Cancer of the esophagus is an aggressive cancer with high mortality. Because of the esophagus’s lack of serosa and its peculiar lymphatic drainage, esophageal cancer is diagnosed in advanced stages. The eighth edition of the TNM (2017) aims to standardize care for esophageal cancer throughout the world; it includes not only patients treated with esophagectomy alone, but also those receiving neoadjuvant chemotherapy and/or radiotherapy. One new development in the eighth edition is that it establishes separate classifications for different time periods, with pathologic stage groups for prior to treatment (cTNM), after esophagectomy (pTNM), and after neoadjuvant therapy (ypTNM). The combined use of endoscopic ultrasound, CT, PET-CT, and MRI provides the greatest accuracy in determining the clinical stage, and these techniques are essential for planning treatment and for evaluating the response to neoadjuvant treatment. Esophagectomy continues to be the main treatment; it is also the elective gastrointestinal surgery that has the highest mortality, and it carries the risk of multiple complications, including anastomotic leaks, pulmonary complications, technical complications, and functional complications
El cáncer esofágico tiene un comportamiento agresivo con alta mortalidad. La ausencia de serosa y su peculiar drenaje linfático hacen que se diagnostique en estadios avanzados. La 8.ª edición TNM (2017) armoniza la atención del cáncer esofágico en todo el mundo e incluye no solo pacientes tratados con esofagectomía aislada, sino aquellos que han recibido tratamiento neoadyuvante con quimioterapia y/o radioterapia. Como novedad establece clasificaciones separadas y relacionadas temporalmente con el cáncer: un estadio clínico previo al tratamiento (cTNM), patológico tras esofagectomía (pTNM) y patológico posneoadyuvante (ypTNM). La combinación de la ecoendoscopia, tomografía computarizada (TC), tomografía por emisión de positrones asociada a TC y resonancia magnética proporciona la mayor precisión posible en la determinación del estadio clínico, y son esenciales para la planificación del tratamiento y evaluación posneoadyuvancia. El tratamiento principal sigue siendo la esofagectomía, que es la cirugía gastrointestinal electiva con mayor mortalidad y acarrea múltiples complicaciones: fugas anastomóticas, complicaciones pulmonares, técnicas y funcionales.
Oesophageal cancer (OC) is the eighth most common type of cancer in the world and the third most common type of cancer of gastrointestinal origin.1 It is seen mainly in older men.2 There are two main types, depending on the cell histopathology.1 Squamous cell carcinoma (SCC) is the more common type overall (90%), although in recent times there has been a significant decrease in Western countries. SCC is associated with alcohol consumption and smoking, and is more common in the upper and middle third of the oesophagus.3 Adenocarcinoma (ADC) is currently the most common OC in Western countries. It is associated with gastroesophageal reflux disease and obesity, and is found in the distal oesophagus in 75% of cases.1 OC is an aggressive form of cancer with a high mortality rate. It is usually diagnosed in advanced stages, partly because of certain particular characteristics of the oesophagus, such as the absence of a serosal layer in the wall of the organ and the complexity of its lymphatic drainage pathways.1,2
The American Joint Committee on Cancer (AJCC) Staging Manual, 8th Edition, contains the best data on staging of cancer of the oesophagus and gastro-oesophageal junction (GOJ) currently available worldwide.4 The TNM system (T: tumour; N: nodes; M: metastasis) that the AJCC uses is the internationally agreed-upon standard system for cancer staging and has significant repercussions for prognosis and treatment decisions. The 8th edition of the TNM unifies OC care worldwide and includes not only patients treated with oesophagectomy alone, but also those who have received adjuvant therapy with chemotherapy and/or radiotherapy.4
The objective of this study is to conduct an updated review of OC using the 8th and latest (2017) edition of the TNM classification. It also reviews the complications that may arise from the primary oesophageal tumour and post-surgical complications from oesophagectomy, which remains the primary treatment for local or locally advanced disease, as well as those in which the radiologist has a fundamental role.
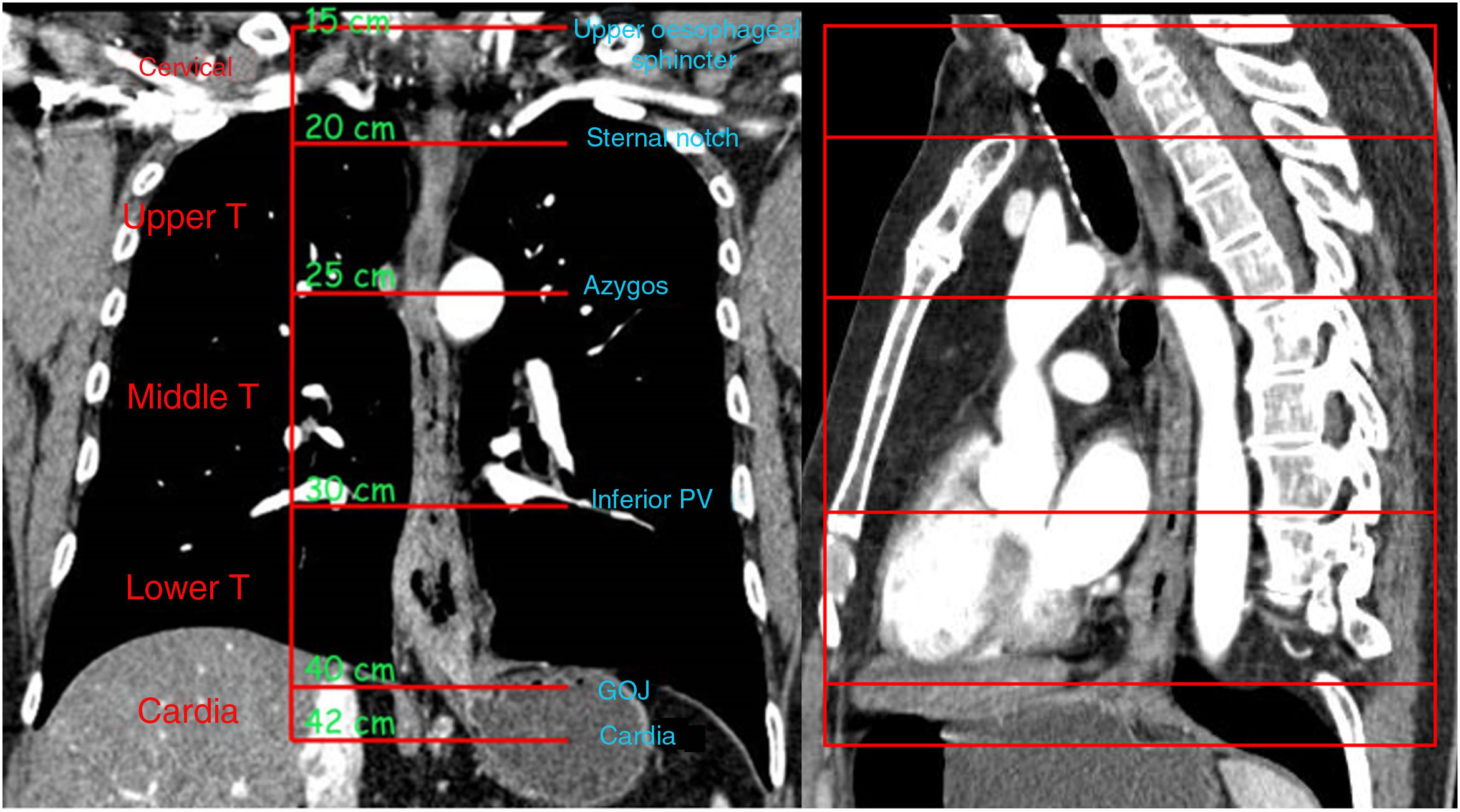
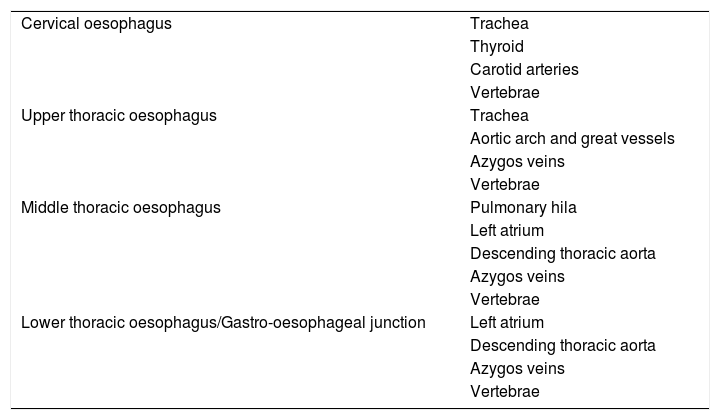
Review of anatomyThe oesophagus is divided into three anatomical compartments: cervical, thoracic and abdominal. The thoracic oesophagus is further divided into the upper, middle and lower thirds (Fig. 1). However, the clinical significance of OC lies in its anatomical relationship to adjacent structures rather than its location in the oesophagus4 (Table 1).
