The association between exposure to heavy metals and cancer has been extensively studied, although the mechanisms involved are far from being completely understood. Concerning renal cell carcinoma, several heavy metals have been implicated as risk factors, namely cadmium (Cd) and lead (Pb).
Herewith, we investigated the presence and distribution of heavy metals in samples of renal cell carcinoma, as well as adjacent renal tissue (control samples), in patients submitted to radical or partial nephrectomy for renal cell carcinoma. Samples from renal tumour and adjacent renal tissue were processed and observed by Scanning Electron Microscopy coupled with X-Ray Microanalysis (SEM-XRM), in order to detect and quantify heavy metals in situ, using the JEOL JSM-6301F microscope.
Our results revealed a significant difference in the composition of heavy metals between the renal adjacent tissue (control) and the tumour tissue. No heavy metal particles were detected in the adjacent (control) tissue, by this technique, but the presence of heavy metals such as chromium (Cr), iron (Fe) and copper (Cu) were detected among other particles which could be seen in the tumour tissue samples.
Our results might suggest a possible role of heavy metals in the oncogenic pathway of renal cell carcinoma. However, there is controversy regarding this topic: 1 - Is this anomalous sequestration of heavy metals just an epiphenomenon or is this a hint of a causal mechanism? 2 - Can heavy metals be used as biomarkers with potential diagnostic or prognostic interest? For example, could they be used to improve the diagnostic value of renal biopsy in clinical practice?
The association between exposure to heavy metals, either occupational or environmental, and cancer has been extensively studied.1,2
The mechanisms involved in heavy metal induced carcinogenicity seem to be diverse and are far from being completely understood.3 Heavy metals are natural elements of the earth's crust. Although not metabolized, some heavy metals participate in some essential metabolic activities: for example, copper (Cu) is essential for the synthesis of hemoglobin and cobalt (Co) is involved in erythropoiesis. However, if the amount of these essential elements exceeds normal levels, they can become toxic and cause serious health risks.4
Prolonged exposure to mercury (Hg), lead (Pb), chromium (Cr), cadmium (Cd), arsenic (As), copper (Cu), vanadium (V), nickel (Ni) and zinc (Zn) can cause deleterious health effects in humans, namely, chronic inflammatory conditions and a higher risk for several cancers, cardiac, pulmonary and neurological diseases.5–7 This scenario has been aggravated by erroneous human intervention that has significantly changed their natural cycle and balance, causing abnormal accumulation and environmental pollution.5–7
Concerning renal cell carcinoma, several heavy metals have been implicated as risk factors, namely Cd8 and Pb.9 Nevertheless, this causal association has not been definitively established yet.10
Herewith, we investigated the presence and distribution of heavy metals in samples of renal cell carcinoma, as well as adjacent renal tissue (control samples), in patients submitted to radical or partial nephrectomy for renal cell carcinoma. We used a semi-quantitative high-resolution method of scanning electron microscopy coupled with X-ray elemental microanalysis (SEM-XRM) to detect particles of Cd, Cr, Cu, iron (Fe), aluminium (Al), Ni and Zn.
Materials and methodsSamples of renal cell carcinoma and adjacent renal tissue (control) from seven patients submitted to radical or partial nephrectomy were fixed in a 3% glutaraldehyde solution (adapted from Silva et al., 198711). An SEM-XRM was used to detect and quantify heavy metals in situ. First, samples were dehydrated in ethanol and critical point-dried in a Balzer's apparatus. The preparation was mounted on metal stubs, coated by carbon under vacuum and examined in a JEOL JSM-6301F scanning electron microscopy, coupled with a Noran Voyager X-ray elemental microanalyser (XRM) with an Energy Dispersive Spectrometry (EDS) detection system. This method involves two main steps: the first consists of processing tissue samples with secondary electrons, in order to visualize white spots that correspond to the deposition of heavy metals (high molecular weight particles). The second consists in the identification of heavy metal contents in all white and/or opaque inclusions. For that purpose we screened all of the spots with X-ray elemental microanalysis to determine which of them contained heavy and/or dense particles. This was achieved with the backscattered electrons technique. Thus, with SEM coupled with X-ray microanalysis, it was possible to reveal the subcellular content and distribution of heavy particles in the collected tissue samples (renal cell carcinoma or adjacent renal tissue). This technique allows in situ identification of heavy metals particles. The micrographs of the samples were derived from secondary and backscattered electron imaging modes.12
This study was approved by the local Ethics Committee of Centro Hospitalar de São João, E.P.E., and all of the patients signed an informed consent.
