To review the main findings for anisakiasis in the different imaging tests that can be used to diagnose it, based on studies done at our center.
ConclusionThe presence of Anisakis species in food consumed in Western countries is becoming more common. Patients with anisakiasis present with acute abdomen; there are no specific clinical signs or laboratory findings. Careful history taking is key to discovering exposure to Anisakis-contaminated food, but this task is hindered by unfamiliarity with the condition and lack of suspicion and is also confounded by the variable latency period after ingestion of Anisakis-contaminated food. Give the nonspecific presentation, patients with anisakiasis often undergo imaging tests to rule out other processes. Thus, radiologists need to be familiar with the spectrum of imaging findings that should lead to the inclusion of anisakiasis in the differential diagnosis, so they can guide clinicians toward directed history taking and specific tests.
Revisar los principales hallazgos radiológicos de la anisakiasis en las diferentes pruebas de imagen que se pueden utilizar en su diagnóstico, basándonos en estudios realizados en nuestro centro.
ConclusiónLa contaminación alimentaria por Anisakis muestra una tendencia creciente en países occidentales. Habitualmente se presenta como un cuadro de patología abdominal aguda, sin datos clínicos o analíticos característicos. Una anamnesis cuidadosa es clave para descubrir el antecedente de exposición, pero se ve dificultada al ser un proceso poco conocido o sospechado y con un periodo de latencia variable. Al tratarse de cuadros poco específicos, a estos pacientes con frecuencia se les realizan pruebas radiológicas para descartar otros procesos. Por ello, es recomendable familiarizarse con los posibles hallazgos en imagen que permitan incluir la anisakiasis en el diagnóstico diferencial, lo que podría guiar al clínico hacia una anamnesis dirigida y pruebas específicas.
Anisakiasis is a parasitic disease of the gastrointestinal tract that is usually associated with ingestion of raw or undercooked fish or seafood that contain live larvae.1 It was first reported in the Netherlands in 1960 by van Thiel et al.2 Each year an average of 20,000 cases are diagnosed, with a high incidence in Asian countries (approximately 90% in Japan), possibly associated with eating habits; however, there is a rising trend in Europe and the United States.3–5 Considering its non-specific symptoms and normally limited suspicion, its actual incidence is probably higher.1
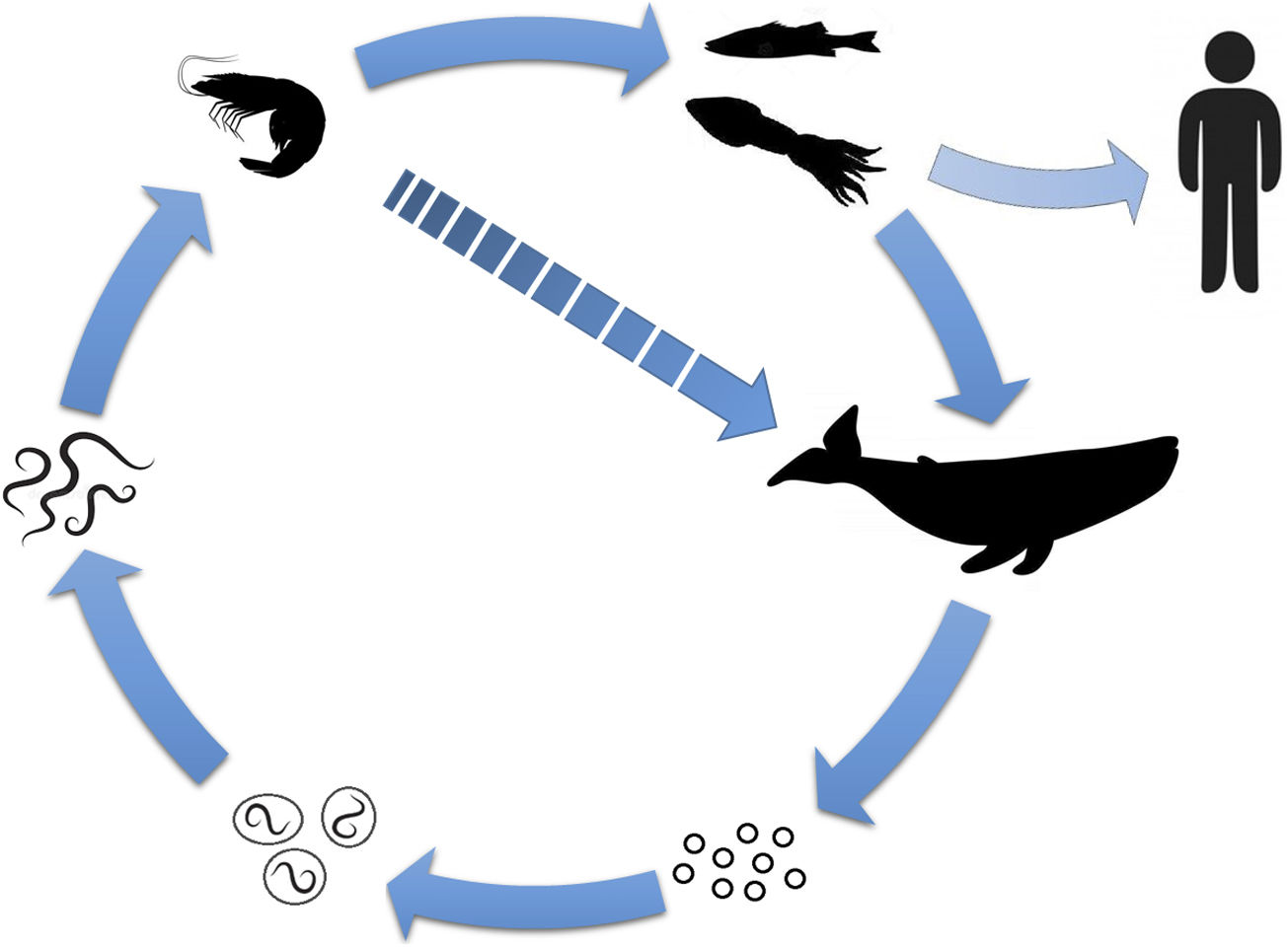
The cause is ingestion of larvae from nematodes belonging to the family Anisakidae (typically Anisakis simplex, with lower rates of Pseudoterranova decipiens and Anisakis pegreffi).4,5 Some fish, cephalopods and small crustaceans are intermediate hosts for the parasite; human beings become accidental hosts by consuming contaminated species (Fig. 1).1,4 It has been associated with species such as hake, anchovies, bonitos, mackerel, salmon and squid.4,6
