Obesity is a growing epidemic worldwide. Evidence so far demonstrates that the bacteria that are commonly found in the human gastrointestinal tract affect nutrient acquisition and energy regulation. This suggests that an important role is played by gut microbiota in the development of obesity.
ObjectivesOur main goal was to assess if a probiotic diet leads to a significant difference in weight change in non-obese and obese people, and in experimental models.
MethodsSearch was undertaken in PubMed, Scopus, ISI Web of knowledge, Cochrane Central Register of Controlled Trials, Google scholar, meta-Register of Controlled Trials, ClinicalTrials.gov and by scanning reference lists of articles, without publication date imposed, for randomised clinical trials studying the administration of probiotics to obese or overweight patients and experimental studies in experimental models and healthy humans. Search terms included probiotics, obesity, weight, BMI, weight gain, weight loss, weight change, probiotic diet and probiotic therapy. In an unblended standardized manner, 2 reviewers analysed the searched studies, using the defined inclusion and exclusion criteria, and performed extraction of data, in an independent way, using predefined data fields.
ResultsWe’ve identified, through searching databases specified in methods, 269 records. A total of 4 clinical trials and 14 experimental studies were included in the systematic review. Among the 4 randomized clinical trials only one showed statistically significant results. L. rhamnosus CGMCC 1.3724 was efficient in reducing weight in females, but not in males - Mean weight loss 12 week/24 week (kg): Males-probiotic: 4/5.4; Males-placebo: 3.05/4.43; Females-probiotic: 4.4/5.2; Females- placebo: 2.6/2.5 (P<0.05 only on females).
ConclusionsIn our systematic review, we found that probiotic effect in body weight is specie and strain specific. L. gasseri BNR17, reduced the weight gain compared to controls; L. gasseri L66-5 promoted weight gain, L. rhamnosus GGMCC is the only one that had a positive effect in weight loss in humans. Probiotic effect in body weight was species and strain specific. On the other hand L. plantarum LG42, L. gasseri SBT2055 and L. plantarum co-therapy with KY103 and L. curvatus HY7601 had an anti-obesity effect in animal models.
Obesity is a growing epidemic worldwide that has nearly doubled since 1980. In 2008, more than 1.4 billion adults over 20 were overweight, almost 35% of the population, and about 11% were obese. More than 40 million children under 5 were overweight in 2011, about 10 million in the developed and 30 million in the developing countries, which demonstrates that this disease is becoming an increasing problem in the latter, that used to fight undernutrition instead. Already being considered the fifth leading risk factor for global deaths, obesity kills at least 2.8 million adults each year.1
It is a major risk factor for the development of cardiovascular diseases, type 2 diabetes mellitus, musculoskeletal disorders (osteoarthritis) and various kinds of cancer (endometrial, breast and colon).1 The enormous social and economic costs of obesity and these associated comorbidities are already threatening to overwhelm health care systems worldwide.2
The World Health Organization (WHO) defines obesity as an abnormal or excessive fat accumulation that may impair health, the result of an energy imbalance between calories consumed and expended. The criteria used by WHO to diagnose obesity is the body mass index (BMI): A person with a BMI greater or equal to 25 is considered overweight; greater or equal to 30 is considered as having obesity. In turn, obesity is also divided into three categories: BMI between 30 and 35 represents 1st degree obesity; BMI between 35 and 40 represents 2nd degree obesity; BMI over 40 represents 3rd degree obesity.1 BMI is a useful indicator of overall adiposity, but different fat compartments are associated with differential metabolic risk. Thus, an evaluation of waist circumference results in a more accurate classification of obesity: visceral/central or subcutaneous obesity. A waist circumference over 88cm for women and over 102cm for men represents that a person has visceral/central obesity.3
However, besides the environmental cause described by WHO, genetic, neural and endocrine factors have been described as causes of obesity,4 as well as infectious agents.5 Evidence so far demonstrates that the bacteria that are commonly found in the human gastrointestinal tract, normally referred to as gut microbiota, affect nutrient absorption and energy regulation, while also being different in an obese person, when compared to a lean one. This suggests that an important role is played by gut microbiota in the development of obesity.2 This information may represent a major advance in obesity therapy, since modifying the gut microbiota, through a diet rich in probiotics (nonpathogenic live microorganisms that, when ingested, confer health benefits to the host), can become an important treatment option for obesity.2 In fact, multiple studies have been researching the effect of the referred probiotics diet in the organism and its possible contribution to treat obesity as well as other comorbidities.6 Amongst the species already studied, the most commonly used are Lactobacillus spp., Bifidobacterium spp. and Enterococcus spp.6
Although there are several studies concerning the effect of different probiotics on weight change and obesity, we were unable to find a systematic review that summarized and discussed them in a global perspective. Thus, the elaboration of this article constitutes a relevant effort to better understand this matter.
Research question and aimsOur main goal was to access whether a probiotic diet leads to a significant difference in weight change in experimental models and non-obese and obese people, thus playing a protective and beneficial role in obesity establishment and in the reduction of obesity-related comorbidities, by performing a systematic revision of randomized, controlled trials and experimental studies that accessed the effect of probiotic therapy on weight change in experimental models and non-obese and obese subjects.
This conducts us to our central question: “What's the effect of probiotic diet on weight change in obese/overweight and/or non-obese subjects?”
MethodsMethods of the analysis and inclusion criteria were documented in a protocol before its execution.
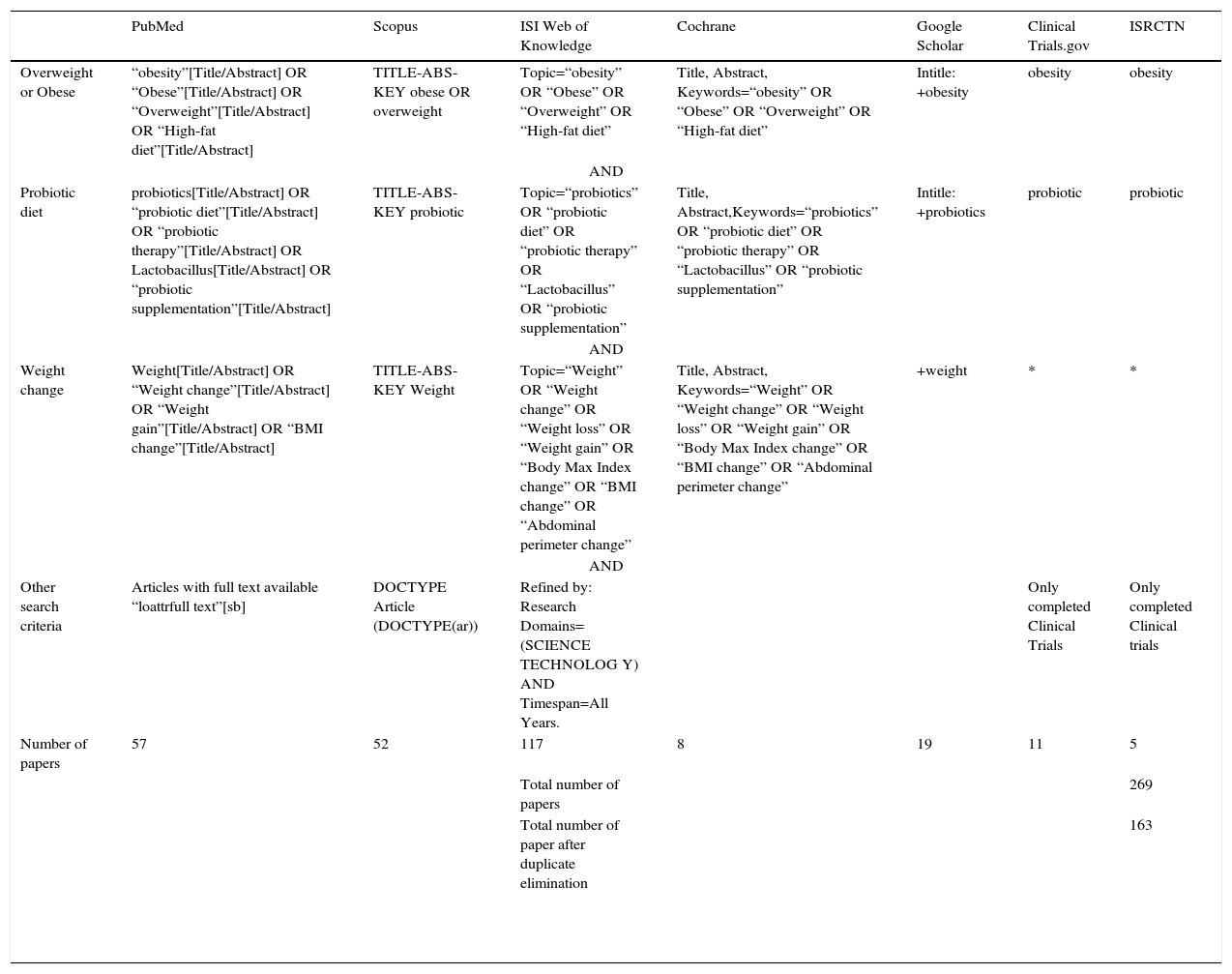
Data sourcesWe followed the PRISMA 2009 statement.7 Studies were identified by searching electronic databases and scanning reference lists of articles. No limits were applied for language and foreign papers were translated. This search was applied to PubMed, Scopus, ISI Web of knowledge. The last search was run on 3 May 2014. A limited update literature search was performed from 3 May 2014 to 18 November 2014.
We searched Google Scholar with several keywords combined to identify grey literature. We also searched for completed and ongoing trials (latest search 7 August 2014) in the following registers: ClinicalTrials.gov (http://clinicaltrial.gov/); and ISRCTN (http://www.controlled-trials.com/isrctn/). The search terms included were: probiotics, probiotic diet and probiotic therapy, Lactobacillus, obesity, weight, BMI, weight gain, weight loss and weight change. There were no new trials identified in the scanning of reference lists of articles. Full details of the search are presented in Table 1.