ResultsOur results clearly show significant differences in heavy metal concentrations between the tumour samples and the controls (surrounding tissue) demonstrating that there is an anomalous sequestration of heavy metals in kidney tumour tissue.
Our results revealed: (i) no heavy metal particles were detected by using the spectrum of X-ray microanalysis technique (Figs. 1A, 1B and 1C) but (ii) the presence of heavy metals such as Ni, Cr, Fe, Ni, tungsten (W), titanium (Ti), Cd, Cu, Mn, Zn and Mg particles could be seen in the tumour tissue samples using spectrum of X-ray microanalysis (Figs. 2A, 2B, and 2C, 2D, 2E). No levels of Pb were detected, either tumour tissue samples or renal adjacent tissue.
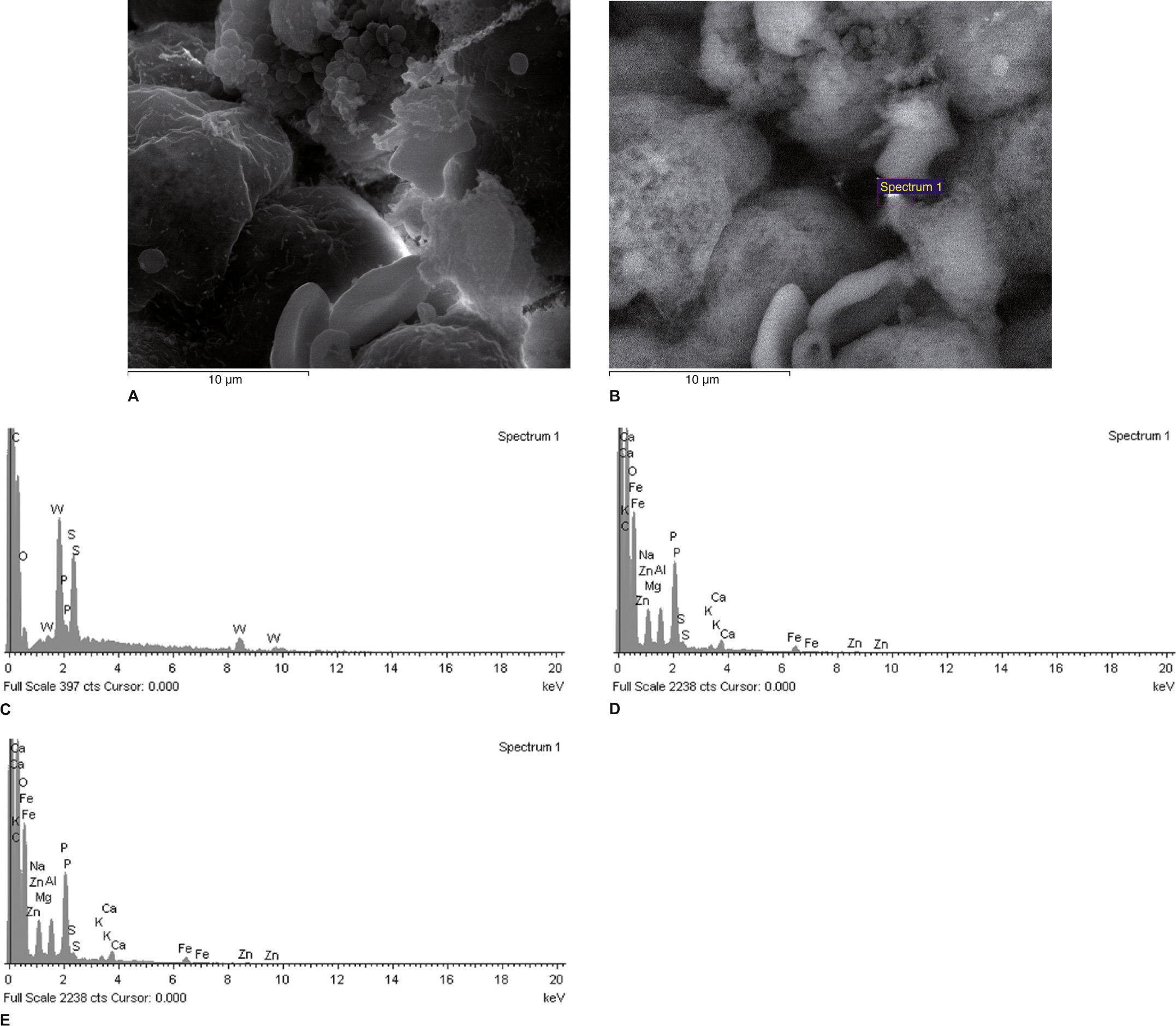
Scanning electron micrographs using secondary (A) and backscattered (B) electrons of an area of a kidney sample from a total nephrectomy renal cell carcinoma adjacent (control) tissue. All of the white spots