Anatomical relationships of the compartments of the oesophagus.
| Cervical oesophagus | Trachea |
| Thyroid | |
| Carotid arteries | |
| Vertebrae | |
| Upper thoracic oesophagus | Trachea |
| Aortic arch and great vessels | |
| Azygos veins | |
| Vertebrae | |
| Middle thoracic oesophagus | Pulmonary hila |
| Left atrium | |
| Descending thoracic aorta | |
| Azygos veins | |
| Vertebrae | |
| Lower thoracic oesophagus/Gastro-oesophageal junction | Left atrium |
| Descending thoracic aorta | |
| Azygos veins | |
| Vertebrae |
Cervical oesophagus: anatomically located in the neck and extends from the hypopharynx to the sternal notch. Although there is some variability, the typical length of the cervical oesophagus measured endoscopically from the incisors is 15−20cm.4
Upper thoracic oesophagus: extends from the sternal notch to the lower border of the azygos vein. It typically measures 20−25cm in endoscopy.4
Middle thoracic oesophagus: extends from the lower border of the azygos vein to the lower border of the inferior pulmonary vein. Endoscopically it measures 25−30cm.4
Lower thoracic oesophagus/gastro-oesophageal junction: extends from the lower border of the inferior pulmonary vein to the stomach. The lower thoracic oesophagus normally passes through the diaphragm into the stomach, but the intra-abdominal part of the oesophagus often varies in length. The abdominal part of the oesophagus is considered part of the lower thoracic oesophagus. It measures 30−40cm in endoscopy.4
The wall of the oesophagus has four layers: mucosa, submucosa, muscularis propria and adventitia. The mucosa comprises epithelium, lamina propria and muscularis mucosae (formed by a longitudinal layer of smooth muscle). The submucosal layer is a layer of fibroelastic connective tissue containing blood and lymph vessels. The muscularis propria consists of an inner layer of circular muscle fibres and an outer layer of longitudinal fibres. There is no serosal layer surrounding the muscle layer; the adventitia, which is a layer of connective tissue, is bounded directly to the muscularis propria.5
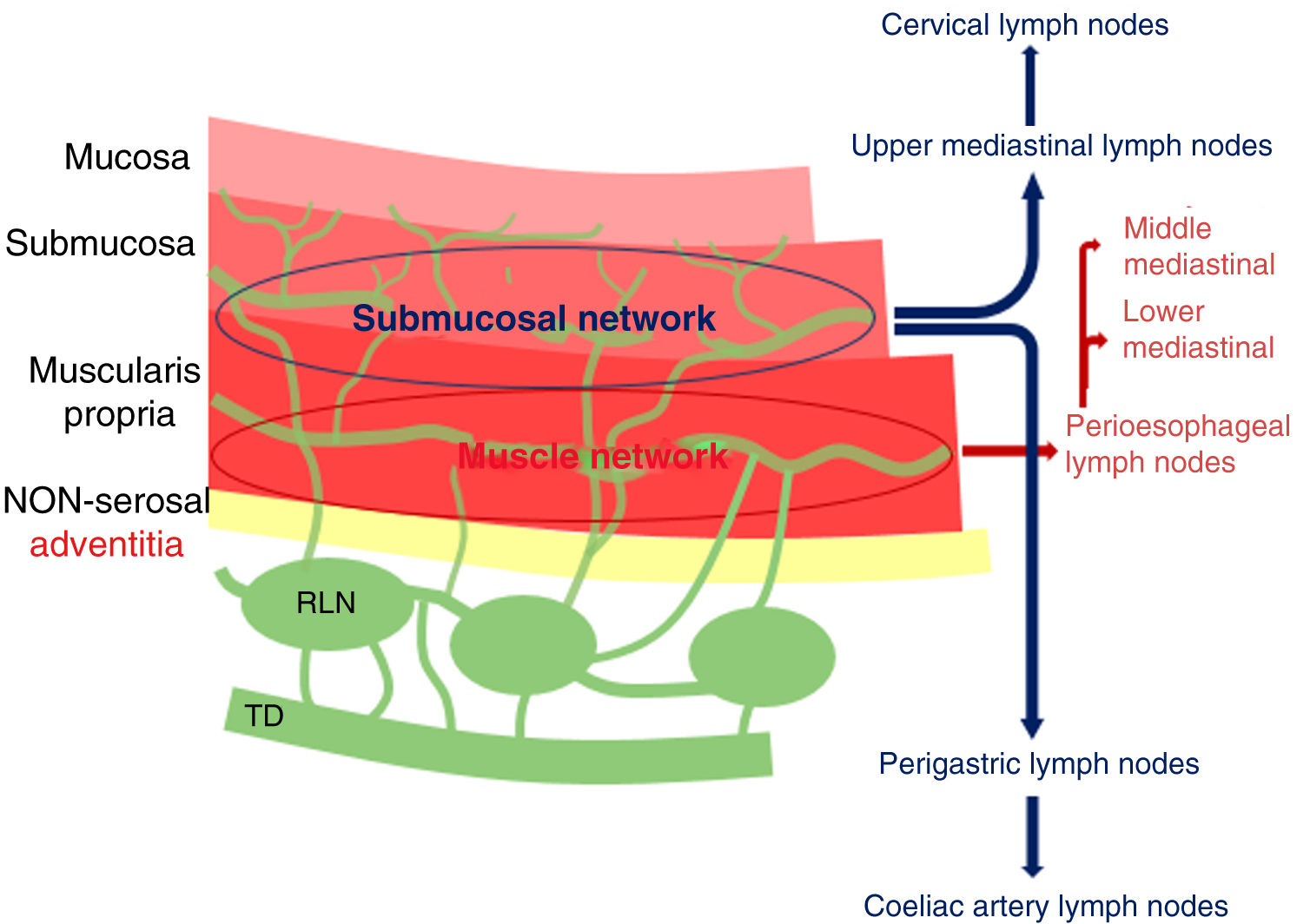
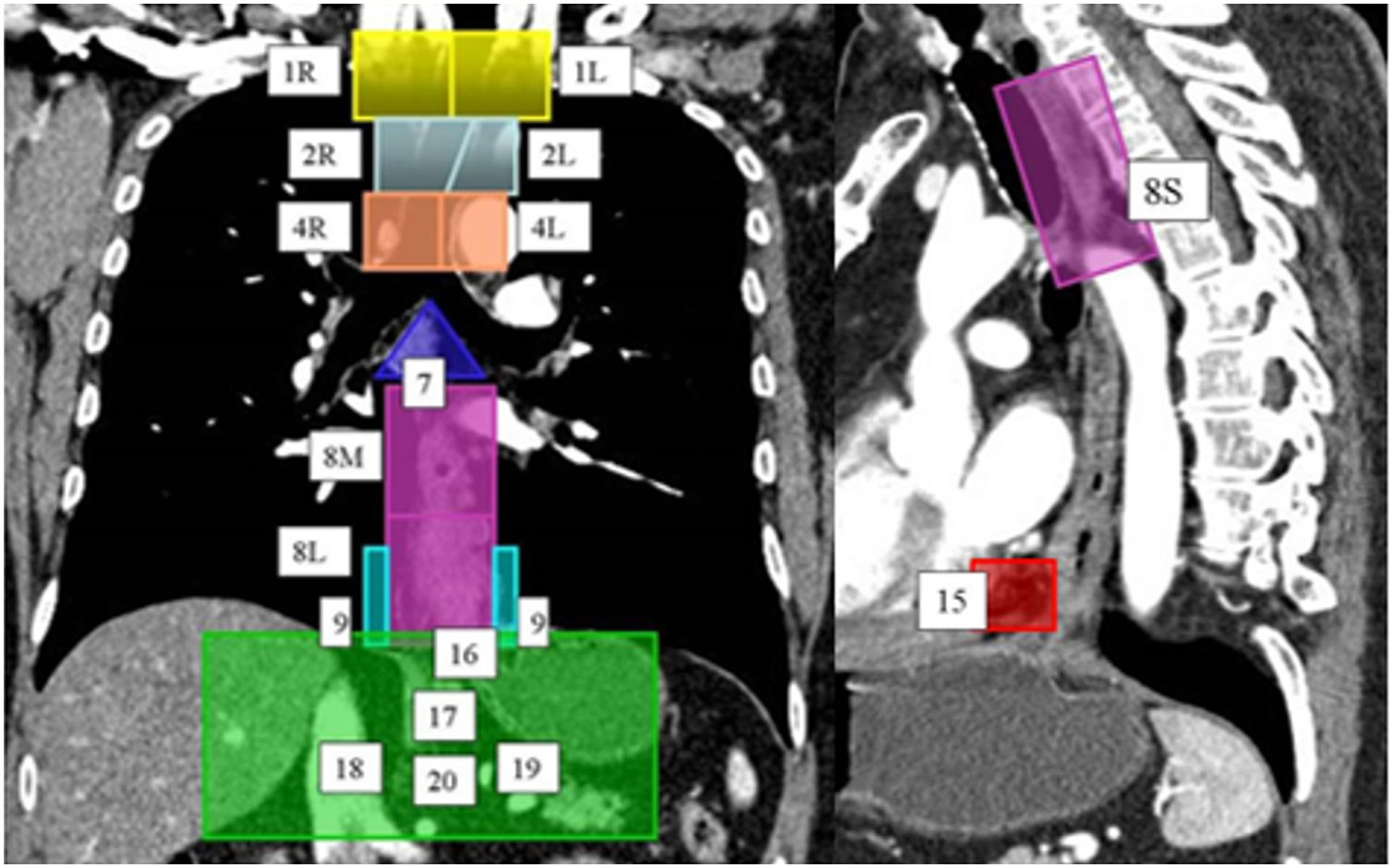
Oesophageal lymphatic drainage is intramural and longitudinal. There are lymphatic plexuses in each layer of the oesophageal wall, but the lymphatic network is mainly developed in the submucosal layer and the lamina propria, and less in the muscle layer and adventitia. The dense lymphatic network of the submucosa is uninterrupted and is continuous with the lymphatic network of the submucosal layer of the pharynx and stomach. In general, the upper two thirds of the oesophagus drain cranially and the lower third drains caudally. The submucosal plexus can bind to regional lymph nodes with no need for connection to the muscular lymphatic plexus. In addition, it can drain directly into the thoracic duct (TD), which occurs in approximately 43% of cases.4,6,7 Lastly, it can also bind to the muscular lymphatic plexus, which is connected to the extramural lymphatic system.6 Thus, even if the oesophageal tumour is limited to the submucosa, craniocaudal spread of tumour cells along the submucosal plexus can cause lymph node involvement away from the location of the primary tumour.4,7 It is worth noting, therefore, that involvement of distant lymph nodes is not indicative of advanced disease. When the tumour invades the muscle layer, this increases the chances that oesophageal lymph nodes and mediastinal lymph node chains will also be affected7 (Fig. 2). The most widely used lymph node map is the AJCC lymph node map for the staging of oesophageal cancer (Fig. 3).