Diagram of the Anisakis life cycle. The adult parasite’s definitive hosts are cetaceans and marine mammals. When they live in the gastric mucosa of these organisms, they expel eggs along with faeces. The embryonic process and a first stage take place in the water, after which the eggs hatch, releasing the larvae. In this second stage, they are ingested by small crustaceans (first intermediate host), where they mature to a third stage. These are then consumed by fish or cephalopods (second intermediate host), in which they migrate to the viscera, peritoneal cavity and musculature. Their consumption by marine mammals (or occasionally by the first host) enables the larvae to proceed to a fourth stage and to maturity. In humans, consumption of the second intermediate host is what causes contamination by the parasite; in this way, humans become accidental hosts.
It is possible to ensure the parasite’s death by heating the specimen to a minimum internal temperature of 60°C for at least one minute, by freezing it at −20°C for one to seven days or by freezing it in an ultra-low temperature freezer at −35°C for more than 15h.4,7,8 Semi-preserves, such as preparations in vinegar or brine, do not ensure the parasite’s death except at high concentrations for at least six weeks, in the absence of prior freezing.9 Currently, regulations in Spain and its Autonomous Communities recommend thermal treatment prior to marketing and in certain cases prior to preparation as a semi-preserve, as well as recall of specimens with visible parasites.8
Signs and symptomsWhen they are ingested, the larvae adhere to the gastrointestinal mucosa. Symptoms usually appear four to 72h after consuming contaminated food, although different latency periods have been reported for gastric involvement (one to 24h) and intestinal involvement (hours to several days).4,5,7 Classically, there has been thought to be a strong predominance of gastric involvement (up to 96%), though subsequent studies have found that it could be weaker.1,5,7,10 On the one hand, larger numbers of intestinal cases have been reported in Europe, which could be due to differences in both epidemiology and diagnostic methods.10–12 On the other hand, the results of published radiology series have shown no such obvious gastric predominance, though this could be related to different needs for radiological tests and time elapsed since exposure depending on the affected segment.12–14 The most commonly affected areas on imaging tests are the stomach and the ileum (with a predominance in the distal ileum) in a variable range.13,15 Involvement of the colon is uncommon (0.1–0.9%), with 50% of cases located in the caecum and right colon.16,17
The clinical presentation may include nausea, vomiting, diarrhoea, fever and abdominal pain of variable location depending on the affected segment (e.g., severe epigastric pain in gastric cases or pain in the right iliac fossa in ileal cases).1,5,18 Symptoms tend to persist for several days.12,13 Due to the location of the pain, associated with non-specific signs and symptoms, acute abdomen (appendicitis, diverticulitis, biliary colic, ulcers, gastritis, etc.) is often suspected.12,14,18
In addition to location, infestation type can account for marked variations in symptoms:4
- 1