in (B) are analyzed in Fig. 1C. It is possible to observe the difference in the qualitative composition of heavy metals of each sample. It is not possible to observe the presence of heavy metals in the control.
Fig. 1C The spectrum reveals that the inclusion contains carbon (C, in the figure), which is used to coat the sample for SEM-XRM viewing, and it is also possible to see other normal chemical elements of cells such as Ca (calcium), P (phosphorus), O (oxygen), sulfur (S) and chlorine (Cl). X-ray microanalysis spectrum of an electron white spot is shown in the previous figure (backscattered electrons, (B).
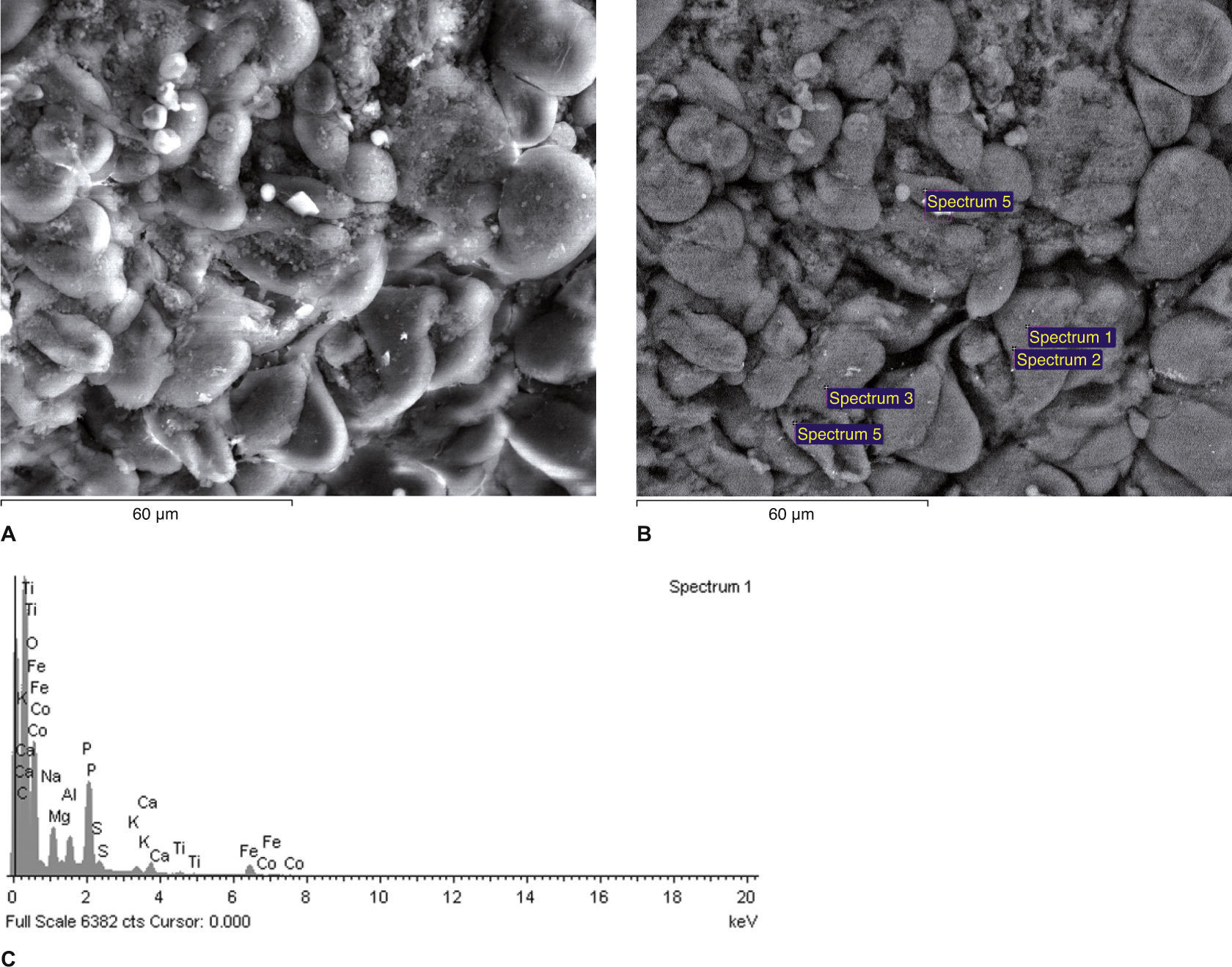
Scanning electron micrographs using secondary (A) and backscattered (B) electrons of an area of a kidney sample from a partial nephrectomy renal cell carcinoma tissue. All of the white spots in (B) are analyzed in Fig. 2C.
Fig. 2C This spectrum (Fig. 2B) reveals that the inclusions contain carbon (C, in the figure), which is used to coat the sample for SEM-XRM viewing, S, P, but also tungsten (W), in amounts greater than 0.2-0.3% (m / m).
Figures 2D and 2E Spectrums of X-ray microanalysis revealing that an intracellular particle observed by scanning electron microscopy contains Fe, Zn, Ca, K, S, P, Al, Mg, Zn and O. The analyzed dots were samples from a total nephrectomy renal cell carcinoma tissue.
The aim of this work was to determine if there were differences in the content of heavy metals in renal cell carcinoma and adjacent renal tissue samples from patients submitted to radical or partial nephrectomy, using the SEM-XRM technique. We found particles of Cr, Cd, Al, Ti, Ni, Fe, Zn, W and manganese (Mn) in renal cell carcinoma samples, but not in the adjacent normal renal parenchyma. These findings corroborate previous data in literature, suggesting that there is a significant difference of metal contents in tumour and non-tumoural tissue.