Layers of the oesophageal wall and distribution of the lymphatic system. Tumour cells can spread cranially to upper mediastinal and cervical lymph nodes, and caudally to perigastric and coeliac lymph nodes. When the muscle network is affected, apart from the lymph nodes described above, it is more common to see perioesophageal and middle and lower mediastinal lymph nodes. RLN: regional lymph node; TD: thoracic duct.
Lymphatic drainage system of the oesophagus. A computed tomography scan with coronal and sagittal reconstruction, using colours and numbers, shows the lymph node map proposed by the American Joint Committee on Cancer for the staging of oesophageal cancer. Right (1 R) and left (1L) lower cervical paratracheal lymph node chains; right (2 R) and left (2L) upper paratracheal lymph node chains; right (4 R) and left (4L) lower paratracheal lymph node chains; sub-carinal lymph node chains (7); upper thoracic paraoesophageal lymph node chains (8 S), middle thoracic lymph node chains (8M) and lower thoracic lymph node chains (8L); right (9 R) and left (9L) pulmonary ligament chains; diaphragmatic lymph node chains (15); paracardiac lymph node chains (16); left gastric lymph node chains (17); common hepatic lymph node chains (18); splenic lymph node chains (19); and coeliac lymph node chains (20).
The AJCC Staging Manual, 8th Edition, for OC is based on updated data, with a significantly larger sample size and a larger number of risk-adjustment variables compared to the 7th edition.4 The data were collected by the Worldwide Esophageal Cancer Collaboration and included information from 33 centres and 22,654 patients.2,4
In summary, the main conclusions are as follows.2 On the one hand, clinical staging, based mainly on imaging, continued to be inaccurate. Clinical staging for decision-making and prognosis was up to then based on the corresponding disease stage groupings. It was found that clinical staging could not be based on post-treatment staging as in the past; it required its own stage grouping. On the other hand, disease staging based on oesophagectomy alone was losing significance in advanced-stage cancer. However, it remained significant for early-stage cancers and as a reference point for staging and survival. Finally, as neoadjuvant therapy affects depth of cancer invasion (T), regional lymph node metastases (N) and distant metastases (M) in different ways, it yields different combinations of TNM, and therefore of survival, compared to treatment with oesophagectomy alone. For this reason, a separate staging was also developed for oesophageal cancer after neoadjuvant therapy.
The 8th edition refers to individual cancer characteristics as categories. OC has anatomical categories (primary tumour [T], regional lymph nodes [N] and distant metastases [M]) and non-anatomical categories (histopathological cell type, histological grade [G] and location [L]). A cancer stage is considered to be the grouping of the categories of a cancer which reflects the prognosis. These stages may be determined at several points in the life and medical care history of the cancer patient, and are designated as cancer classifications. In this latest TNM edition, the main new elements are separate, temporally related classifications for cancers of both the oesophagus and OGJ: a clinical stage prior to treatment (cTNM); a pathological stage following oesophagectomy (pTNM); and a post-neoadjuvant pathological stage (ypTNM).2,4
The cTNM stage is based on physical examination and imaging, and rarely on microscopic examination of biopsy samples. It is limited by the resolution and accuracy of each imaging technique. For this reason, the composition of the clinical stage grouping and the survival profiles differ from those of the pathological stage grouping. Current recommendations for clinical stage include supplemental use of endoscopic ultrasound (EUS) with or without fine needle aspiration (FNA) biopsy, multidetector computed tomography (MDCT), positron emission tomography/computed tomography (PET/CT) and magnetic resonance imaging (MRI).2
The ypTNM stage is based on preoperative treatment (chemotherapy±radiation therapy) followed by oesophagectomy. The post-neoadjuvant pathological categories are determined by pathology assessment of oesophagectomy samples.2
The pTNM stage is based on histological examination after oesophagectomy alone. With the increasing use of neoadjuvant therapy for most advanced cancers, this staging is likely to be most relevant for early-stage cancers.2
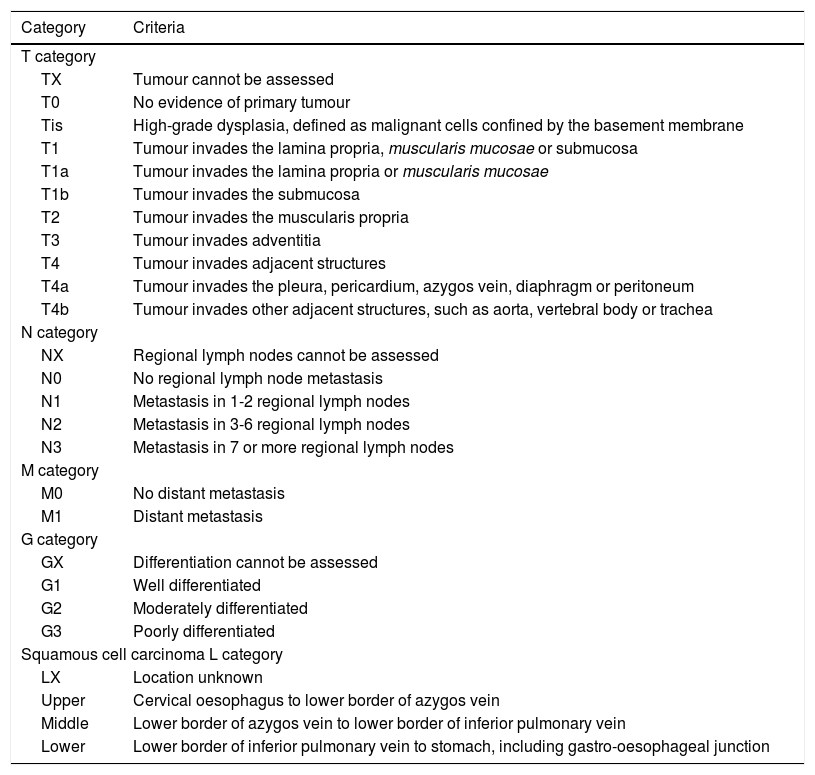
The 8th edition of the TNM for oesophageal SCC and ADC remains unchanged in the T, N and M categories and subcategories, except for the addition of peritoneal invasion to the T4a criteria (Table 2). This edition changes the definition of tumour location for all cancers; in the previous edition it was the upper border, and in the present edition it is the epicentre (geometric centre of tumour tissue) of the lesion. Location has a prognostic value in SCC for stages IIA-IIB.2,4 There is also a change in the definition of OGJ cancers which replaces the Siewert-Stein classification. Cancers affecting the OGJ whose epicentre is less than 2cm into the cardia (Siewert type I/II) are classified as oesophageal. Those affecting the OGJ whose epicentre is more than 2cm into the cardia (Siewert type III) and those not affecting the OGJ whose epicentre is less than or equal to 2cm into the cardia are classified as stomach cancers.2,4
8th edition of the TNM of the American Joint Committee on Cancer for squamous-cell carcinoma and adenocarcinoma of the oesophagus.