Keywords used to perform the query in the 7 databases used in this study.
| PubMed | Scopus | ISI Web of Knowledge | Cochrane | Google Scholar | Clinical Trials.gov | ISRCTN | |
|---|---|---|---|---|---|---|---|
| Overweight or Obese | “obesity”[Title/Abstract] OR “Obese”[Title/Abstract] OR “Overweight”[Title/Abstract] OR “High-fat diet”[Title/Abstract] | TITLE-ABS-KEY obese OR overweight | Topic=“obesity” OR “Obese” OR “Overweight” OR “High-fat diet” | Title, Abstract, Keywords=“obesity” OR “Obese” OR “Overweight” OR “High-fat diet” | Intitle: +obesity | obesity | obesity |
| AND | |||||||
| Probiotic diet | probiotics[Title/Abstract] OR “probiotic diet”[Title/Abstract] OR “probiotic therapy”[Title/Abstract] OR Lactobacillus[Title/Abstract] OR “probiotic supplementation”[Title/Abstract] | TITLE-ABS-KEY probiotic | Topic=“probiotics” OR “probiotic diet” OR “probiotic therapy” OR “Lactobacillus” OR “probiotic supplementation” | Title, Abstract,Keywords=“probiotics” OR “probiotic diet” OR “probiotic therapy” OR “Lactobacillus” OR “probiotic supplementation” | Intitle: +probiotics | probiotic | probiotic |
| AND | |||||||
| Weight change | Weight[Title/Abstract] OR “Weight change”[Title/Abstract] OR “Weight gain”[Title/Abstract] OR “BMI change”[Title/Abstract] | TITLE-ABS-KEY Weight | Topic=“Weight” OR “Weight change” OR “Weight loss” OR “Weight gain” OR “Body Max Index change” OR “BMI change” OR “Abdominal perimeter change” | Title, Abstract, Keywords=“Weight” OR “Weight change” OR “Weight loss” OR “Weight gain” OR “Body Max Index change” OR “BMI change” OR “Abdominal perimeter change” | +weight | * | * |
| AND | |||||||
| Other search criteria | Articles with full text available “loattrfull text”[sb] | DOCTYPE Article (DOCTYPE(ar)) | Refined by: Research Domains=(SCIENCE TECHNOLOG Y) AND Timespan=All Years. | Only completed Clinical Trials | Only completed Clinical trials | ||
| Number of papers | 57 | 52 | 117 | 8 | 19 | 11 | 5 |
| Total number of papers | 269 | ||||||
| Total number of paper after duplicate elimination | 163 | ||||||
Note: In the PubMed central database the keywords were used as a [Title/Abstract] to ensure that they were defined as key word of the articles searched.
* The search didn’t include the outcome reference word in order to maximize the results in these databases. The 16 studies searched in Clinical Trials.gov and ISRCTN were excluded because there were no available results.
Inclusion was limited to randomised clinical trials studying the administration of probiotics to obese or overweight patients, with or without diabetes, cardiovascular diseases, hyperlipidaemia or metabolic syndrome and experimental studies with experimental models and healthy humans. No publication date was imposed. We excluded pregnant women, probiotics given only or simultaneously to the mother, hosts affected by gastro-enteric diseases, such as diarrhoea, colitis or irritable bowel syndrome, any article that evaluated symbiotics or non-direct fed, non-viable or recombinant probiotics, experimental studies in farm animals without clinical purpose, articles with unavailable statistical data, unavailable results or not yet completed, and articles not published in medical sciences journals. Articles without original data, not written in English or Portuguese and double publications were excluded. Articles that we didn’t had access (articles without free full text available or of journals not included in the catalogue access of our faculty) were also excluded.
Data extractionObesity was defined and subclassified according to the WHO definition cited above.
Probiotic was defined as “Live microbial DIETARY SUPPLEMENTS which beneficially affect the host animal by improving its intestinal microbial balance. Antibiotics and other related compounds are not included in this definition” according to MeSH terms (Index Medicus “Medical Subject Heading” terminology).
We analysed, as primary outcomes, differences in weight: significant weight loss; significant weight gain; weight change from baseline, weight at the end of the study, weight/age ratio, BMI changes and weight percentile.
As secondary outcomes, we measured adverse events of probiotic treatment modification on lipidaemia levels, glycaemic levels, blood pressure differences, PCR levels, inflammation levels modification, asthma improvement, other diseases improvement, leptin levels and mortality.
Data retrieved also includes intervention duration, type, dose and frequency; versus placebo or versus of control treatment (duration, type, dose and frequency); outcome duration, improvement in quality of life score [using a validated scale, when there was available information], effect on daily activities, when avaliable; characterization of the publication: year of publication; journal, authors, country of origin, study quality; methodological characteristics such as sample size, study design, control groups and if it is presented the CONSORT statement flow diagram or any written information discriminating the sample in a similar way (http://www.consort-statement.org/)8; characterization of the participants including gender, age, ethnicity, initial BMI (and consequent classification), initial abdominal circumference, when avaliable.
Study selection and data collection processEligibility assessment and data extraction was performed in an unblended standardized manner by 2 reviewers that analyzed titles and abstracts of searched studies, using the defined inclusion and exclusion criteria. Discrepancies were discussed between the 2 reviewers and, if required, a third reviewer was involved. This same process, performed after analysis of the entire article, was performed on a data extraction sheet (based on the Cochrane Consumers and Communication Review Group's data extraction template), and refined after a pilot- test on 5 randomly-selected included studies.9
Risk of bias assessmentThe pair of reviewers, independently, with adequate reliability determined the adequacy of randomization and concealment allocation, blinding of patients, health care providers, data collectors, and outcome assessors; proportion of patients lost to follow- up; stopping of trials early for benefit; if outcome data is complete; whether the analysis followed the intention-to-treat principle; if there's the possibility of selective outcome reporting and other potential threats to validity of the study. Each criterion for risk bias (based on the Cochrane Consumers and Communication Review Group's “risk of bias assessment tool”) was judged as Low, Unclear or High risk.9
AnalysisIndividual participants in each clinical trial were the unit of analysis. Results are expressed as mean differences between intervention group and control group with standard deviations, in a summary table, when those values were available.
We performed a meta-analysis for clinical trials only, for weight change and HDL change parameters, and assessed the heterogeneity using the Cochran's Q statistic with a P value ≤0.1 interpreted as statistically significant. We obtained further information on the impact of statistical heterogeneity on the study results by calculating the I2 statistic.9 We used values of the I2 statistic above 50% as a cut-off for considerable heterogeneity.9 We used a random-effects model. Meta-analysis was performed using RevMan5.
Had there been a sufficient amount of studies (i.e. at least 10 RCTs, as outlined in Chapter 10 the Cochrane Handbook for Systematic Reviews of Interventions),9 we would have constructed funnel plots to assess for any publication bias.
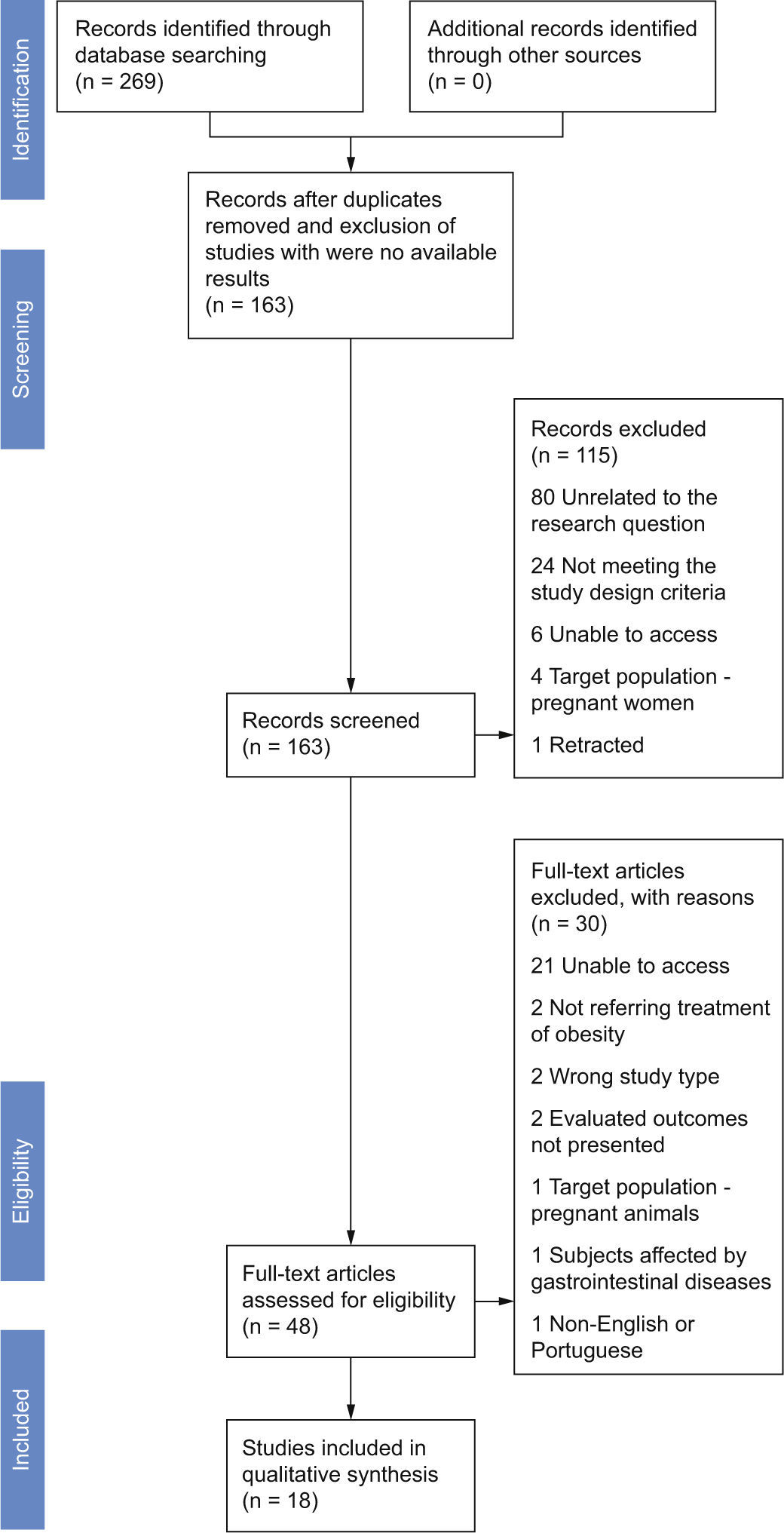
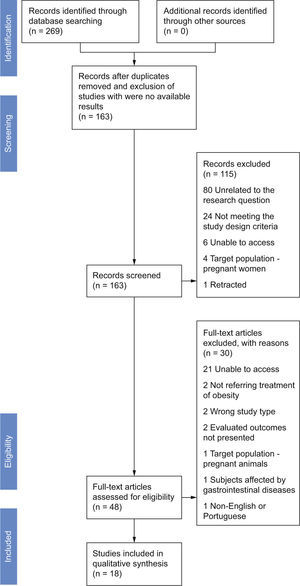
ResultsStudy selectionThe search identified a total of 269 records, prior to removal of duplicates, with 59 of the records retrieved from the search of MEDLINE, 52 records from SCOPUS, 117 records from ISI, 8 records from CENTRAL, 19 records from Google Scholar, 11 from Clinical Trials.gov and 5 from ISRCTN; 163 records were left after duplicates were removed (Fig. 1). Scanning reference lists of review articles, contacting content experts and contacting authors produced no extra trials. We only included completed trials.