SEM-XRM is a qualitative and semi-quantitative (0.2-0.3% m/m) technique used to detect metal particles at the subcellular level. This method12 allows the precise and specific identification of individual particles of metals at high resolution and can offer complementary information to the data obtained by previously used methods, such as atomic absorption spectrophotometry,13 cold vapour atomic absorption spectrometry,14 or synchrotron radiation induced X-ray emission (SRIXE)15 or Particle Induced X-ray Emission Analysis (PIXE).16
Environmental factors have been considered responsible for at least 80% of the incidence of neoplastic diseases.17 It is well known that heavy metals, in particular Cd, have an oncogenic role in tumourigenesis.3,18 Cd was identified as a human carcinogen, being associated with lung cancer after occupational exposure.2 In addition, it has been implicated in kidney, breast and prostate cancers.19 Although the mechanisms underlying renal carcinogenesis were not the objective of this work, several hypotheses can be raised. In fact, there is strong evidence of chronic inflammation induced by heavy metals as one of the main underlying mechanisms.18
Other authors using the technique of atomic absorption spectrophotometry reported high levels of Cd and Pb in samples of renal cell carcinoma.13
Our results show different contents of heavy metals in renal cell carcinoma and in the adjacent tissue in all analysed samples. Nevertheless, the results obtained by other authors and using other techniques are not in agreement with our preliminary data. Dobrowolski Z, et al.,15 used Synchrotron Radiation Induced X-ray Emission (SRIXE), to identify trace elements (Cd, Ti, Pb and Rb) which can be used to distinguish between normal and malignant tissue in renal cell kidney cancer and to assess changes in the concentration of trace elements in tissue with progressing malignant disease. They found that the concentration of some trace elements such as Cd and Pb is lower in malignant tissue than in control kidney cortex tissue obtained during autopsy in which the cause of death was trauma. Cerulli et al.,17 evaluated the amounts of Cd and Pb in neoplastic tissue and in the adjacent normal part of the kidney excised for carcinoma and compared them with those in renal tissue of fetuses, newborns and subjects that died of non-neoplastic diseases. To accomplish that task, they used the Inductively Coupled plasma Atomic Emission Spectrometry and Atomic Absorption Spectrometry with Electrothermal Atomization. The metallothionein immunoperoxidase staining technique was used to localize the accumulation of Cd and Zn in the nephron. They concluded that (i) contents of Cd and Pb in kidneys of fetuses and newborns was extremely low, (ii) they were significantly increased in adjacent-normal tissue of kidneys with carcinomas and these amounts were significantly higher compared to kidneys of individuals that died of non-neoplastic diseases, (iii) in tumoural tissue, Cd level was low and Pb level was high. Karcioglu et al.,16 using Particle Induced X-ray Emission Analysis (PIXE), could not detect Cd in neoplastic renal tissue.
We should recall that the purpose of this work was not to elucidate the role of heavy metals in a putative oncogenic pathway, but only to characterize the relative content of heavy metals in renal carcinoma cell compared with the control surrounding tissue using a different technique, as previous data in literature showed controversial results.
Although our results might suggest a possible role of heavy metals in the oncogenic pathway of renal cell carcinoma, there is controversy regarding this topic. Firstly, these results have to be confirmed by other studies using the same technique. If our results are to be confirmed by other authors, we should raise the following issues: 1 - is this anomalous sequestration of heavy metals just an epiphenomenon or is this a hint to a causal mechanism? 2 - Can heavy metals be used as biomarkers with potential diagnostic or prognostic interest? For example, could they be used to improve the diagnostic value of renal biopsy in clinical practice?
ConclusionAccording to these results, heavy metals might have a role in renal cell carcinogenesis. In fact, the chemical composition of the tumour tissue and the adjacent tissue is different. Inversely to the adjacent tissue, there was an anomalous sequestration of heavy metals in kidney tumour samples.
These findings open a new venue for future studies on the detailed kinetics of microscopic particles of heavy metals and could be fundamental for understanding the impact of heavy metals in renal carcinogenesis.
The authors would like to thank CEMUP, Centro de Materiais da Universidade do Porto, Engineer Carlos Sá and Dr. Daniela Silva for the technical support and availability during the execution of this work. The authors are also grateful to Prof. Carlos Silva, and his team, Urology, HSJ, for their expertise, samples collection and all clinical support.