| Category | Criteria |
|---|---|
| T category | |
| TX | Tumour cannot be assessed |
| T0 | No evidence of primary tumour |
| Tis | High-grade dysplasia, defined as malignant cells confined by the basement membrane |
| T1 | Tumour invades the lamina propria, muscularis mucosae or submucosa |
| T1a | Tumour invades the lamina propria or muscularis mucosae |
| T1b | Tumour invades the submucosa |
| T2 | Tumour invades the muscularis propria |
| T3 | Tumour invades adventitia |
| T4 | Tumour invades adjacent structures |
| T4a | Tumour invades the pleura, pericardium, azygos vein, diaphragm or peritoneum |
| T4b | Tumour invades other adjacent structures, such as aorta, vertebral body or trachea |
| N category | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in 1-2 regional lymph nodes |
| N2 | Metastasis in 3-6 regional lymph nodes |
| N3 | Metastasis in 7 or more regional lymph nodes |
| M category | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| G category | |
| GX | Differentiation cannot be assessed |
| G1 | Well differentiated |
| G2 | Moderately differentiated |
| G3 | Poorly differentiated |
| Squamous cell carcinoma L category | |
| LX | Location unknown |
| Upper | Cervical oesophagus to lower border of azygos vein |
| Middle | Lower border of azygos vein to lower border of inferior pulmonary vein |
| Lower | Lower border of inferior pulmonary vein to stomach, including gastro-oesophageal junction |
Radiology plays a fundamental role in the clinical stage, as it is mainly based on imaging. Given the disparity in results when comparing cTNM and pTNM, the need for more accurate clinical staging methods is clear. Below is a summary of the current role of the main imaging techniques used in the diagnosis of OC.
Endoscopic ultrasoundThis is the preferred imaging test for locoregional OC staging due to its reliability and accessibility. The overall accuracy of EUS for OC staging ranges from 73% to 93%, depending on the stage.8 It is considered to be the most accurate technique in assessing cT.1
Superficial OC is defined as malignancy limited to the mucosa or submucosa — in other words, an invasive depth between Tis and T1b.8 Advanced disease is considered any tumour extending beyond the submucosa (≥T2). The overall accuracy of EUS for assessment of category T is 79%9 and increases to 100% for T3 tumours.10 EUS is ineffective in stenosing tumours, which account for 20%–36% of cases of OC.8
EUS is the most accurate method available for determining locoregional lymph node involvement. Ultrasound criteria suggestive of malignancy include a hypoechoic ultrasound pattern, well-defined borders, rounded contours and a diameter greater than 1cm. When these four characteristics are present, the diagnosis of lymph node malignancy in histopathology can be predicted with an accuracy of 80%–100%.8 However, only 25% of pathological lymph nodes show all four characteristics. EUS can detect pathological cervical, perioesophageal and perigastric lymph nodes.8
Another limitation of EUS is staging after neoadjuvant therapy. A recent meta-analysis11 found low sensitivity and specificity for T and N staging in patients having EUS after they had undergone chemotherapy. This is due to the local inflammation and fibrosis caused by the treatment.11 It is important to stress, however, that EUS is operator-dependent.
Computed tomographyMDCT is the main imaging test in clinical staging. It allows non-invasive study of the primary tumour, as well as lymph node metastases and visceral metastases. It is also the most widely used technique in assessing response to neoadjuvant therapy. A CT scan for OC clinical staging usually involves a study of the thorax and abdomen following administration of oral and intravenous contrast. A first helical scan of the thorax is usually performed 30s after the contrast is injected, extending from the lower cervical region to the costophrenic angles, followed by a helical scan of the abdomen and pelvis 70s after said contrast injection, from the dome of the diaphragm to the ischial tuberosities.1
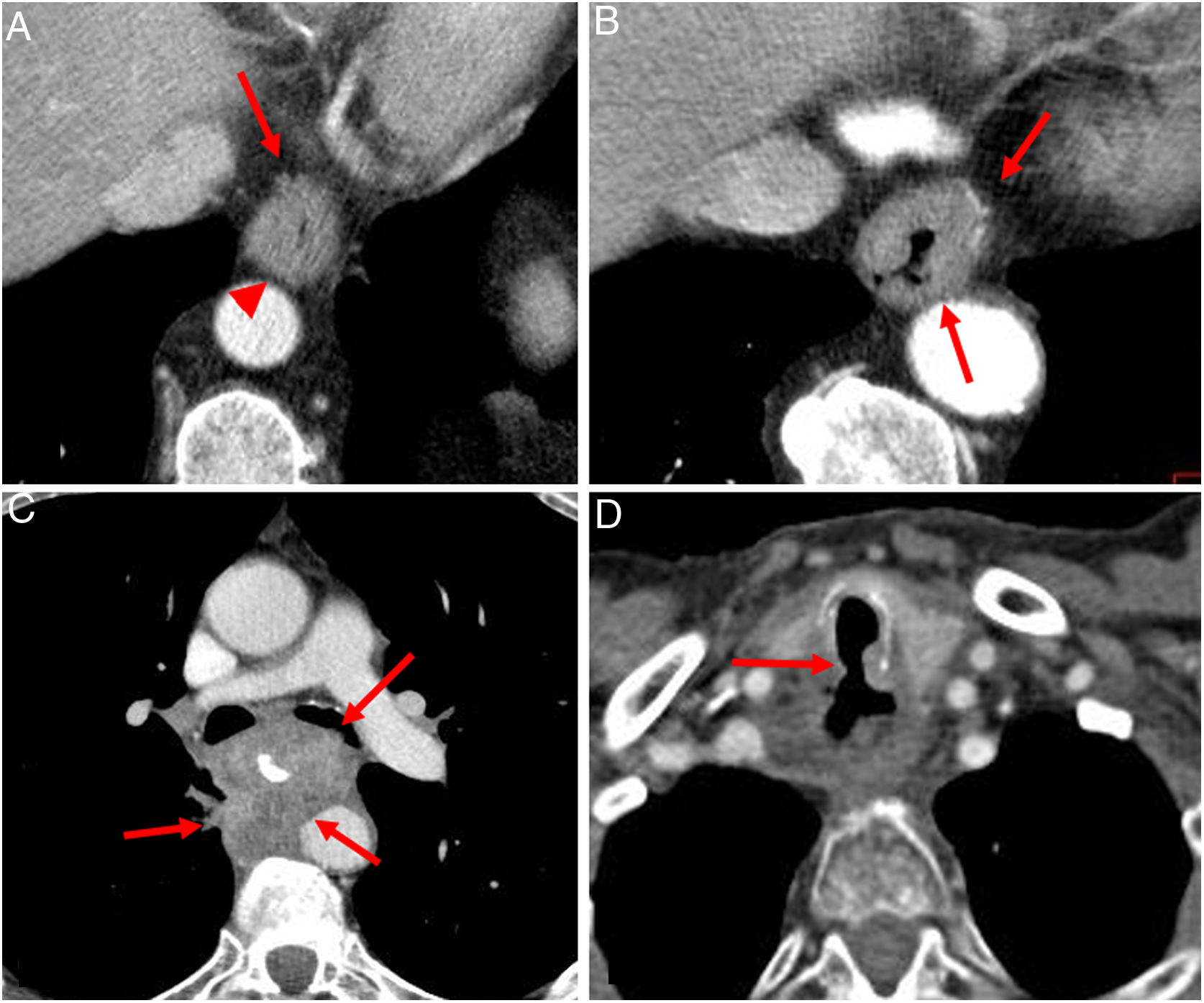
Radiological findings suggestive of an oesophageal tumour are an irregular thickening of the oesophageal wall greater than 5mm, eccentric light stenosis and proximal dilation of the oesophagus12 (Fig. 4). The role of CT in determining cT is limited. Most comparative studies have shown that accuracy for category T assessment is lower with CT than with EUS.12 CT does not adequately distinguish between cT1, cT2 and cT3.12 However, it is important in cT3 and cT4 assessment. Signs of T3 include extramural extension without infiltration of neighbouring structures and preservation of fatty planes. T3 is considered tumour tissue indented in mediastinal fat. Signs of T4 are loss of fatty planes between the tumour and mediastinal structures and displacement or deformity of other structures. The most important role of MDCT in the assessment of category T lies in ruling out cT4 involvement4,12 (Table 3 and Fig. 5). The sensitivity and specificity of CT in detecting mediastinal invasion in oesophageal cancer are 88 %–100 % and 85 %–100 %, respectively.12
Oesophageal carcinoma centred in the middle thoracic oesophagus with the characteristic radiological findings seen on multidetector computed tomography: irregular thickening of the oesophageal wall greater than 5mm (arrow in coronal reconstruction), stenosis of the lumen (arrow head in sagittal reconstruction) and proximal dilation (arrow in sagittal reconstruction).