Luminal form. The larvae adhere to the surface of the mucosa, generally in a gastric location. It is usually asymptomatic, though it can cause gastritis.19
- 2
Invasive form. The larvae penetrate the gastrointestinal mucosa and submucosa. This leads to direct tissue damage with local inflammatory signs and symptoms associated with severe abdominal pain of sudden onset (diffuse or colicky, of variable location depending on the affected segment), nausea, vomiting and diarrhoea.13,19
- 3
Allergic reaction or gastroallergic anisakiasis. The infestation can trigger a severe IgE-mediated systemic allergic reaction in individuals who are already sensitised; it seems that for said sensitisation to occur, the parasite must have previously penetrated the gastrointestinal mucosa.1,20 As this is an immune response to an antigen (which is sometimes thermostable) present in the nematode, it is not necessary for the parasite to be viable; therefore, the allergic reaction may occur with ingestion of either raw fish or properly cooked or preserved fish.11 Its prevalence varies widely across the different series available.7 It usually presents as urticaria, angio-oedema or anaphylactic shock, though signs and symptoms may overlap with other forms of presentation of anisakiasis.1,11
- 4
Ectopic form. The literature features rare reports of extraintestinal involvement (pleuropulmonary, liver or lymph nodes) due to migration of larvae through the gastrointestinal wall, as well as cases of chronic anisakiasis leading to the formation of granuloma and adhesions in intraperitoneal organs, the mesentery and the peritoneal cavity.14,18,20–22 The latter occur as a result of chronic local inflammation, since the larva tends to die within a maximum of 14 days, with non-specific signs and symptoms of mild, intermittent abdominal pain.14,18 They sometimes constitute incidental findings in surgery for another reason or in tumour extension studies.14,18,20
Occasionally, atypical symptoms may appear, such as syncope, dyspnoea, dizziness, fever or abdominal distension — in any case, at lower rates. Rarely, upper gastrointestinal bleeding or signs of peritonitis may be seen.1
In general, signs and symptoms tend to be self-limiting, with spontaneous elimination of the parasite through the gastrointestinal tract after several days or through the immune response itself in the case of tissue infiltration.7 Complications secondary to anisakiasis are uncommon. The most common is obstruction, secondary to a decrease in intestinal lumen due to parietal oedema.19,22,23 Intestinal intussusception occasionally develops, with no known cause; it has been proposed to be related to oedema, as well as to abnormal peristalsis caused by intestinal wall irritation.16,17,24 Isolated cases of intestinal ischaemia (due to direct inflammation or vasoconstriction secondary to allergic reaction), perforation and abscesses have also been observed.19,23–25
Remember: the clinical presentation is non-specific, with signs and symptoms of acute abdomen and pain of variable location that depends on the affected segment. Symptoms of a severe allergic reaction may appear, either in isolation or associated with gastrointestinal signs and symptoms.