The 16 studies searched in Clinical Trials.gov and ISRCTN were excluded from screening because there were no available results. We discarded 115 after reviewing the abstracts, in its majority for being unrelated to the research question – did not mention researching the effect of probiotics in weight neither in the title, or in the abstract (80) – or for not meeting the study type or the interventions design criteria (24). Others were excluded due to being unavailable to us,6 their target population being pregnant women4 or for being retracted.1 The assessment for eligibility yielded 48 articles. Of these, 30 did not meet the following inclusion criteria: unable to access (21), not referring treatment of obesity,2 not corresponding to the study type we intended to include – not being neither a RCT or an experimental study in experimental models,2 not mentioning the primary outcome searched in this review,2 treatment applied to pregnant animals,1 including in the sample subjects affected by gastrointestinal diseases1 or unavailable in English or Portuguese.1 A total of 4 clinical trials and 14 experimental studies were included in the systematic review.
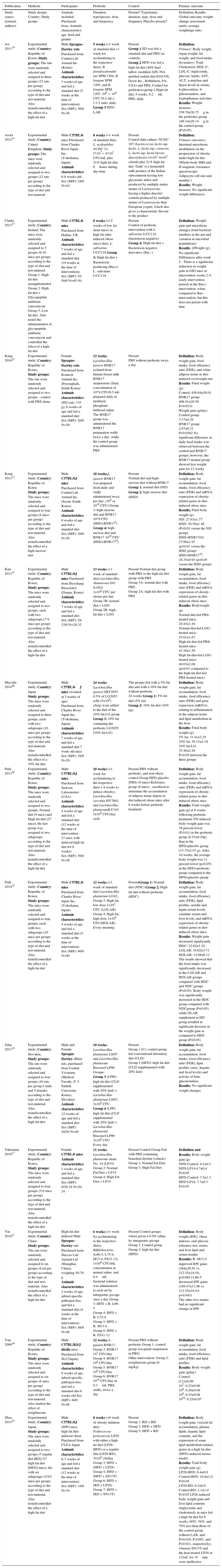
Study characteristics and results of individual studiesIn Table 2 we describe the data extracted from included human studies. We included four RCTs. All were published between 2013 and 2014. The sample size of these studies ranged from 50 to 125 (total 307). Two RCTs had the duration of 8 weeks. One study only enrolled female subjects10 and another enrolled male and female subjects but did not report data according to the gender.11
Data extracted from included human studies (4 RCTs).
| Publication | Methods | Participants | Sample | Criteria | Probiotic | Control | Primary outcome |
|---|---|---|---|---|---|---|---|
| Study source (author year) | Location; period of inclusion; mono or multicentre; study design; control groups | Subjects included; age, gender and ethnicity; classification according to initial BMI or initial abdominal circumference | Sample size: eligible (n), randomized(n), allocated for intervention (n) allocated for control group (n), loss of follow up in intervention group (n), loss of follow up in control group (n), analysed (n); | Inclusion criteria, Exclusion criteria | Duration, type/species, dose and frequency | Present? Type/name, duration, type, dose and frequency Placebo present? | Definition; Results: Global outcome; weight change assessment (unit); average weight/age ratio; |
| Jung 201313 | Monocentre; Randomized, Double-Blind Clinical Trial Country: Republic of Korea | 19 to 60 year old, male and female, obese fasting blood sugar ≥100 mg/dL, Sex (M/F): 22/35 | Eligible (83), randomized(62), allocated for intervention (31-2 lost of follow up for primary outcome) allocated for control group (31-6 lost of follow up for primary outcome), loss of follow up in intervention group (3), loss of follow up in control group (2) | Inclusions: men and nonpregnant women aged between 19 and 60 with body mass index(BMI) ≥23 kg/m2 and fasting blood sugar (FBS) ≥100 mg/dL Exclusions: taking drugs that could affect weight change, including anti-diabetic drugs, lipidlowering drugs, orantiphycotic drugs, or if they had endocrine, cardiovascular, thyroid, or chronic liver disease. Subjects were excluded if they had received surgery to reduce weight, were taking probiotics or antibiotics within one month, or if their weight had changed over five percent within three months | 12 weeks (no wash-out before the initiation of treatment), Lactobacillus gasseri BNR17, 6 capsules per day (2 capsules, 3 times a day), 1010 cfu of Lb. gasseri BNR17. Excipient: filler powder (50% trehalose, 25% skim milk, and 25% fructooligosaccharide) | Present Placebo capsules were packaged only with the filler produced by the pharmaceutical factory, 6 capsules per day (2 capsules 30minutes before breakfast, lunch, and dinner) | Definition: Primary outcomes: reduction of body fat (%) and body weight (kg) Secondary outcomes: fasting blood sugar, 2 hour postprandial blood sugar, Insulin, total cholesterol, HDL, LDL, triglyceride, blood pressure and pulse rate, hematology parameters and blood chemistry; Results: Final weight (kg): Placebo: 77.2±11.6 Intervention group: 76.1±10.0 P value=0.07 |
| Lee 201410 | Randomized, double-blinded, placebo controlled study Country: Republic of Korea | 19 to 65 year old, female, overweight and obese, Sex (M/F): 0/64 | Eligible (64), randomized(50), allocated for intervention (25) allocated for control group (25) | Inclusions: female subjects aged 19-65 years meeting the criteria of BMI>25 kg/m2 and waist circumference>85 cm Exclusions: Hypothyroidism; Cushing's syndrome; Heart diseases; Cancer;Lung diseases; Severe renal dysfunction (Cr>2.0 mg/dl); Liver dysfunction (ALT, AST 2.5 fold upper limit of normal); Non-insulin dependent diabetes mellitus with FBS>140 mg/dL;neuropsychiatric diseases; Eating disorder; Subjects who underwent anatomical change such as incision; Pregnancy, breast feeding, or planning of pregnancy; Subjects who have lost 10% of body weight within six months of the study | 8 weeks, Bofutsushosan(BTS) and probiotics (n=25) Bofutsushosan(BTS) and probiotics (n=25), Streptococcus thermophiles, Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium lactis, Bifidobacterium longum and Bifidobacterium breve, co-administered with BTS extracts, 5×109 cells per species, twice per day | Present BTS and placebo (n=25). Placebo capsules were identical in appearance, except for the bacterial strains and were also given twice per day, co-administered with BTS extracts. Bofutsushosan (BTS),is an oriental herbal medicine containing 18 components | Definition: Primary outcomes: weight (calculated as difference of weight) and gut permeability (through the level of mannitol and lactulose) Secondary outcomes: blood lipid level, including triglyceride, total cholesterol, HDL, and LDL cholesterol; waist circumference, blood pressure, BMI, main bacterial strains of intestinal microbiota, endotoxin serum level, and KOQOL (The Korean version of obesity-related quality of life) Results: Body weight (kg): Probiotics: 70.66±5.90 Placebo: 70.59±6.97 P value: 0.974 |
| Sanchez 201412 | Randomised, double-blind, placebo-controlled trial Country: Canada; Quebec city; January 2010- May 2012 | 18 to 55 year old, male and female, obese; Sex (M/F): 48/77 | Eligible (153), randomized(153), after randomization they excluded 28 dyslipidaemic patients allocation 125 patients, allocated for intervention (62), allocated for control group (63), loss of follow up in intervention group phase I/phase II (10/7), loss of follow up in control group phase I/phase II (10/5) | Inclusions: age between 18 and 55 years; stable body weight (body-weight change<5kg for 3 months before screening); BMI between 29 and 41kg/m2; without associated co-morbidities (hypertension 140/90mmHg or more, obstructive sleep apnoea, type 2 diabetes or CVD, or family history of dyslipidaemia); absence of pregnancy, breast-feeding or menopause (determined by the cessation of menstruation); no abnormal thyroid hormone levels; no immunocompromised conditions or anaemia; no use of vitamin and mineral supplementation within 6 months of screening; no use of medication affecting body weight, energy expenditure, or glucose control or antibiotic treatment for the last 3 months; no smoking, drug or alcohol (>2 drinks/d) problem; no consumption of 5 or more cups of coffee/d (1250 ml/d). Participants with allergy to the ingredients in the study product and placebo or experiencing nausea, fever, vomiting, bloody diarrhoea or severe abdominal pain or currently participating or having had participated in another clinical trial during the last 6 months before the beginning of the study Exclusions: Participants with allergy to the ingredients in the study product and placebo or experiencing nausea, fever, vomiting, bloody diarrhoea or severe abdominal pain or currently participating or having had participated in another clinical trial during the last 6 months before the beginning of the study. Dyslipidaemea was considered as an exclusion criteria after randomization | 24 weeks (+2 weeks of wash-out before the initiation of treatment): Two-phase intervention protocol: Phase 1: weight-loss period, supervised dietary restriction with or without probiotic LPR supplementation (12 weeks). Phase 2: weight maintenance with supervision of dietary habits without restriction during which LPR or placebo supplementation was continued (12 weeks). Lactobacillus rhamnosus CGMCC1.3724 (LPR), 1.62×108 CFU/capsules The probiotic capsules contained a formulation consisting of 10mg of a LPR powder, 300mg of a mix of oligofructose and inulin (70:30, v/v) and 3mg of magnesium stearate. Two capsules per day | Present The placebo capsules were of the same colour and size as the LPR capsules and contained 250mg of maltodextrin and 3mg of magnesium stearate. Two capsules per day | Definition: Primary outcome: weight loss and % fat mass Secundary outcome: energy balance and physiological parameters (resting energy expenditure, respiratory quotient, heart rate, blood pressure); metabolic and inflammatory markers (fasting glucose, fasting insulin, cholesterol - total, LDL, HDL and TAG, leptin, adiponectin, glycerol, NEFA, beta-Hydroxybutyrate, C-reactive protein); microbiota composition (Lachnopiracea family) Results: Mean weight loss 12 week/24 week (kg): Males- probiotic: 4/-5.4 Males- placebo: 3.05/4.43 Females- probiotic: 4.4/5.2 Females- placebo: 2.6/2.5 (P<0.05 only on females) |
| Zarrati 201311 | Randomized double-blind, controlled clinical trial. Country: Iran; Tehran. Department of Nutrition in Tehran University of Medical Science, August 2011-June 2012, monocentre, Randomized double-blind controlled clinical trial | 20 to 50 years olds, male and female, n/a, obese and overweight. Sex (M/F): 24/51 | Eligible (75),randomized(75), allocated for intervention (25) allocated for control group(25), loss of follow-up in intervention group (2) | Inclusions: 75 obese and overweight men and women, aged 20 to 50 years old, who were referred to the Department of Nutrition in Tehran University of Medical Science. Exclusions: clinical signs of acute inflammation, lactose intolerance, cow's milk allergy, acute gastrointestinal disorders for 3 months before the study initiation, drug and alcohol consumption, diabetes mellitus, pregnancy, lactation, menstruation at the time of blood sampling, history of metabolic disorders, and autoimmune diseases, infectious disease during the study time and having a weight loss diet for 6 months before the study initiation. “Two of the participants became pregnant during the study period and thus were excluded from the trial” - we concluded that this two participants were excluded before randomization - as written in the flow diagram of patient recruitment and randomization process and in the results description. | 8 week (+ 2 weeks of wash-out before the initiation of treatment), Probiotic yogurt was prepared with the starter cultures containing Streptococcus thermophiles + Lactobacillus bulgaricus, and enriched with Lactobacillus acidophilus LA5 + Lactobacillus casei DN001 + Bifidobacterium lactis Bb12, 108 CFU in 200mL yogurt and a low fat diet, daily. They studied 3 groups. Two interventional: Group 2 (n=25) who received probiotic yogurt with a LCD [PLCD]. Group 3 (n=25) who consumed probiotic yogurt without any low calorie diet [PWLCD]. | Low calorie diet+Yogurt prepared with the starter cultures containing Streptococcus thermophiles + Lactobacillus bulgaricus, without the probiotics in investigation, 8 week, 200mL, daily Yes. Yougurts had PH range of 4.3–4.5 and a fat content of 1.5%. Group 1 (n=25) who consumed regular yogurt as part of a low calorie diet [RLCD] | Definition: Primary outcome: expression of the FOXP3, T-bet, GATA3, TNF-a, IFN-c, TGF-b, and ROR-ct in peripheral blood mononuclear cells(PBMCs) genes. Secundary outcome: body weight, BMI, waist and hip circumference, mid-upper arm circumference, waist to hip ratio, blood pressure. Results: Mean weight loss; 4.23kg in probiotic group 4.87kg in control group P>0.05 |
One study had the duration of 24 weeks, reporting the results of 12 and 24 weeks of treatment12; another study had the duration of 12 weeks.13 Species of probiotics were very different between the 4 studies: 2 studies used only one strain12 (– L. rhamnosus CGMCC1.3724 and Jung 2013 – L.grasseri BNR17), the other 2 used a combination of Lactobacillus and Bifidobacterium. Among the 4 randomized clinical trials only one showed statistically significant results12 – L. rhamnosus CGMCC 1.3724 was efficient in reducing weight in females, but not in males. Validity of these was satisfactory.