Multidetector computed tomography findings suggestive of cT4 local invasion.
| Aorta | Contact ≥90 |
| Obliteration of the fatty triangle between oesophagus, aorta and vertebra | |
| Trachea and bronchi | Displacement or deformity of the posterior wall |
| Tracheoesophageal fistula | |
| Growth of the tumour in the lumen | |
| Pericardium | Pericardial thickening |
| Pericardial effusion | |
| Cardiac deformity with loss of pericardial fatty plane | |
| Peritoneum | Ascites |
| Nodules or plaques of soft tissue on peritoneal surface | |
| Striation of intra-abdominal fat | |
| Peritoneal thickening and/or enhancement |
Multidetector computed tomography assessing depth of tumour invasion in oesophageal cancer. A) Asymmetric thickening+irregularity of perioesophageal fat (arrow) (cT3 due to invasion of the adventitia). Fatty plane of separation with the aorta (arrow head). B) Thickening+irregularity of the fat+contact with aorta less than 90° (arrows): cT3. C) cT4b OC: infiltration of the aorta, left bronchus and adjacent lung (arrows). D) Squamous cell carcinoma with tracheal fistula (arrow) (cT4b).
Although CT can be used for assessing locoregional nodal extension, it provides suboptimal detection due to its low accuracy (46 %–58 %).12 The criteria for lymph node invasion are a short axis greater than 10mm for thoracic and abdominal lymph nodes or 5mm for supraclavicular lymph nodes, morphological abnormalities (rounded, without fatty hilum, with irregular contours) and changes in density (hyperuptake and necrosis)4,12 (Fig. 6).
MDCT is very useful for detecting distant metastases. It is the most widely used imaging test to rule out M1, with a sensitivity of 90% for the detection of liver metastases larger than 1cm.12 Some 20 %–30 % of cases of OC feature metastasis at diagnosis and are inoperable.12 The main locations are the liver, lungs, bone, adrenal glands, peritoneum and brain.4
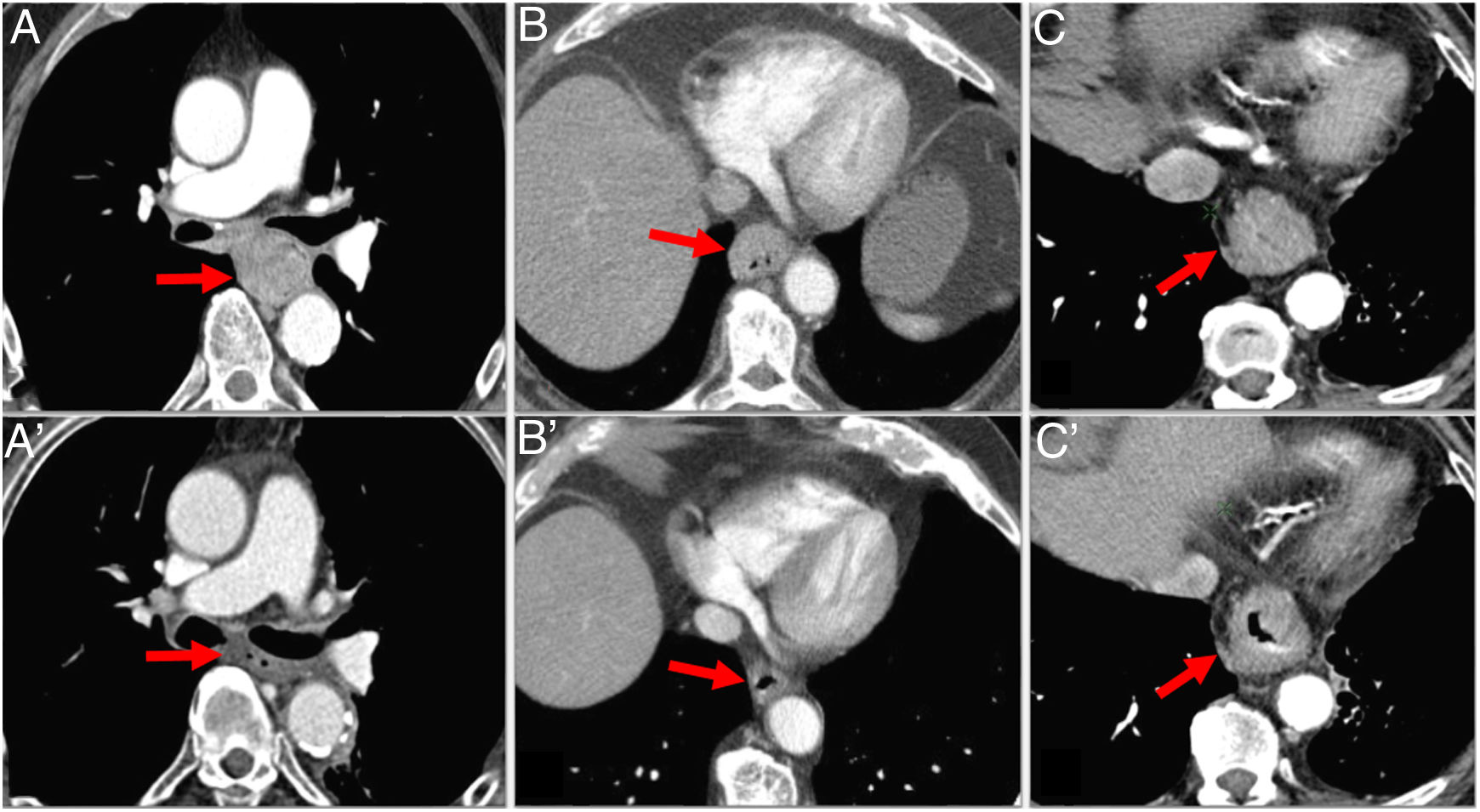
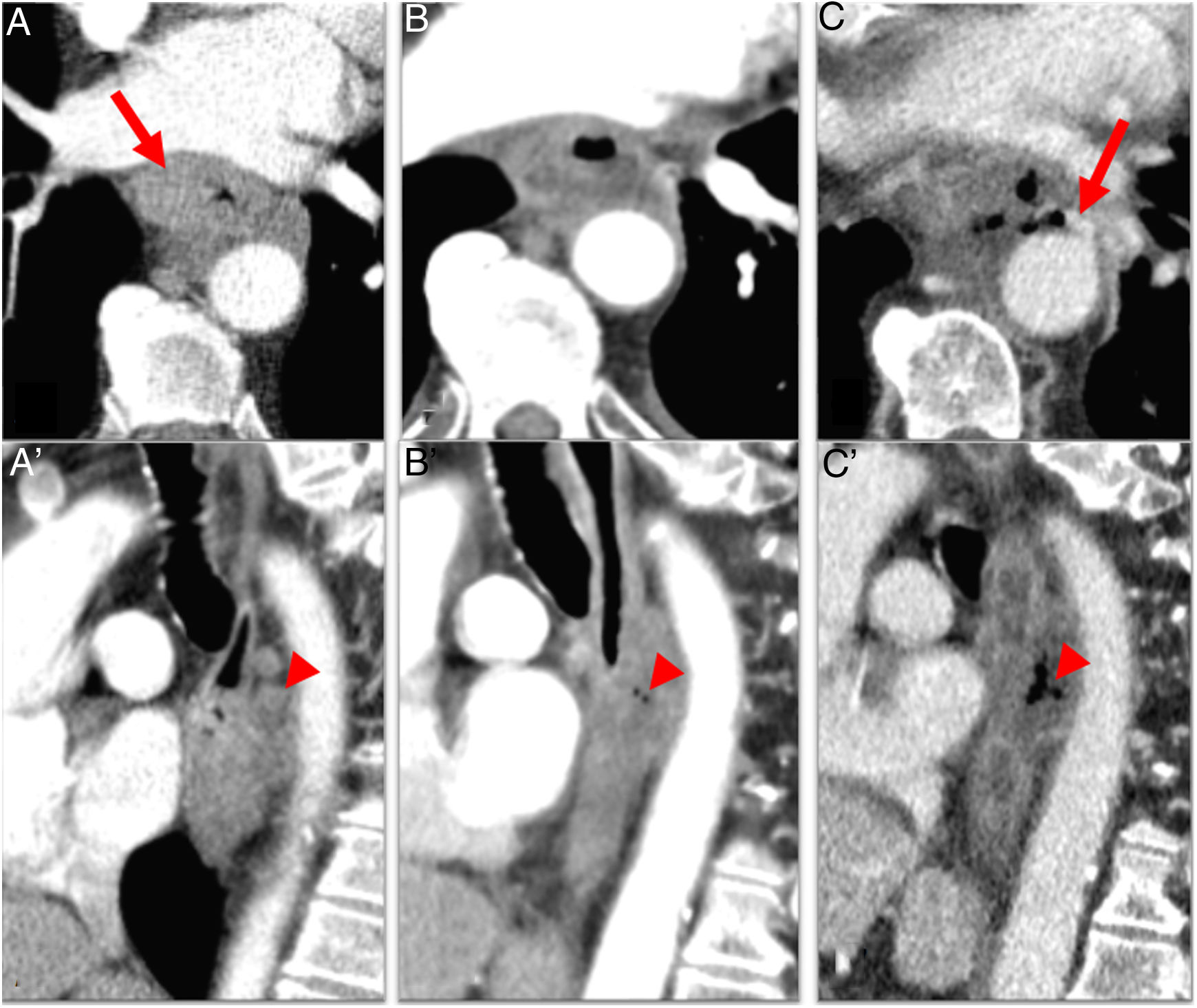
MDCT is the most commonly used test to assess post-neoadjuvant response.1 The limitations of CT are due to post-treatment changes, such as an increase in density of the fatty planes and the lack of difference between fibrosis/inflammation and viable tumour.12 This makes overestimation the most common error. Response to neoadjuvant therapy may be complete, partial or absent (stable disease) (Fig. 7). It is important to realise that not all changes are about size, and that the post-treatment changes mentioned above should be reflected in the radiology report.