DiagnosisIt is necessary to take a thorough medical history when faced with a case featuring consistent symptoms, focused on assessing possible recent exposure to contaminated foods.5,13 It is not rare for a targeted medical history to be initiated by a radiologist upon observing suspicious findings. Abnormalities on laboratory tests are non-specific. It may be associated with elevated acute-phase reactants, usually with mild or no leukocytosis; in the presence of an allergic reaction, peripheral eosinophilia may be seen, but it is variable in all other cases.1,7,26 The presence of eosinophilia in ascitic fluid has also been reported.3
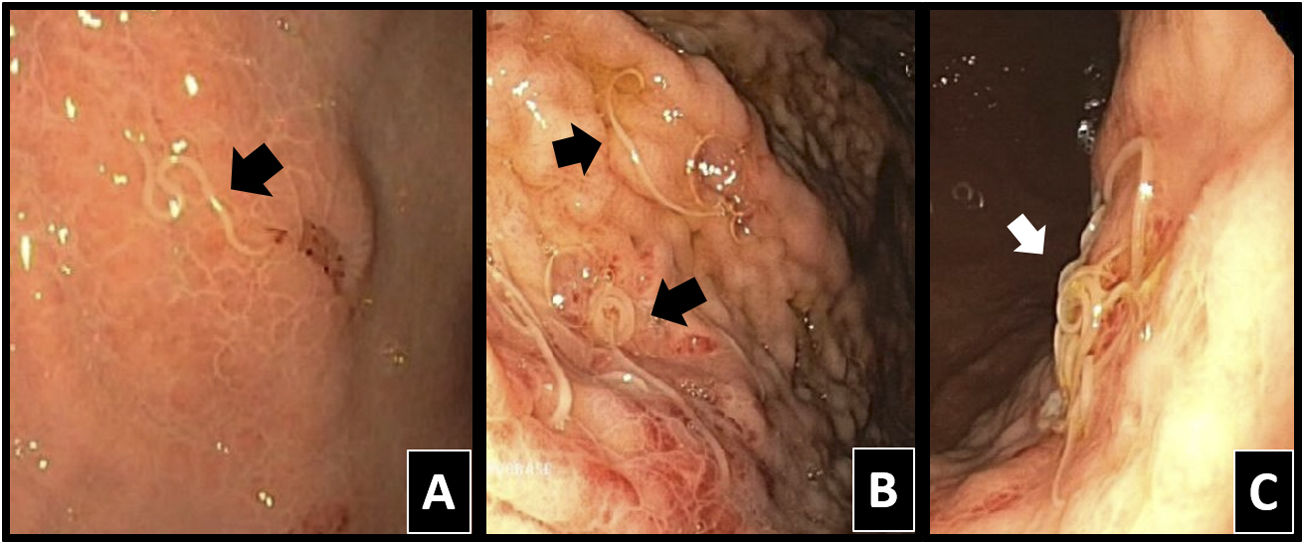
Classically, a definitive diagnosis would be made with direct observation of parasites in endoscopic tests or surgery (Fig. 2).1,4,6,7 Local erythema, mucosal/submucosal oedema and local phlegmonous changes may be seen, as may eosinophilia in tissue samples, all in relation to a severe local allergic reaction due to penetration by the parasite.4,7,13
It is possible to detect exposure by means of serology tests with determination of specific IgE antibodies, polymerase chain reaction tests to detect nematode DNA or skin tests with a specific antigen, though they are only performed in cases of high suspicion and specificity is not always optimal.4,7,13,26 It may be possible to detect specific antibodies in blood after the first day of infestation, persisting for six to 12 months; however, due to the time it takes to obtain their results, their usefulness in emergency cases is limited.5,27,28