Other relevant outcomes include an alteration of the profile of cytokine production by peripheral blood mononucleated cells (PBMCs) after dietary treatment with probiotics: significant decrease in Interferon gamma (IFNγ) and T-bet gene expression and significant increase in Interleucin 10 (IL10) production11; a substantially and significantly reduction of the abundance of bacteria of the Lachnospiraceae family in women at both week 12 and week 24 taking probiotics12; a reduction in Leptin serum levels of 25% after 24 weeks of intervention, independently of fat mass reduction, preferentially in women who had about 3-fold higher baseline leptin concentrations(Sanchez 2014); and also a significant difference in the change in HDL cholesterol level between the probiotics and placebo groups.10
In the Table 3 we describe the data extracted from included non-human studies.
Data extracted from included non-human studies (14 experimental studies).
| Publication | Methods | Participants | Probiotic | Control | Primary outcome |
|---|---|---|---|---|---|
| Study source (journal, authors) | Study design; Country; Study groups | Animals included; Purchased from; Animals characteristics: age, feed and gender | Duration, type/species, dose and frequency | Present? Type/name, duration, type, dose and frequency Placebo present? | Definition; Results: Global outcome; weight change assessment (unit); average weight/age ratio |
| An 201119 | Experimental study; Country: Republic of Korea; Study groups: The rats were randomly selected and assigned to three groups (12 rats per group) according to the type of diet and test-material. Also tested/controlled the effect of a high-fat diet | Male Sprague-Dawley rats Purchased from Central Lab Animal Inc. (Korea) Animals characteristics: 3 weeks of age and fed a standard diet (5 weeks at the time of intervention); Sex (M/F): 36/0 N=36 | 5 weeks (+1 week of standard diet +1 week for acclimatizing to the respective diets), B. pseudocatenulat um SPM 1204, B. longum SPM 1205, and B. longum SPM 1207, 108 ∼ 109 CFU (0.2 mL); 1:1:1 ratio; daily Group 3 HFD-LAB | Present Group 1 SD was fed a standard diet and PBS (as control); Group 2 HFD was fed a high fat diet (40% beef tallow modified AIN-76A purified rodent diet #101556, Dyets Inc., Bethlehem, PA, USA) and PBS; Control for probiotics=group 2 High fat diet, 5 weeks, 0.2mL PBS, daily | Definition: Primary: Body weight, organ weight, fat weight, and food intake Secundary: Total Cholesterol, HDL-C, LDL-C, triglyceride, glucose, leptin, AST, ALT, a-amylase and lipase levels in serum; b-glucosidase, b-glucuronidase, and tryptophanase activities Results: Weight increase; 339.70±26.75g in the probiotics group 349.14±29.14g in the control-group (P>0.05) |
| Arora 201214 | Experimental study; Country: United Kingdom; Study groups: The mice were randomly selected and assigned to two groups (12 rats per group) according to the type of diet and test-material | Male C57BL/6 mice Purchased from Charles River Japan Inc. (Yokohama, Japan) Animals characteristics: 6-8 weeks old; Sex (M/F): 24/0 N=24 | 8 weeks (+1 week of standard diet), L. acidophilus NCDC 13, 5*107∼ 9*107 CFU/mL plus 21% high-fat diet, 6hours during day-time | Present Control dahi culture: NCDC 167 (Lactococcus lactis ssp. lactis, L. lactis ssp. cremoris, L. lactis ssp. lactis biovar. diacetylactis) (3×107-4×107 cfu/ml) plus 21% high-fat diet ‘Dahi’ is a fermented milk product of the Indian subcontinent having low glycaemic index and produced by multiple starter strains of Lactococcus, having a higher diacetyl content produced by multiple strains of Lactococcus than European yogurt, which also gives a characteristic flavour to the product | Definition: Primary outcomes: Intestinal microbiota modulation on the progression of obesity under high-fat diet (Whole-body MRI and H magnetic resonance spectroscope; Adipocyte cell size and number.) Results: Weight increase; No significant weight differences |
| Clarke 201327 | Experimental study; Country: Ireland; The mice were randomly selected and assigned to 5 groups (9-10 mice per group) according to the type of diet and test-material Group 1: High fat diet unsupplemented Group 2: High fat diet + Glycopeptide antibiotic vancomycin Group 5: Low fat diet. Also tested the administration of glycopeptide antibiotic vancomycin and controlled the effect of a high fat diet | Male C57BL/6 mice Purchased from Harlan, UK Animals characteristics: 7 weeks of age and fed a standard diet (19 weeks at the time of intervention); Sex (M/F): 45-50/0 N=45-50 | 8 weeks (+12 weeks of low fat (lean mice) or high fat (diet-induced obese mice) diet), L. salivarius UCC118 Group 3: High fat diet + Bacteriocin producing (Bac+) L. salivarius UCC118 | Present Control of probiotic intervention with L. salivarius UCC118 (bacteriocin negative) Group 4: High fat diet + Bacteriocin negative derivative (Bac–) | Definition: Weight gain and microbiota changes (total bacterial numbers in the gut and variation in microbial populations) Results: ΔWeight (g); No significant Differences after week 4 - There is a significant reduction in weight gain in DIO mice at intervention weeks 2-4 (early intervention period) in the Bac+ intervention, when compared to Bac- intervention, but this does not persist with time |
| Kang 201023 | Experimental study; Country: Republic of Korea; Study groups: The rats were randomly selected and assigned to two groups - control with PBS alone | Female Sprague-Dawley rats Purchased from Koatech Animal Inc. (Pyeongtaek, South Korea) Animals characteristics: (SD) rats: 135 g), 6 weeks of age and fed a standard diet Sex (M/F): 20/0 N=20 | 12 weeks, Lactobacillus gasseri BNR17 isolated from human breast milk BNR17 suspensions (final, concentration of 10^9 CFU/0.5 ml) prepared daily in sterilized phosphate-buffered saline. The BNR17 group was administered the BNR17 preparation orally twice a day, while the control group was administered PBS | Present PBS without probiotic twice a day | Definition: Body weight gain, food intake, food efficiency ratio (FER), and white adipose tissue in diet-induced overweight rats Results: Final weight (g): Control: 436.64±26.01 BNR17 group: 408.91±20.59 P=0.0331 Weight gain (g/day): Control group: 3.17±0.29 BNR17 group: 2.87±0.21 P=0.0282 No significant difference in daily food intake was observed between the control and BNR17 groups; however, the BNR17-treated group showed less weight gain for 12 weeks |