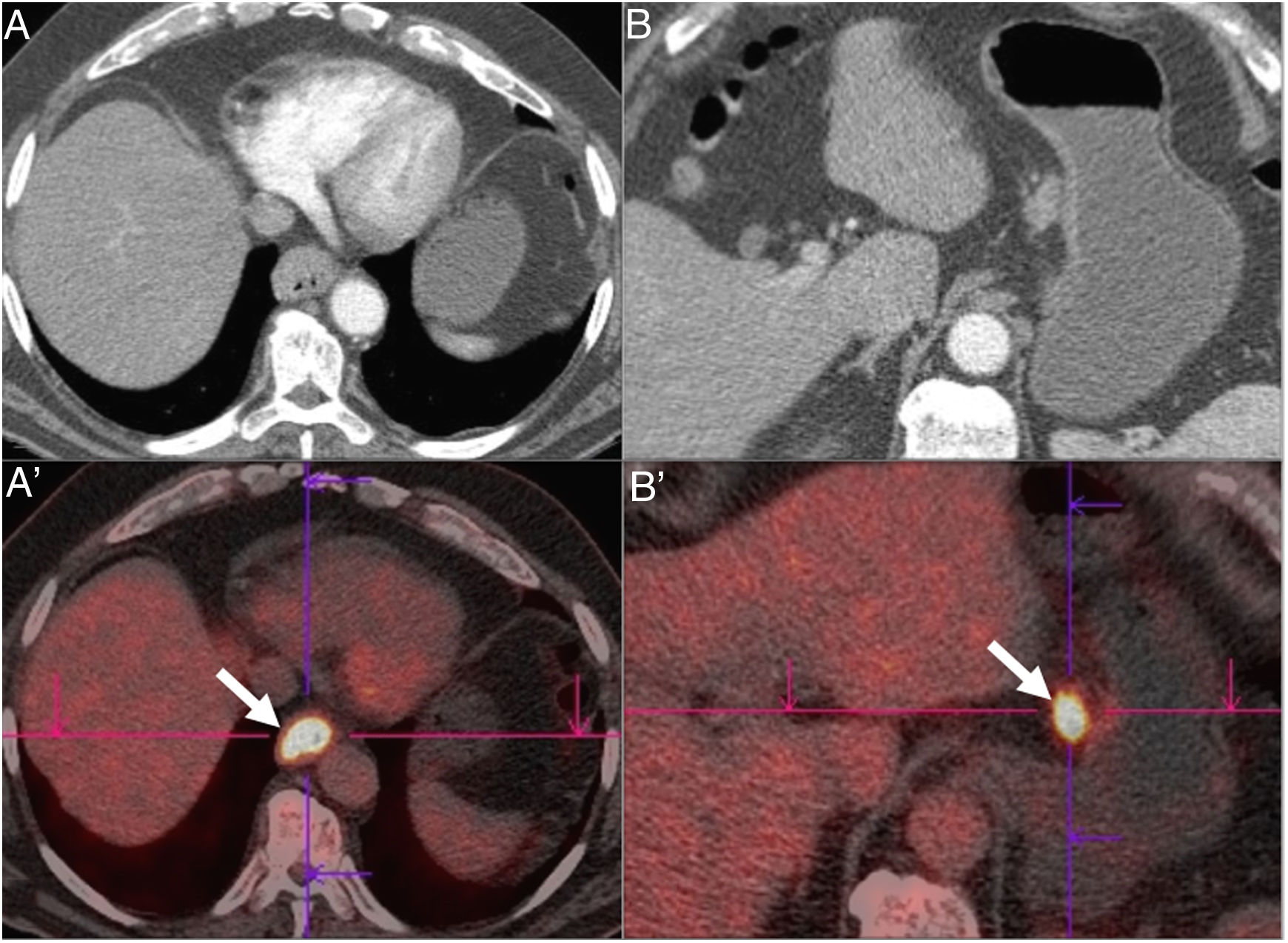
Assessment of response with multidetector computed tomography after neoadjuvant therapy in 3 different cases of oesophageal cancer (OC). A) Middle thoracic OC (arrow) with complete radiological response after neoadjuvant therapy (arrow in A'). B) OC (arrow) showing partial shrinkage after neoadjuvant therapy consistent with partial response (arrow in B'). C) Lower thoracic OC (arrow) showing no significant changes after neoadjuvant therapy (C arrow) consistent with stable disease.
18F-fluorodeoxyglucose PET/CT (18F-FDG PET/CT) is useful in identifying metastases not visible on CT (approximately 15% of patients), synchronous tumours and tumour recurrence1 (Fig. 8). However, it should be noted that it has some limitations in terms of spatial resolution for the assessment of T and N.4 For example, intense tumour uptake may obscure lymphadenopathy adjacent to the lesion.
Distal oesophageal squamous cell carcinoma with clinical staging cT3N1M0 by multidetector computed tomography (A and B). The same findings are confirmed in 18F-FDG PET (A' and B'): increased uptake of 18F-FDG in the primary oesophageal tumour and in the lymph node adjacent to the lesser curvature of the stomach (arrows). gr8.