Imaging findingsNon-specific symptoms in a clinical context of acute abdomen mean that it is common to resort to diagnostic imaging tests in these cases. Their number-one objective is therefore to rule out other possible diagnoses.14 However, consideration of anisakiasis based on imaging findings may be key to guiding the clinician in unsuspected cases, primarily in intestinal cases given the longer latency period following ingestion, rendering the link more difficult to identify.6,13,14,19
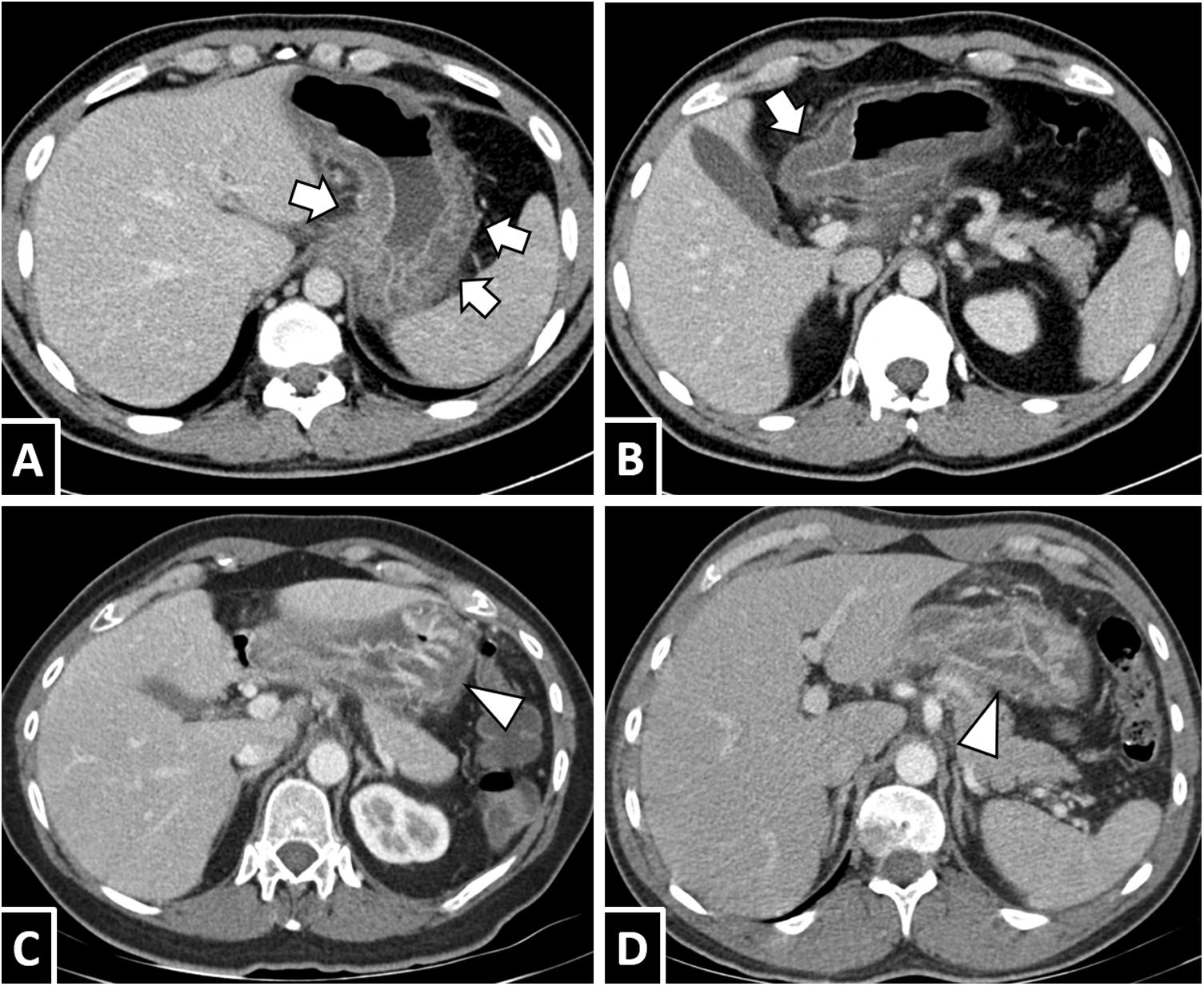
In computed tomography (CT), the most typical protocol would be a portal phase with intravenous contrast; administration of oral contrast is unlikely to yield useful data for diagnosis. A high prevalence has been reported of cases that show wall thickening and marked submucosal oedema of the affected segments, forming a “double halo” or “target” image; this can be striking in the stomach, with thickening associated with gastric folds (Figs. 3 and 4).5,6,14,19 Other common findings are oedema of adjacent mesenteric fat, ascites (from scant adjacent fluid to generalised ascites) and proximal dilation of loops with fluid contents (Figs. 4–8) (Figs. 5–8).14,15,28 The presence of lymphadenopathy varies from publication to publication; rarely, intestinal intussusception may appear (Fig. 9).14,16,24
Gastric anisakiasis. A and B) A 31-year-old man with suspected biliary disease. Axial slices from a contrast-enhanced CT scan show marked gastric wall thickening with submucosal oedema (white arrows) and slight blurring of adjacent fat. C) A 50-year-old man with severe abdominal pain. D) A 63-year-old man with epigastric pain and vomiting. Both cases showed gastric wall thickening with significant thickening of folds (white arrow tips) and submucosal oedema.