| Kang 201313 | Experimental study; Country: Republic of Korea Study groups: The mice were randomly selected and assigned to four groups (8 mice per group) according to the type of diet and test-material. Also tested/controlled the effect of a high-sucrose diet | Male C57BL/6J mice Purchased from Central Lab Animal Inc. (Seoul, South Korea) Animals characteristics: 6 weeks of age and fed a standard diet Sex (M/F): 20/0 N=20 | 10 weeks,L. gasseri BNR17 was prepared fresh daily and orally administered twice per day. (109 or 1010 CFU) Group 3: high-sucrose diet and BNR17 10^9 CFU (HSD+BNR179) Group 4: high-sucrose diet and BNR17 1010 CFU (HSD+BNR1710) | Present Normal diet and high-sucrose diet without BNR17 Group 1: normal diet (ND) Group 2: high sucrose diet (HSD) | Definition: Body weight gain, fat accumulation, food intake, food efficiency ratio (FER) and mRNA expression of obesity-related genes in diet-induced obese mice Results: Final body weight (g): ND: 27.63±1.77 HSD: 30.59±1.46 (P<0.01 versus the ND group) HSD+BNR17(9): 27.98±1.93 (p<0.01 versus the HSD group) HSD+BNR1710: 28.35±0.93 (p<0.05 versus the HSD group) |
| Kim 201313 | Experimental study; Country: Republic of Korea; Study groups: The mice were randomly selected and assigned to two groups, each with two subgroups (7-8 mice per group) according to the type of diet and test-material. Also tested/controlled the effect of a high-fat diet | Male C57BL/6J mice Purchased from Hyochang Bioscience (Daegu, Korea) Animals characteristics: 7 weeks of age and fed a standard diet Sex (M/F): 28-32/0 N=28-32 | 13 weeks (+1 week of standard diet) Lactobacillus rhamnosus GG (LGG) 1×108 CFU per mouse per day Group 1B: normal diet + LGG Group 2B: high-fat diet + LGG | Present Normal diet group with PBS or the high-fat diet group with PBS Group 1A: normal diet with PBS Group 2A: high-fat diet with PBS | Definition: Body weight gain, fat accumulation, food intake, food efficiency ratio (FER) and mRNA expression of obesity-related genes in diet-induced obese mice Results: Bodyweight (g) Normal diet-fed PBS-treated mice: 24.43±1.44 Normal diet-fed LGG-treated mice: 25.01±1.67 High-fat diet-fed PBS-treated mice: 43.38±1.50 High fat-diet-fed LGG-treated mice: 40.03±2.08 (p<0.01 compared to the high-fat diet-fed PBS-treated mice) |
| Miyoshi 201426 | Experimental study; Country: Japan Study groups: The mice were randomly selected and assigned to three groups, each with two subgroups (10 mice per group) according to the type of diet and test-material. Also tested/controlled the effect of a 10%-fat diet | Male C57BL/6J mice (weaned at 3 weeks of age) Purchased from Charles River Japan Inc. (Yokohama, Japan) Animals characteristics: 7 weeks of age and fed a standard diet 7 week old mice Sex (M/F): 29/0 N=29 | 24 weeks Lactobacillus gasseri SBT2055 0.5% of LG2055 cells (5×108) cfu/g) were added to the diet of the 10%-fat-LG group Group 3: 10% fat containing the probiotic LG2055 (10% fat-LG) | The groups fed with a 5%-fat diet and with a 10%-fat diet without probiotic 24 weeks Group 1: 5%-fat diet (5% fat) Group 2: 10%-fat diet (10% fat) | Definition: Body weight gain, fat accumulation, food intake, food efficiency ratio (FER) and gene expression (mRNA) relating to inflammation in the adipose tissue and lipid metabolism in the liver Results: Final body weight (g): 5% fat: 31.41±2.25 10% fat: 39.15±2.18 10% fat-LG: 35.48±2.26 P<0.05 between the three groups |
| Park 201320 | Experimental study; Country: Republic of Korea; Study groups: The mice were randomly selected and assigned to two groups, Normal diet (9 mice) and High fat diet (27 mice), the last group was assigned to two subgroups according to the type of diet and test-material. Also tested/controlled the effect of a high-fat diet | Male C57BL/6J mice Purchased from Jackson Laboratories (USA) Animals characteristics: 4 weeks of age and fed a standard diet (12 weeks at the time of intervention - 27 mice with induced high fat diet for 8 weeks) Sex (M/F): 36/0 N=36 | 10 weeks (+1 week for acclimatizing to the respective diets + 8 weeks to induce obesity), Lactobacillus curvatus HY7601 and Lactobacillus plantarumKY103 5x109 CFU/day each | Present PBS without probiotic, and non-obese control Group HFD-placebo (PBS) (9 mice) Reference group (9 mice) - sacrificed to determine the accumulation of adipose tissue depots in diet induced obese mice after 8 weeks before probiotic treatment | Definition: Body weight gain, fat accumulation, food intake, food efficiency ratio (FER) and mRNA expression of obesity-related genes in diet-induced obese mice Results: Total weight gain (g) at 8 weeks following probiotic treatment, FD-induced body weight gain was 38 percent lower (P,0.01) in the probiotic group (8.53±0.20g) than in the HFD+placebo group (13.75±1.07 g). After 10 weeks, the average body weight was 11 percent lower (p<0.05) in the HFD+probiotic group compared to the HFD+placebo group |
| Park 201415 | Experimental study; Country: Republic of Korea; Study groups: The mice were randomly selected and assigned to four groups, each with two subgroups (10 mice per group) according to the type of diet and test-material. Also tested/controlled the effect of a high-fat diet | Male C57BL/6 mice Purchased from Charles River Japan Inc. (Yokohama, Japan) Animals characteristics: 4 weeks of age and fed a standard diet (5 weeks at the time of intervention) Sex (M/F): 40/0 N=40 | 12 weeks (+1 week of standard diet) Lactobacillus plantarum LG42, Group 3: High fat, low dose 1×107 CFU (LGLAB) Group 4: High fat, high dose 1×109 CFU(HGLAB) Every morning | PresentGroup 1: Normal diet (NDC) Group 2: High fat diet without probiotic (HDC) | Definition: Body weight gain, fat accumulation, food intake, food efficiency ratio (FER), lipid profiles, insulin and leptin serum levels, carnitine serum and liver levels, and mRNA expression of obesity-related genes in diet-induced obese mice Results: Weight gain decreased significantly HDC: 22.62±1.24 LGLAB: 16.62±3.71 HGLAB: 14.08±6.11 The results showed that the food intake was significantly decreased in the LGLAB and HGLAB groups compared with HDC and NDC groups (P<0.05). Body weight was significantly increased in the HDC group compared with NDCgroup (P<0.05), while GLAB supplement in HD group resulted in significant decrease in the weight gain as compared to HDC group (P<0.05) |
| Salaj 201318 | Experimental study; Country: Slovakia; Study groups: The rats were randomly selected and assigned to four groups (10 rats per group,5 male and 5 female) according to the type of diet and test-material. Also tested/controlled the effect of a high-fat diet | Male and Female Sprague Dawley albino rats, purchased from Central Vivarium, (Medical Faculty, P. J. Safárik University, Kósice, Slovakia) Animals characteristics: 12 weeks of age and fed a standard diet Sex (M/F): 20/20 N=40 | 10 weeks, Lactobacillus plantarum LS/07 and Lactobacillus plantarum Biocenol LP96 Groups: Group 3 (LPH): high fat diet (CLD supplemented with 20% lard) + Lactobacillus plantarum LS/07, 3×109 CFU Group 4 (LPP): high fat diet (CLD supplemented with 20% lard) + Lactobacillus plantarum Biocenol LP96 3×109 CFU Every day | Present Group 1 (C): control group fed conventional laboratory diet (CLD) Group 2 (HFD): high fat diet (CLD supplemented with 20% lard) | Definition: Body weight gain, fat accumulation, food intake, food efficiency ratio (FER), lipid profiles (seric, hepatic and fecal levels) and activity of beta-glucuronidase Results: No significant weight changes |