This technique is also useful in post-neoadjuvant staging. The quantitative decrease in FDG uptake after neoadjuvant therapy appears to correspond to the pathological response to the therapy.12 However, false positives due to inflammatory changes with treatment are common, as are false negatives due to partial or poor response.12–14 Nevertheless, FDG-PET plays an important role in the detection of interval metastasis in 8%–17% of patients.12
Magnetic resonance imagingTechnological advances leading to new protocols with faster sequences and cardiac and respiratory synchronisation have brought about a significant improvement in MRI quality. Tissue contrast resolution is even higher in MRI than in CT and PET/CT.13–15 STIR and DWI sequences improve the detection of regional lymph nodes (cN) and distinguish them from tumour invasion of the wall and peri-oesophageal tissue.16,17 MRI has also been shown to be useful in detecting liver metastases with liver-specific contrast agent and DWI, with a sensitivity of around 90%.18,19 Recent studies have reported that MRI is useful in assessing response to neoadjuvant therapy when CT and PET/CT are contradictory,20,21 and in planning radiotherapy.22
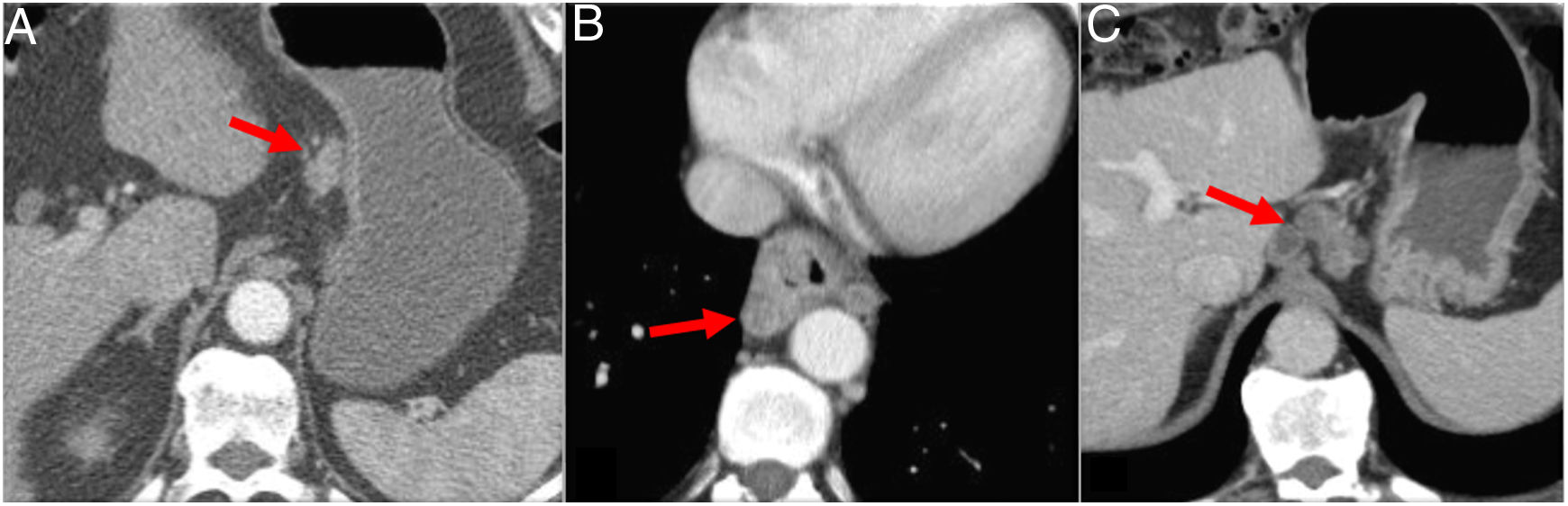
Complications of oesophageal cancerComplications of the primary tumourOC can lead to a number of potentially fatal complications. One of the most serious complications is perforation of the oesophagus. Although more commonly caused by endoscopic instruments or surgical thoracic procedures, progression of the primary tumour can also be a cause.23 The lack of a serosal layer can make the oesophagus more susceptible to injury than other parts of the gastrointestinal tract.24 Chest X-ray can help diagnose oesophageal perforation with indirect signs, such as pneumomediastinum, left pneumothorax and pleural effusion. In oral contrast oesophagram, contrast extravasation to the mediastinum is an unequivocal sign of perforation, although 10% of patients can have a false negative result.23 CT can be used as a complementary imaging method, but these days, thanks to its availability and speed, it may be the preferred technique in many cases.
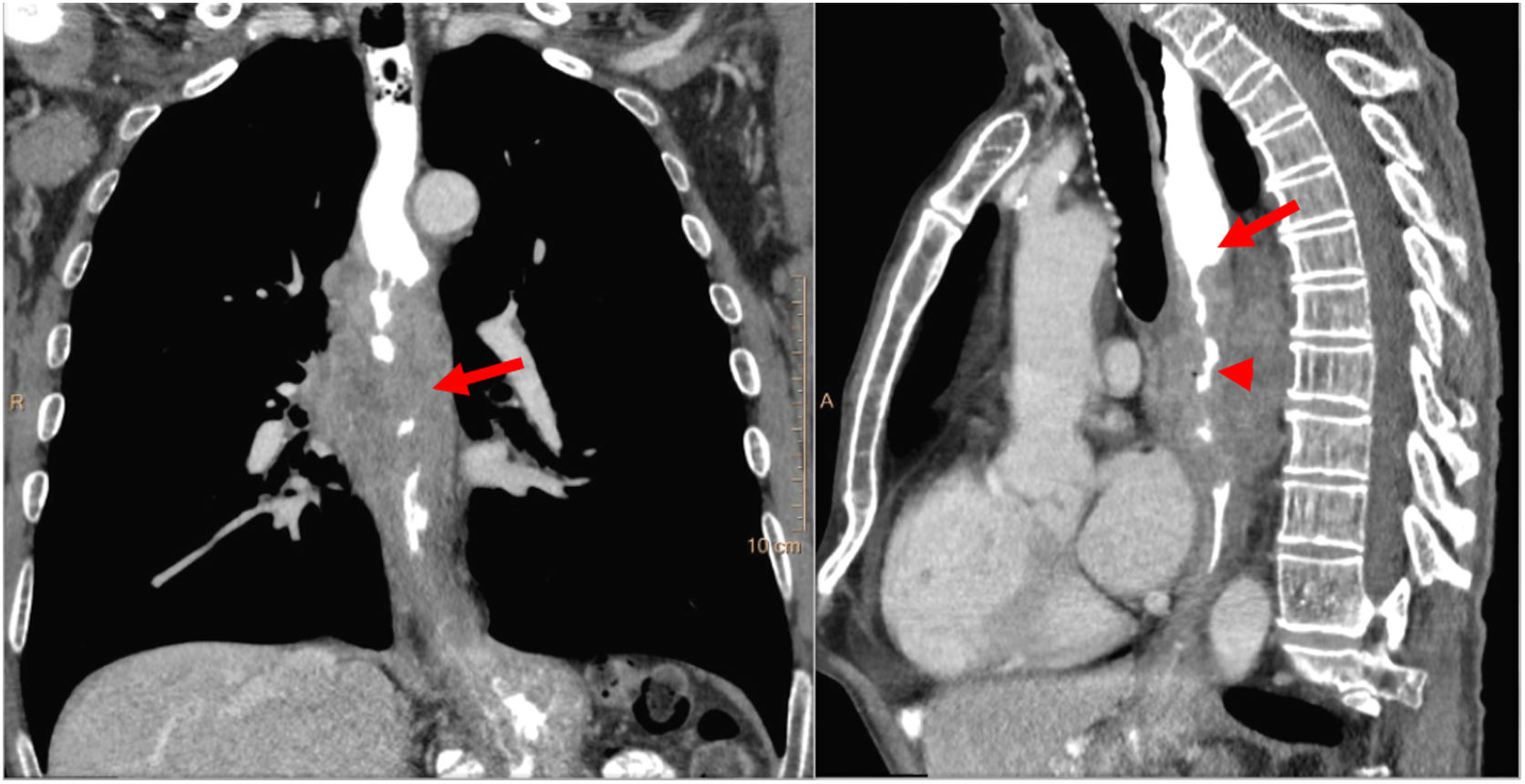
The formation of fistulas between the oesophagus and the trachea, bronchus or lung occurs in 5 %–10 % of cases of advanced OC. The risk increases in the case of previous radiotherapy. A fistula should be suspected in any patient with recurrent pneumonia. A diagnosis can be made by an oesophagram, and CT will show the fistulous tract23 (Fig. 5d). Oesophageal-pleural fistula is commonly associated with advanced OC. A chest X-ray may show air in the pleura or hydropneumothorax. CT is the test of choice for its diagnosis. Aorto-oesophageal fistula has also been described as an uncommon complication of tumour progression25 (Fig. 9).
Complication of advanced oesophageal cancer. A and A') Computed tomography (CT) images showing cT3N1M1a squamous cell carcinoma of the middle thoracic oesophagus; primary tumour (arrow) and mediastinal lymphadenopathy (arrow head). B and B') Post-neoadjuvant therapy CT: partial tumour response and fistulisation between tumour and lymphadenopathy (arrow head). C and C') CT 10 days later with aortoesophageal fistula: perforation of the oesophagus with extraluminal gas bubbles (arrow head) and active bleeding (arrow).
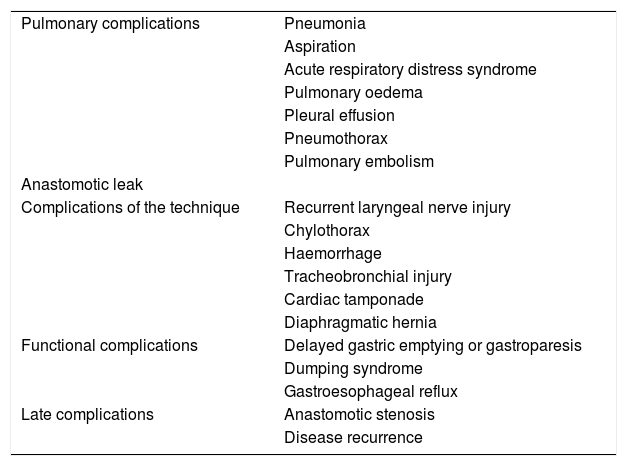
Oesophagectomy is currently the main treatment for local or locally advanced disease. It is the type of elective gastrointestinal surgery with the highest mortality rate (8%–23%).26 There are multiple surgical techniques for oesophagectomy. The database of the Society of Thoracic Surgeons for general thoracic surgery lists 14 different methods.26 The most common involve transthoracic oesophagectomy, including the Ivor Lewis and McKeown techniques, and a left thoracoabdominal approach; transhiatal oesophagectomy; and oesophagectomy with intestinal interposition (with the colon or small intestine). Regardless of the technique used, oesophagectomy can lead to multiple complications (Table 4).