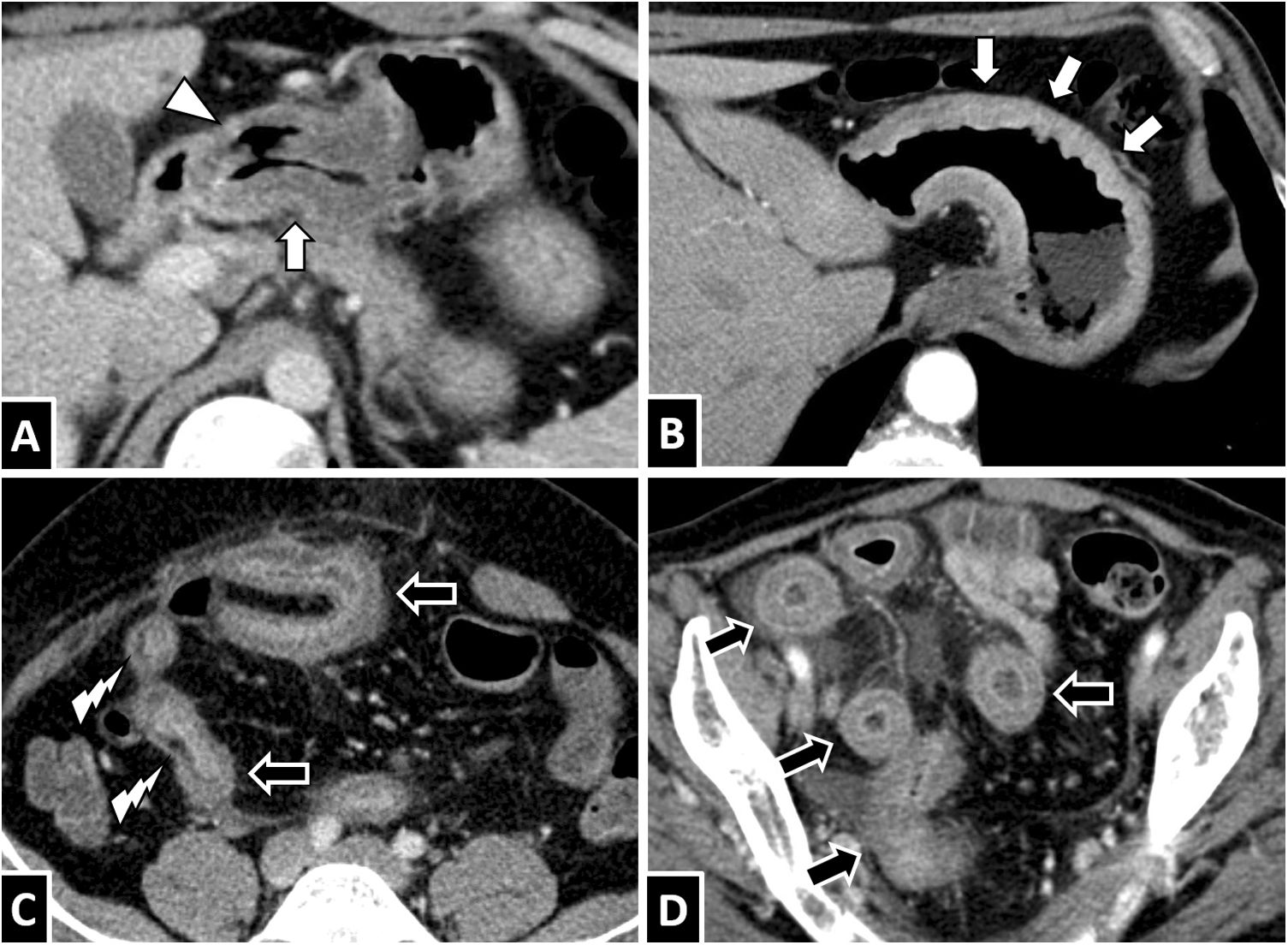
Anisakis in the small intestine. A and B) A 35-year-old woman with suspected diverticulitis. C and D) A 58-year-old woman with suspected appendicitis. E and F) A 68-year-old woman with suspected diverticulitis. An intravenous contrast-enhanced CT scan of the abdomen in all three cases showed circumferential wall thickening of a lengthy segment of ileum with submucosal oedema (white arrows). To different extents, ascites (B and C, white arrow tips), decreased lumen calibre in affected segments with proximal lumen dilation associated with fluid contents (B and D, black arrows) and oedema of adjacent mesenteric fat (E and F, white lightning bolts) are also seen. All cases resolved with conservative treatment.
Anisakiasis in the colon. A 37-year-old man with suspected appendicitis (A) and a 30-year-old man with severe abdominal pain (B). Both showed significant circumferential wall thickening in the right colon with submucosal oedema (white arrows) and adjacent free fluid (white arrow tip).