| Takemura 201017 | Experimental study; Country: Republic of Korea; Study groups: The mice were randomly selected and assigned to four groups (5-6 mice per group) according to the type of diet and test-material. Also tested/controlled the effect of a high-fat diet | Female C57BL/6 mice Animals characteristics: 3 weeks of age and fed a standard diet Sex (M/F): 0/20-24 N=20-24 | 11 weeks, Lactobacillus plantarum strain No. 14 (LP14) Group 2: Normal Fat Diet + LP14 Group 4: High Fat Diet + LP14 | Present Control Group Fed with PBS containing branched dextrin (vehicle) Group 1: Normal Fat Diet Group 3: High Fat Diet | Definition and Results: Body weight gain (g) NFD-Control: 4.1±0.5 NFD-LP14:4.7±0.4 P>0.05 HFD-Control: 7.5±1.3 HFD-LP14: 7.7±0.7 P>0.05 |
| Yin 201021 | Experimental study; Country: China Study groups: The rats were randomly selected and assigned to six groups (8 rat per group) according to the type of diet and test-material. Also tested/controlled the effect of a high-fat diet | High-fat diet induced Male Sprague-Dawley rats Purchased from Slaccas Lab Animal Ltd (Shanghai, China), weighing 50-70 g Animals characteristics: 3 weeks of age, inbred-specific, pathogen-free, and fed a standard diet (4 weeks at the time of intervention) Sex (M/F): 48/0 N=48 | 6 weeks (+1 week for acclimatizing to the respective diets), Bifidobacteria (L66-5, L75-4, M13-4, FS31-12) 1×108 CFU/mL concentration in neutral saline, and 0.4mL bacterial solution was administered to each rat by intragastric gavage once a day Group 3: HFD + B. L66-5 Group 4: HFD + B. L75-4 Group 5: HFD + B. M13-4 Group 6: HFD + B. FS31-12 | Present Control groups where given a 0.9% saline by intragastric gavage Group 1: Control group Group 2: high-fat diet (HFD) | Definition: Body weight (BW), obese indexes, oral glucose tolerance test, serum and liver lipid and serum insulin Resulds: B. M13-4 improved BW gains (264±26.91 vs 212.55±18.54; p=0.001) L66-5 decreased BW gains (188.47±11.96 vs 212.55±18.54; p=0.043) The other two strains had no significant change in BW |
| Yun 200916 | Experimental study; Country: Republic of Korea; Study groups: The mice were randomly selected and assigned to six groups (8 mice per group) according to the type of diet and test-material. Also studied the effect of rosiglitazone | Male C57BL/KS/J db/db mice Purchased from SLC(Japan) Animals characteristics: 6 weeks of age, inbred-specific, pathogen-free, and fed a standard diet 6 weeks old Sex (M/F): 48/0 N=48 | 12 weeks, L. gasseri BNR17, Group 3: BNR17 107 CFU/day Group 4: BNR17 108 CFU/day Group 5: BNR17 109 CFU/day Group 6: BNR17 1010 CFU/day in 0.3mL PBS, orally, twice a day | Present PBS without probiotic Group 1: control group (excipient suspension in PBS) Other intervention: Group 2: rosiglitazone group (8 mg/kg) | Definition: Body weight gain, fat accumulation, food intake, food efficiency ratio (FER) and lipid profiles Results: Body weight gain (g/day) Control: 0.21±0.09 107: 0.22±0.06 108: 0.20±0.09 109: 0.25±0.08 1010: 0.25±0.07 |
| Zhao 201222 | Experimental study; Country: Japan; Study groups: The mice were randomly selected and assigned to two groups (7 regular diet [RD] /27 high fat diet [HFD] mice), the with six subgroups (3/3/2 mice per group) according to the type of diet and test-material. Also tested/controlled the effect of a high-fat diet | Male C57BL/6J (SPF) mice, high fat diet-induced obese Purchased from CLEA Japan Animals characteristics: 6-7 weeks of age and fed a standard diet (12 weeks at the time of intervention) Sex (M/F): 34/0 N=34 | 8 weeks (+6 week of obesity indution diet) Pediococcus pentosaceus LP28 with either a high fat diet (LP28-HFD) or a regular diet (LP28-RD), 3×109 cfu/day Group 3: HFD + (HFD + LP28) Group 4: HFD + (HFD + SN13T) Group 6: HFD + (RD + LP28) Group 7: HFD + (RD + SN13T) | Present Group 1: RD + RD Group 2: HFD + HFD Group 5: HFD + RD | Definition: Body weight gain, visceral fat accumulation, plasma lipids, hepatic lipid contents, and the expression of some lipid metabolism-related genes in a high fat diet (HFD)-induced mouse model. Results: Total body weight gain (g) LP28-HFD: 6.4±0.9 Control-HFD: 10.8±1.0 P<0.05 LP28-RD: 0.2±0.8 Control-RD: 1.1±1.0 P>0.05 LP28 reduced body weight gain and liver lipid contents (triglyceride and cholesterol), in mice fed a high fat diet for 8 weeks (40%, 54%, and 70% less than those of the control group without LAB, and P=0.018, P,0.001, and P=0.021, respectively), whereas SN13T and the heat treated LP28 at 121uC for 15min were ineffective |
We included fourteen experimental studies. All were published between 2009 and 2014. The sample size of these studies ranged from 20 to 50 (total 481 animals). The minimum interventional duration was of 5 weeks14 and maximum interventional duration was of 24 weeks.15 Four studies used as experimental models Sprague Dawley albino rats (SDr) (total of 154 rats; 20 female/130 male), one used C57BL/KS/J db/db mice16 and the other 10 studies used C57BL/6J mice (total of 337 mice; 24 female/ 313 male). One study only enrolled female animals - C57BL/6J mice17 and another enrolled male and female animals – SDr,18 all the other studies only enrolled male animals. Four studies applied a combination of probiotics.19–22 Among these four studies, one used Bifidobacterium combination19 and another also used four Bifidobacterium strains but as mono-intervention.21 The other two used Lactobacillus combinations. One other study applied two Lactobacillus strains separately.18
Among the experimental animal trials, L. gasseri BNR17 in one study showed weight loss capability in rats, as well as reduced visceral fat accumulation.23 In another study, of the same authors,24 probiotic fed mice also showed reduced body weight. Opposing these results, other authors using the same probiotic were not supportive of these findings, showing instead a non-significant weight gain capability of this probiotic.16
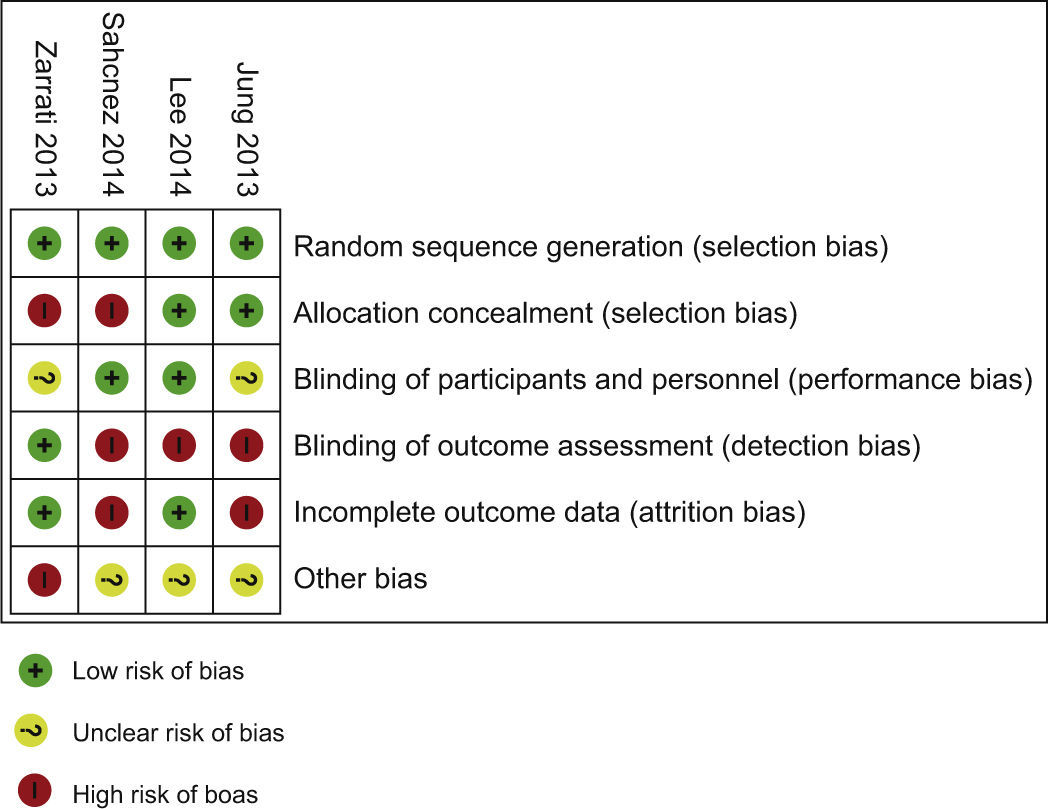
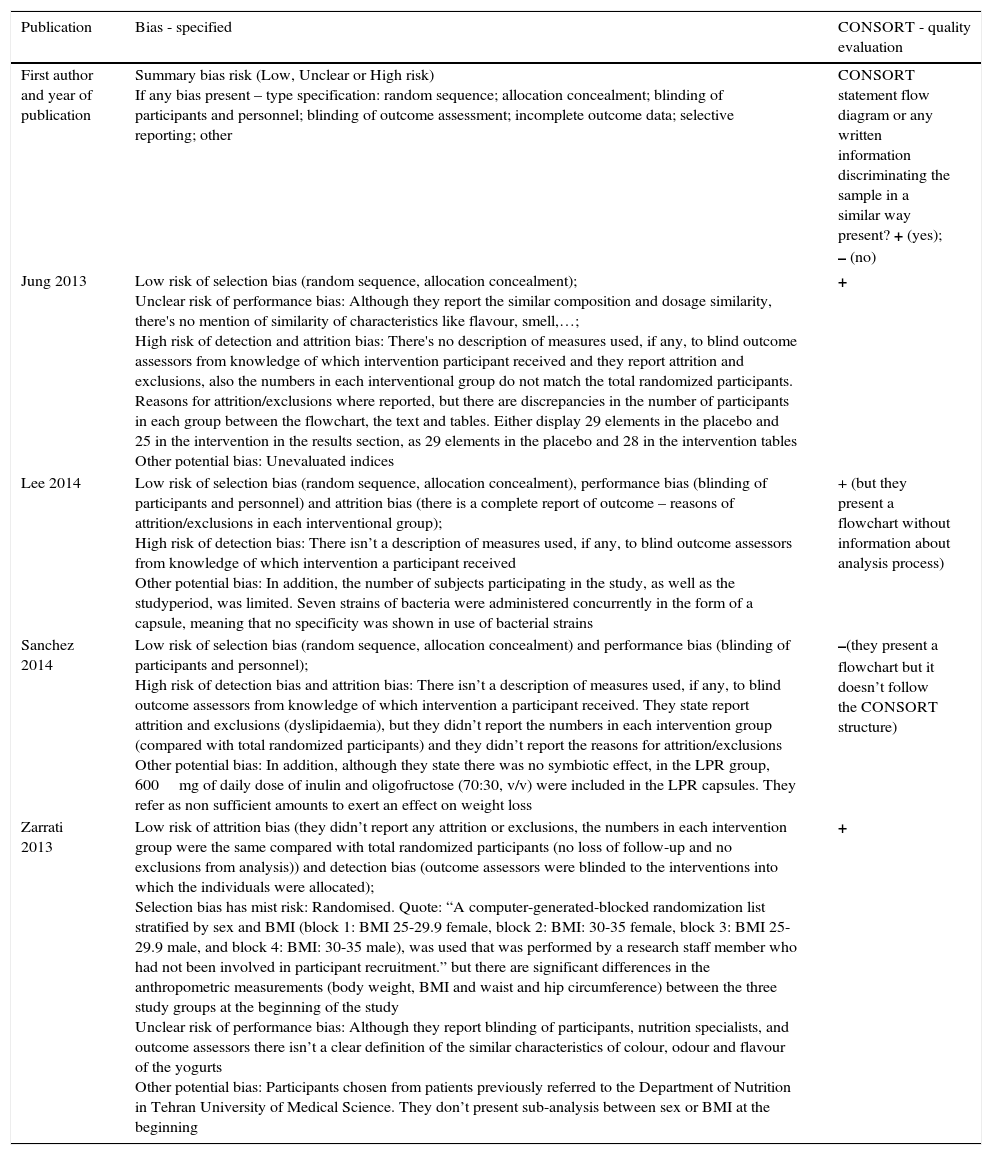
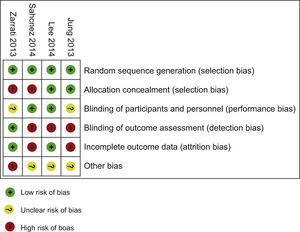
Risk of bias within studiesBias assessment for RCT included is described in the Table 4, according to the Cochrane Consumers and Communication Review Group's “risk of bias assessment tool”, judged as Low, Unclear or High risk.9
Bias assessment – 4 RCT.
