion and, consequently, persistence of chronic infections and tumors. That seems to be the case of H. pylori infection and GC.
H. pylori infection persistence has been associated with less responsive T-cells and, while other mechanisms have been proposed, PD-L1 expression, favoring a status of peripheral tolerance to H. pylori, might play an important role by 1 - reducing the level of CD4+ proliferation and cytokine production, 2 - inducing T-cells apoptosis and 3 - turning naïve T-cells into Tregs. Interestingly, PD-L1 expression on H. pylori exposed gastric epithelial cells occurs without a direct contact between them and independently of VacA, CagA and ureaseB production, raising the possibility for the existence of a new, yet unidentified, molecular determinant of virulence. Another interesting possibility is that the H. pylori PD-L1 induced expression might open up a path for transformed cells to evolve without adequate immune surveillance mechanisms, favoring tumor escape and progression. To investigate PD-L1 expression along the lesions of the Correa model could enlighten these possibilities.
Another pathogen involved in gastric carcinogenesis is EBV. Gastric tumors infected by this virus characteristically show a dense lymphoid stromal infiltration (gastric carcinoma with lymphoid stroma) and PD-L1 overexpression. These two findings add rationale for testing immune checkpoint inhibitors in EBV-positive GC.
During carcinogenesis and cancer progression, tumors develop genomic alterations that are able to generate tumor-specific antigens and trigger an immune response. However, the relationship between tumor cells and tumor immune infiltrates is not straightforward, that is, it is not always one of antagonism, in which tumor immune-mediated elimination occurs, but can also be one of synergism, favoring tumor progression and immune escape – an emerging hallmark of cancer. This dual-interaction refers to the concept of cancer immunoediting. One mechanism that tumors develop to escape immune surveillance is through constitutive and inducible up-regulation of PD-L1, a ligand of PD-1 on T lymphocytes. This signaling pathway is responsible for a less effective anti-tumor immunity, namely 1 - decreased lymphocyte proliferation and cytokine production and 2 - increased lymphocyte apoptosis, and has been extensively studied as a potential target for immunotherapy. To this date, two anti-PD-1 mAbs have already been approved for the treatment of unresectable and metastatic melanoma (nivolumab and pembrolizumab). As for the anti-PD-L1 mAbs, they have already shown some unparalleled good results on advanced incurable cancers, such as NSCLC, melanoma, renal cell carcinoma and bladder cancer, amongst others. However, its role on GC has only been addressed in a few studies, leaving a door open for further developments.
PD-L1 expression in GC is significantly correlated with advanced clinico-pathological features and poor survival. A single nucleotide polymorphism at the PD-L1 miR-570 binding site was associated with an increased risk for the development of GC, further underlining the importance of PD-L1 expression on GC biology and its potential as a molecular target for advanced stage GC treatment. As a result, the first challenge is to identify predictive biomarkers that may help identify suitable GC patients for therapy with available anti-PD-L1 mAbs. An obvious candidate is the expression of PD-L1 in GC tissues. As evidenced by recent phase I clinical trials, there is a tendency for tumors expressing PD-L1 to respond better to anti-PD-L1 mAbs. However, by current immunohistochemical assays, there are tumors that do not express PD-L1 but still respond to anti-PD-L1 mAbs; conversely, there are tumors that express PD-L1 but do not respond to anti-PD-L1 mAbs. Therefore, an immunohistochemical assay with a cut-off that better correlates with response to these antibodies is necessary.
The second challenge is to develop combinatorial approaches. Phase I clinical trials with anti-PD-L1 mAbs showed not only biological activity in some forms of cancer but also that they are fairly tolerable, making them a good fit for combinatorial approaches. Furthermore, many cancer therapies, such as vaccines or more traditional chemotherapies, rely on the immune system to target tumor cells. Since tumor cells can adapt to this reinforced immune response via up-regulation of PD-L1 (adaptive immune resistance), synergistic effects can be expected when combining such immune stimulating therapies with anti-PD-L1 mAbs in advanced stage GC.