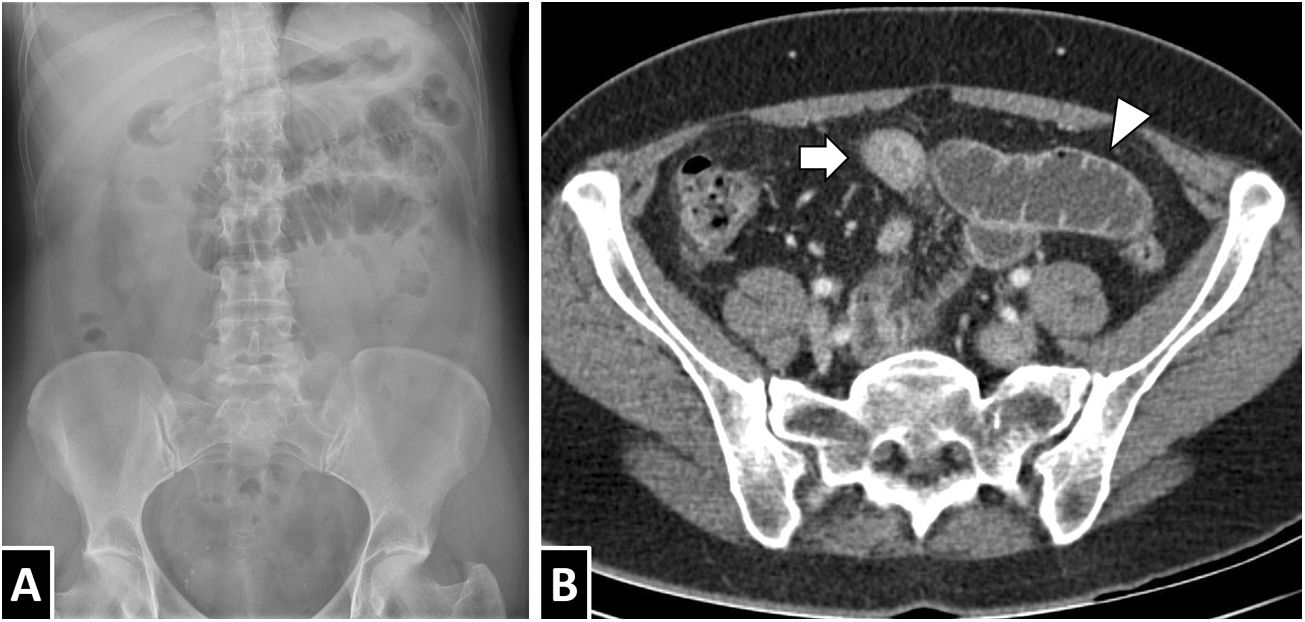
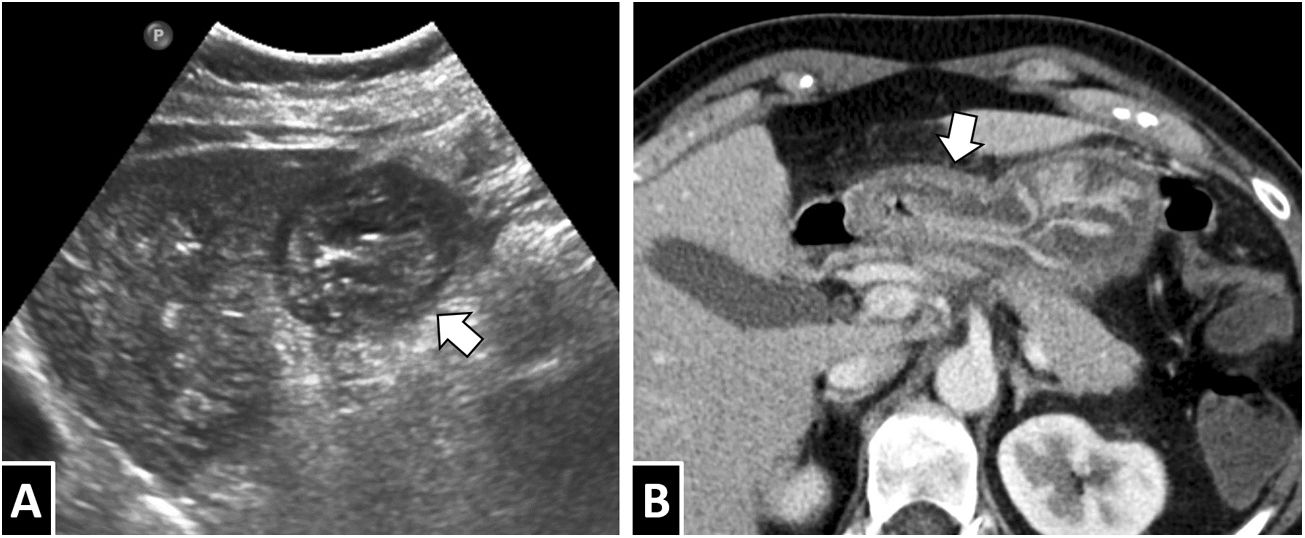
A 55-year-old woman with abdominal pain and a definitive diagnosis of intestinal anisakiasis. Conventional X-ray of the abdomen (A) showed focal dilation of loops in the left hypochondrium. A CT scan of the abdomen with intravenous contrast (B) showed circumferential thickening of a short segment of ileum with homogeneous wall hyperdensity with a minimal mucosal predominance (white arrow) and proximal intestinal dilation with fluid contents (white arrow tip). While