| Publication | Bias - specified | CONSORT - quality evaluation |
|---|---|---|
| First author and year of publication | Summary bias risk (Low, Unclear or High risk) If any bias present – type specification: random sequence; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting; other | CONSORT statement flow diagram or any written information discriminating the sample in a similar way present? + (yes); – (no) |
| Jung 2013 | Low risk of selection bias (random sequence, allocation concealment); Unclear risk of performance bias: Although they report the similar composition and dosage similarity, there's no mention of similarity of characteristics like flavour, smell,…; High risk of detection and attrition bias: There's no description of measures used, if any, to blind outcome assessors from knowledge of which intervention participant received and they report attrition and exclusions, also the numbers in each interventional group do not match the total randomized participants. Reasons for attrition/exclusions where reported, but there are discrepancies in the number of participants in each group between the flowchart, the text and tables. Either display 29 elements in the placebo and 25 in the intervention in the results section, as 29 elements in the placebo and 28 in the intervention tables Other potential bias: Unevaluated indices | + |
| Lee 2014 | Low risk of selection bias (random sequence, allocation concealment), performance bias (blinding of participants and personnel) and attrition bias (there is a complete report of outcome – reasons of attrition/exclusions in each interventional group); High risk of detection bias: There isn’t a description of measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received Other potential bias: In addition, the number of subjects participating in the study, as well as the studyperiod, was limited. Seven strains of bacteria were administered concurrently in the form of a capsule, meaning that no specificity was shown in use of bacterial strains | + (but they present a flowchart without information about analysis process) |
| Sanchez 2014 | Low risk of selection bias (random sequence, allocation concealment) and performance bias (blinding of participants and personnel); High risk of detection bias and attrition bias: There isn’t a description of measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received. They state report attrition and exclusions (dyslipidaemia), but they didn’t report the numbers in each intervention group (compared with total randomized participants) and they didn’t report the reasons for attrition/exclusions Other potential bias: In addition, although they state there was no symbiotic effect, in the LPR group, 600mg of daily dose of inulin and oligofructose (70:30, v/v) were included in the LPR capsules. They refer as non sufficient amounts to exert an effect on weight loss | –(they present a flowchart but it doesn’t follow the CONSORT structure) |
| Zarrati 2013 | Low risk of attrition bias (they didn’t report any attrition or exclusions, the numbers in each intervention group were the same compared with total randomized participants (no loss of follow-up and no exclusions from analysis)) and detection bias (outcome assessors were blinded to the interventions into which the individuals were allocated); Selection bias has mist risk: Randomised. Quote: “A computer-generated-blocked randomization list stratified by sex and BMI (block 1: BMI 25-29.9 female, block 2: BMI: 30-35 female, block 3: BMI 25-29.9 male, and block 4: BMI: 30-35 male), was used that was performed by a research staff member who had not been involved in participant recruitment.” but there are significant differences in the anthropometric measurements (body weight, BMI and waist and hip circumference) between the three study groups at the beginning of the study Unclear risk of performance bias: Although they report blinding of participants, nutrition specialists, and outcome assessors there isn’t a clear definition of the similar characteristics of colour, odour and flavour of the yogurts Other potential bias: Participants chosen from patients previously referred to the Department of Nutrition in Tehran University of Medical Science. They don’t present sub-analysis between sex or BMI at the beginning | + |
All the four included RCT as a ‘high’ risk of bias across one10 or two of the domains. Three of the four RCTs had high risk of bias in blinding of outcome (Fig. 2). The study limitations, differences in probiotics administered and participants, and small sample sizes across the included studies mean that the power to detect a trend of overall effect may be limited and chance findings cannot be excluded.
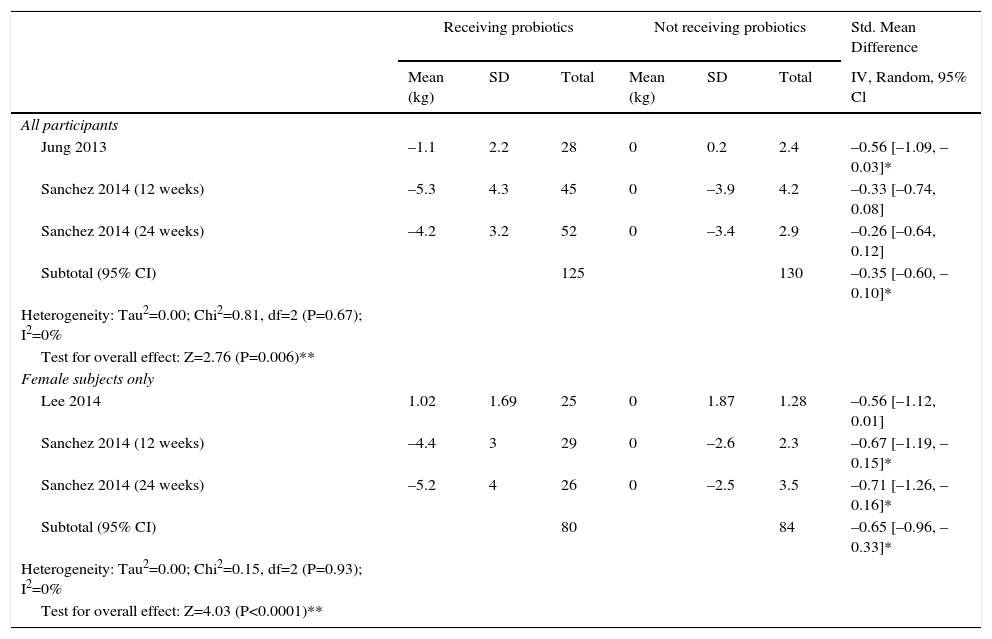
Synthesis of resultsPrimary outcome: weight change (Table 5)We performeda meta-analysis of weight change. This includes 3,10,12,13 of the 4 trials which reported the difference of weight (n=255) in the same measure [difference between baseline body weight and post-intervention body weight in kg]. We included data of 12 and 24 weeks analysis of Sanchez 2014 RCT. One study was only included in the subgroup analysis of female body weight changes (n=164), since it only enrolled female subjects.10 There was a significant difference (P value=0.006) between various probiotics and the control group for weight loss, with a standardized mean difference of –0.35kg in the probiotic treatment group (95% confidence interval (CI) –0.60 to –0.10) for all gender participants and in the subgroup analysis of female body weight changes (SMD –0.65; P value<0.0001; 95% CI –0.96 to –0.33).
ITT analysis of changes in weight loss: probiotics versus control.
| Receiving probiotics | Not receiving probiotics | Std. Mean Difference | |||||
|---|---|---|---|---|---|---|---|
| Mean (kg) | SD | Total | Mean (kg) | SD | Total | IV, Random, 95% Cl | |
| All participants | |||||||
| Jung 2013 | –1.1 | 2.2 | 28 | 0 | 0.2 | 2.4 | –0.56 [–1.09, –0.03]* |
| Sanchez 2014 (12 weeks) | –5.3 | 4.3 | 45 | 0 | –3.9 | 4.2 | –0.33 [–0.74, 0.08] |
| Sanchez 2014 (24 weeks) | –4.2 | 3.2 | 52 | 0 | –3.4 | 2.9 | –0.26 [–0.64, 0.12] |
| Subtotal (95% CI) | 125 | 130 | –0.35 [–0.60, –0.10]* | ||||
| Heterogeneity: Tau2=0.00; Chi2=0.81, df=2 (P=0.67); I2=0% | |||||||
| Test for overall effect: Z=2.76 (P=0.006)** | |||||||
| Female subjects only | |||||||
| Lee 2014 | 1.02 | 1.69 | 25 | 0 | 1.87 | 1.28 | –0.56 [–1.12, 0.01] |
| Sanchez 2014 (12 weeks) | –4.4 | 3 | 29 | 0 | –2.6 | 2.3 | –0.67 [–1.19, –0.15]* |
| Sanchez 2014 (24 weeks) | –5.2 | 4 | 26 | 0 | –2.5 | 3.5 | –0.71 [–1.26, –0.16]* |
| Subtotal (95% CI) | 80 | 84 | –0.65 [–0.96, –0.33]* | ||||
| Heterogeneity: Tau2=0.00; Chi2=0.15, df=2 (P=0.93); I2=0% | |||||||
| Test for overall effect: Z=4.03 (P<0.0001)** | |||||||
Mean = Mean difference between baseline body weight and post-intervention body weight in kg. SD = standard deviation. Heterogeneity was assessed using the Cochran's Q statistic with a P value = 0.1 interpreted as statistically significant (#). I2 statistics was calculated. Standard mean difference was used as effect measure, Confidence interval (95%CI) calculated using inverse variance method and random effects model, not including 0-zero was interpreted as statistically significant (*). Test for overall effect was interpreted as statistically significant (**) when P value = 0.05.
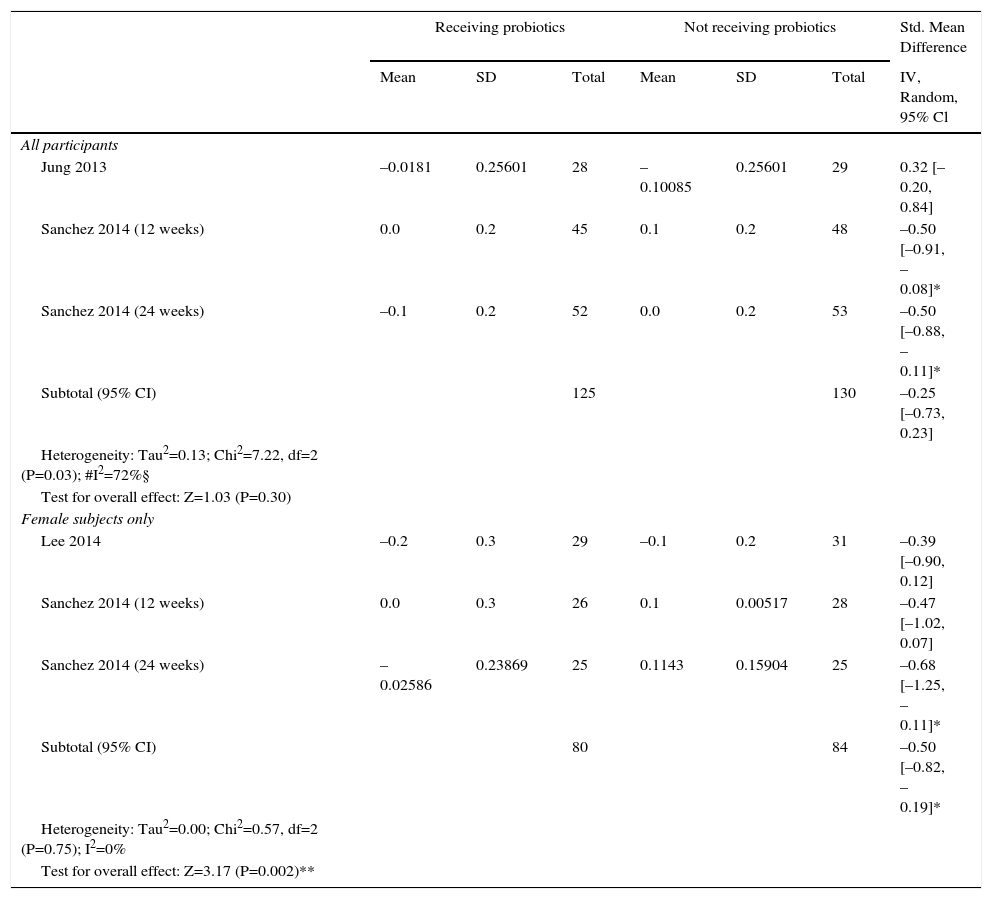
Weperformed a meta-analysis of weight change. This includes 3,10,12,13 of the 4 trials which reported the difference of HDL serum levels (n=255) in the same measure [difference between baseline HDL and post-intervention HDL Sanchez 201412 in mmol/L; Jung 201313 and Lee 201410 (they didn’t specify unit, we presumed according to values presented) in mg/dL – we converted it in mmol/L]. We included data of 12 and 24 weeks analysis of Sanchez 201412 RCT. One study was only included in the subgroup analysis of female body weight changes (n=164), once it only enrolled female subjects.10 There was a significant difference (P value=0.002) between various probiotics and the control group for HDL serum levels, with a SMD of –0.50 mmol/L in the probiotic treatment group (95% CI –0.82 to –0.19) for the subgroup analysis of female participants. In the all gender participants analysis, there was no significant difference between various probiotics and the control group (SMD −0.25, 95% CI −0.73 to 0.23), with results demonstrating considerable levels of heterogeneity (Tau2=0.13; Chi2=7.22, df=2 (P value=0.03); I2=72%).