Post-oesophagectomy complications.
| Pulmonary complications | Pneumonia |
| Aspiration | |
| Acute respiratory distress syndrome | |
| Pulmonary oedema | |
| Pleural effusion | |
| Pneumothorax | |
| Pulmonary embolism | |
| Anastomotic leak | |
| Complications of the technique | Recurrent laryngeal nerve injury |
| Chylothorax | |
| Haemorrhage | |
| Tracheobronchial injury | |
| Cardiac tamponade | |
| Diaphragmatic hernia | |
| Functional complications | Delayed gastric emptying or gastroparesis |
| Dumping syndrome | |
| Gastroesophageal reflux | |
| Late complications | Anastomotic stenosis |
| Disease recurrence |
These are the most common complications, and are responsible for two-thirds of postoperative deaths.26 They increase when thoracotomy is performed.27 They include: pneumonia, aspiration, acute respiratory distress syndrome, pulmonary oedema, pleural effusion, pneumothorax and pulmonary embolism.
Anastomotic leakThis occurs in 10%–44% of patients after surgery, and is responsible for 40% of postoperative deaths.26,27 This normally occurs within the first 10 days. It is attributed to inadequate blood pressure,28 whether too low or too high. Insufficient blood pressure in the anastomosis causes poor tissue apposition, ultimately leading to extravasation. High blood pressure can lead to ischaemia and necrosis, which is the most feared complication, and this can lead to leakage. Most leaks occur at the oesophagogastric anastomosis. They are more common when the anastomosis is cervical and occur more often with gastric than colonic conduits.28,29 Leaks are graded according to the Lerut classification. A grade 1 leak is a small, previously unsuspected leak seen on an X-ray in an asymptomatic patient. It does not require treatment. A grade 2 leak is a small, contained leak with minimal symptoms. It requires conservative treatment with drainage and antibiotic therapy. A grade 3 leak is a large leak with major symptoms. Grade 4 is reserved for conduit necrosis. The latter two grades require emergency surgery.27,30
Technical complicationsTechnical complications include recurrent laryngeal nerve injury, which occurs in 10%–20% of cases with cervical anastomosis, and chylothorax, which occurs in 1%–5% of oesophagectomies.31Haemorrhage usually occurs as a result of an intra-operative injury to the spleen, azygos vein, intercostal vessels, right gastric artery or lung parenchyma.31
Functional complicationsGastroparesis or delayed gastric emptying occurs in 10% of cases, usually in association with vagotomy and anatomical reorganisation. It tends to be more pronounced with the Ivor Lewis technique.3,27o0Dumping syndrome occurs in up to 50% of cases, due to a rapid emptying of hyperosmolar content from the stomach into the small intestine.29Gastroesophageal reflux is an expected complication in most cases; it is more common after pyloroplasty. It can cause ulcers and stenosis.32
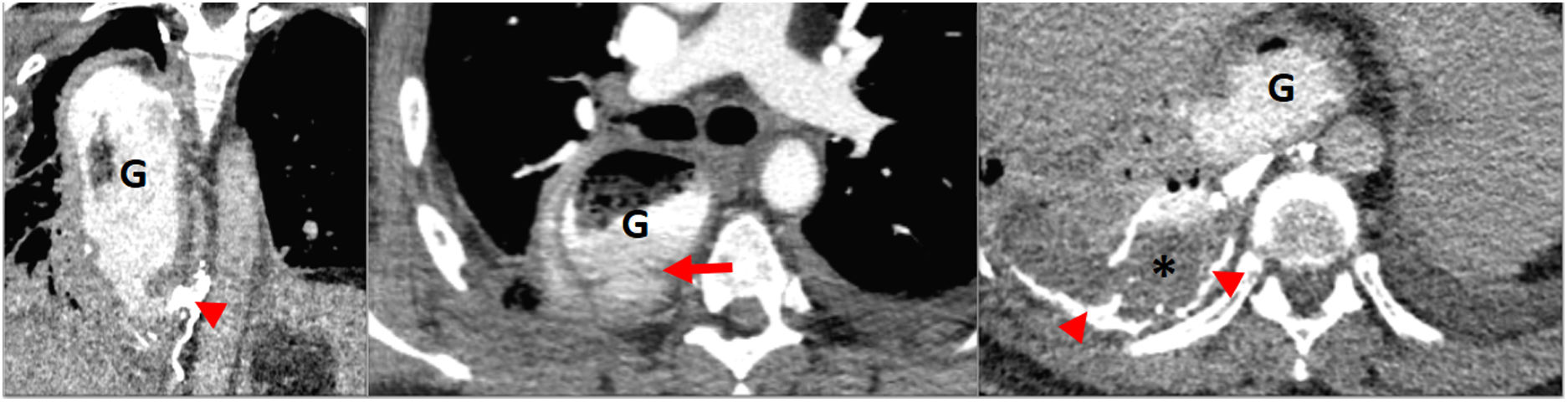
Early detection of complications is important for improving the prognosis of patients after oesophagectomy, and an essential part of assessing patients for most of these complications is radiological study. The most commonly used imaging tests are oesophagrams and CT scans of the chest. The oesophagram is used to assess leakage, obstruction and delayed gastric emptying. It is part of the routine postoperative assessment in most centres and is usually done six to ten days after surgery.26 It is important to perform the test with the patient in multiple positions if leakage is suspected. To rule out leakage, an initial oesophagram with a low-osmolar non-ionic water-soluble contrast administered by nasogastric tube or orally is recommended, as patients who have undergone surgery for oesophageal cancer are at higher risk of aspiration, and high-osmolar water-soluble contrasts have been associated with massive pulmonary oedema in the event of aspiration.26 If no leakage is observed, it is advisable to repeat the study with barium.27,33,34 The use of water-soluble contrast alone may not show a leak if it is small. Barium has a higher density than water-soluble contrast and can adhere more strongly to the leakage site.26 A chest CT scan is useful for visualising a number of the postoperative complications, especially in unstable patients, as a complement to an oesophagram. In the event of anastomotic leakage, CT will show extravasation of oral contrast in the mediastinum or pleural space27,33 (Fig. 10). Abscesses are easily identifiable in CT; they tend to develop adjacent to the suture lines and they predispose the patient to developing fistulas. CT can also be used to guide drainage procedures in leaks, abscesses, pleural effusions and pneumothorax.26
Postoperative complications of oesophageal cancer (OC). Patient who had undergone surgery for OC with oesophagectomy (Ivor Lewis technique) 7 days earlier. Emergency chest CT performed due dyspnoea: break in continuity observed caudal to the cervical oesophagogastric anastomosis, consistent with leakage from the gastric conduit (G) (arrow). The extravasation continues behind the gastric conduit like a column with a density similar to endoluminal density (determined using oral gastrografin), mixing with the contents of the patient's pleural effusion and right chylothorax component (star). Dense matter consistent with Lipiodol from previous embolisation (arrow heads) after sectioning of the thoracic duct during surgery.
The classifications in the 8th edition of the TNM for oesophageal cancer are no longer shared. There are separate classifications for clinical (cTNM), pathological (pTNM) and post-neoadjuvant pathological (ypTNM) stage groupings. Radiologists must be familiar with both the particular characteristics and the current classification of oesophageal cancer. Proper reading of radiological images is essential for planning of patient management. The role of the radiologist is fundamental not only in the clinical staging of the cancer, but also in post-treatment follow-up.
Authorship- 1
Responsible for study integrity: PLS, NAA, IFF and JSB.
- 2
Study conception: PLS, NAA IFF and JSB.
- 3
Study design: PLS, NAA, IFF and JSB.
- 4
Data acquisition: PLS, NAA, IFF and JSB.
- 5
Data analysis and interpretation: PLS, NAA, IFF and JSB.
- 6
Statistical processing: not applicable.
- •
Literature search: PLS and IFF.
- •
Drafting of the article: PLS, NAA, IFF and JSB.
- •
Critical review of the manuscript with intellectually significant contributions: PLS, NAA, IFF and JSB.
- •
Approval of the final version: PLS, NAA, IFF and JSB.
Please cite this article as: López Sala P, Alberdi Aldasoro N, Fuertes Fernández I, Sáenz Bañuelos J. Cáncer de esófago: revisión actualizada del TNM y de sus complicaciones. Radiología. 2020. https://doi.org/10.1016/j.rx.2020.09.003