ITT analysis of changes in HDL: probiotics versus control.
| Receiving probiotics | Not receiving probiotics | Std. Mean Difference | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Total | Mean | SD | Total | IV, Random, 95% Cl | |
| All participants | |||||||
| Jung 2013 | –0.0181 | 0.25601 | 28 | –0.10085 | 0.25601 | 29 | 0.32 [–0.20, 0.84] |
| Sanchez 2014 (12 weeks) | 0.0 | 0.2 | 45 | 0.1 | 0.2 | 48 | –0.50 [–0.91, –0.08]* |
| Sanchez 2014 (24 weeks) | –0.1 | 0.2 | 52 | 0.0 | 0.2 | 53 | –0.50 [–0.88, –0.11]* |
| Subtotal (95% CI) | 125 | 130 | –0.25 [–0.73, 0.23] | ||||
| Heterogeneity: Tau2=0.13; Chi2=7.22, df=2 (P=0.03); #I2=72%§ | |||||||
| Test for overall effect: Z=1.03 (P=0.30) | |||||||
| Female subjects only | |||||||
| Lee 2014 | –0.2 | 0.3 | 29 | –0.1 | 0.2 | 31 | –0.39 [–0.90, 0.12] |
| Sanchez 2014 (12 weeks) | 0.0 | 0.3 | 26 | 0.1 | 0.00517 | 28 | –0.47 [–1.02, 0.07] |
| Sanchez 2014 (24 weeks) | –0.02586 | 0.23869 | 25 | 0.1143 | 0.15904 | 25 | –0.68 [–1.25, –0.11]* |
| Subtotal (95% CI) | 80 | 84 | –0.50 [–0.82, –0.19]* | ||||
| Heterogeneity: Tau2=0.00; Chi2=0.57, df=2 (P=0.75); I2=0% | |||||||
| Test for overall effect: Z=3.17 (P=0.002)** | |||||||
Mean = Mean between baseline HDL and post-intervention HDL Sanchez 2014 (12) in mmol/L; Jung 2013 (13) and Lee 2014 (10) (they didn’t specify unit, we presumed according to values presented) in mg/dL – we converted it in mmol/L]. SD = standard deviation. Heterogeneity was assessed using the Cochran's Q statistic with a P value ≤ 0.1 interpreted as statistically significant (#). I2 statistics was calculated, cut-off for considerable heterogeneity = 50% (§). Standard mean difference was used as effect measure, Confidence interval (95%CI) calculated using inverse variance method and random effects model, not including 0-zero was interpreted as statistically significant (*). Test for overall effect was interpreted as statistically significant (**)
In this review, it is not sensible to perform any other meta-analysis or to present forest plots due to the very large difference in presentation of outcomes, the variability of the species and strains used, without reproduction in a consistent stratified results related to weight change and due the limited number of studies involved.
DiscussionProbiotic effect in body weight is not only species specific, but also strain specific. The 4 clinical trials and 14 experimental used different species, different combinations, had different study durations and, particularly, the experimental studies used different species participants. Outcomes were not reported in a padronized measure, not allowing to perform other meta-analysis.
Regarding other probiotic that showed promising results, L. rhamnosus GG fed mice with a high fat diet showed reduced weight when compared to high fat diet animals without this probiotic. The same did not happen when animals were fed a normal diet, having instead non-significant results.25L. gasseri SBT2055 was effective in preventing weight gain in 10% fat diet-fed mice.26 Simultaneous feeding of animals with L. curvatus HY7601 and L. plantarum KY103 also resulted in a reduced weight gain.20L. plantarum LG42 fed mice had a smaller weight gain than control animals, with the weight gain being smaller with higher doses of this probiotic.15 Finally, Lactobacillus L66-5 strain also showed a significant body weight decreasing power.21
Overall most attempts to use probiotics to reduce body weight, or to slow body weight gain did not have the desired results. Even though several Lactobacillus strains have been studied and some have results that support a possible beneficial weight reducing capability, with only L. rhamnosus CGMCC 1.3724 at the moment having positive results in humans.
Probiotics beneficial mechanism in host metabolism is explored Kim 2013,25 where authors propose that LGG (the probiotic strain used) sensitizes insulin action is co-mediated by an enhanced adiponectin production and AMPK activation and at least in part associated with increased expressions of GLUT4, and lipid oxidative genes in responsible tissues, such as adipose tissue, skeletal muscle and the liver and in Kang 2013,24 consistent with the hypothesis put forward in Kim 2013,25 reported that the anti-obesity effect of BNR17 is responsible for the increased expression of fatty acid metabolism-related genes rather than reduced fatty acid synthesis and fat intake in the liver.
It is relevant to mention that there is evidence that the presence of two different bacterial species modifies the mutual metabolic activity. This aspect must be taken in consideration during the establishment of the species that will integrate a probiotic. We emphasize, concerning this topic, the results of Arora 2012,14 in which the lactobacilli administration promoted an increase of colonization by Bifidobacterium, which other studies have demonstrated to be positively correlated with improved metabolic profile and normalization of inflammatory tone.
Prebiotic carbohidrates enhance the survival in the gastric (low pH) and duodenal (bile salts) conditions and proliferation of probiotics, being an useful tool to ensure the presence proliferation in the gut in the desired quantities.
Potential biases in the review processOwing to the limited number of studies involved, it was impossible to detect publication bias. Only four RCTs met the inclusion criteria in our analysis, thus the power to detect the trend of overall effect in humans was limited and a chance finding cannot be excluded. Also, although we included 14 experimental studies, the animals species and the mode of probiotic administration and the specific strains used vary widely between studies. As the efficacy of a probiotic is highly strain-specific, the mentioned fact could account for the differences found in the outcomes of those different studies and the power to detect the trend of overall effect in experimental studies.
LimitationsDue to the variability of the species and strains used and each population specification, the majority of the included studies have low generalizability. It represented a limitation to perform a meta-analysis and to present a forest plot.
Although we included major databases, according to PRISMA statement,7 our review may not have been exhaustive because: in the PubMed central database the keywords were confined to [Title/Abstract], in the SCOPUS database were confined to TITLE- ABS-KEY, Cochrane Central Register of Controlled Trials - CENTRAL database were confined to Title, Abstract and Keyword to ensure that they were defined as key word of the articles searched; the 16 studies searched in Clinical Trials.gov and ISRCTN because these were excluded because there were no available results. In Google Scholar and ISI Web of knowledge the search did not include the outcome reference word in order to maximize the results in these databases. Our findings were also limited by the inherent limitations of the literature itself. We also consider a limitation of the search the restriction to English and Portuguese languages.
With Mesh some of our quote phrases would not be included in the real query performed by pubmed: “Weight change”[Mesh]; “BMI change”[Mesh]; “probiotic diet”[Mesh]; “probiotic therapy”[Mesh]; “probiotic supplementation”[Mesh]; “high-fat diet”[Mesh]
The correspondent Mesh terms don’t have all the potential entries correspondent to the search term we used (i.e. the Mesh term Probiotics does not consider probiotic therapy as entry terms). High-fat diet has a similar Mesh term, but it was only introduced in 2012. This expression is mainly used in experimental studies, some of which have been published and indexed before 2012.
With all fields classification, we would include some quote phrases not meaningful for the study: Weight would be translated by the search engine as “weights and measures” and “body weight”. The Mesh term “body weight” does not consider weight alone as a potential entry term. With “weight”, our search also included studies that referred to “body weight” as “weight” alone.
The same rationale can be applied to other databases in addition to the fact that the query should present the minimum variability between databases.
ConclusionsIn our systematic review, we found that probiotic effect in body weight is specie and strain specific. L. gasseri BNR17, reduces weight gain compared to controls; L. gasseri L66-5 promoted weight gain, L. rhamnosus GGMCC is the only one that had positive effect in weight loss in humans. In other hand, L. plantarum LG42, L. gasseri SBT2055 and L. plantarum co-therapy with KY103 and L. curvatus HY7601 had an anti-obesity effect in animal models.
The combined therapy of Lactobacillus acidophilus LA5, Lactobacillus casei DN001 and Bifidobacterium lactis Bb12 had a modulating effect in the immune system, improving the inflammatory status. L. rhamnosus CGMCC, while promoting weight gain, also reduced leptin levels, reflecting a sensitizing effect for this adipokine which may prove useful in weight gain in a less harmful form. L. gasseri BNR17 improved lipidaemia in a clinically significant way and, in an experimental study performed on Male Sprague-Dawley rats, it presented a beneficial modulation of adipose accumulation, promoting the reduction of the weight gain rate and the reduction of the accumulation of visceral adiposity.
The investigation of the usefulness of these microbes in shaping weight, and their actions as growth-promoting agents has had great developments in agriculture during the last decade.1 However, the level of clinical trials or research performed with guidance for implementing clinically isn’t yet sufficient or consensual. Thus, further research is needed to assess the effect of probiotic therapy in human weight change. Finally, specific probiotics could represent important agents in the management of the two of the major and emerging epidemics worldwide: malnutrition and obesity.
Author contributionsStudy concept and design: Rouxinol-Dias, AL. Acquisition of data: Pinto, AR. Analysis and interpretation of data: Rouxinol-Dias, AL, Pinto, AR, Rodrigues, D, Dias, J and Moreira, M. Drafting of the manuscript: Rouxinol-Dias, AL, Pinto, AR, Janeiro, C, Rodrigues, D, Dias, J, Moreira, M, Pereira, P. Critical revision of the manuscript for important intellectual content: Rouxinol-Dias, AL and Dias, J. Statistical analysis: Rouxinol-Dias, AL. Study supervision: Rouxinol-Dias, AL.
Competing interest statementAll authors have completed the Unified Competing Interest form at http://www.icmje.org/downloads/coi_disclosure.pdf and declare that 1 - no authors have support from probiotics companies for the submitted work; 2 - no authors have relationships with probiotics companies that might have an interest in the submitted work in the previous 3 years; 3 - their spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and 4 - no authors have non-financial interests that may be relevant to the submitted work.
We would like to thank the Immunology Lab and Service for facilitating the development of this work, with special regards to our teacher, PhD. André Moreira.