submucosal oedema is a common finding in anisakiasis, as can be seen in this case, it is not a constant.
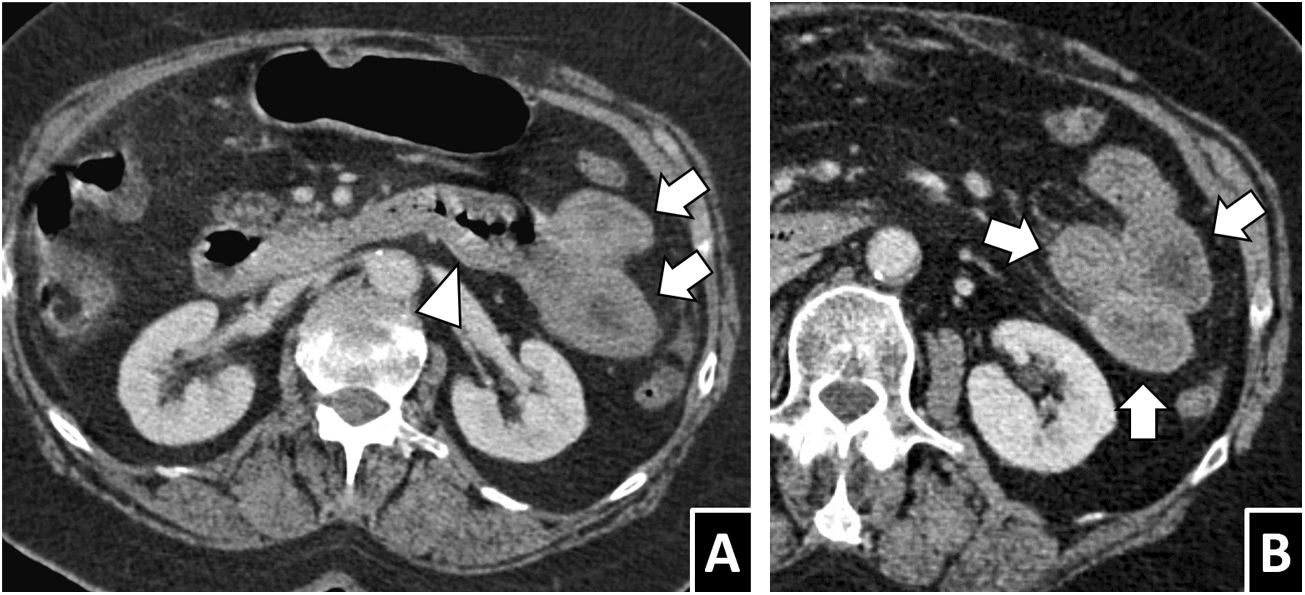
A 76-year-old woman with abdominal pain, with a definitive diagnosis of anisakiasis. An intravenous contrast-enhanced CT scan showed circumferential wall thickening in the fourth duodenal segment (A, arrow tip) and jejunum (arrows). A mixed pattern could be seen with a predominance of transmural hyperdensity in the most proximal area (A) and mild submucosal oedema in the most distal area (B).
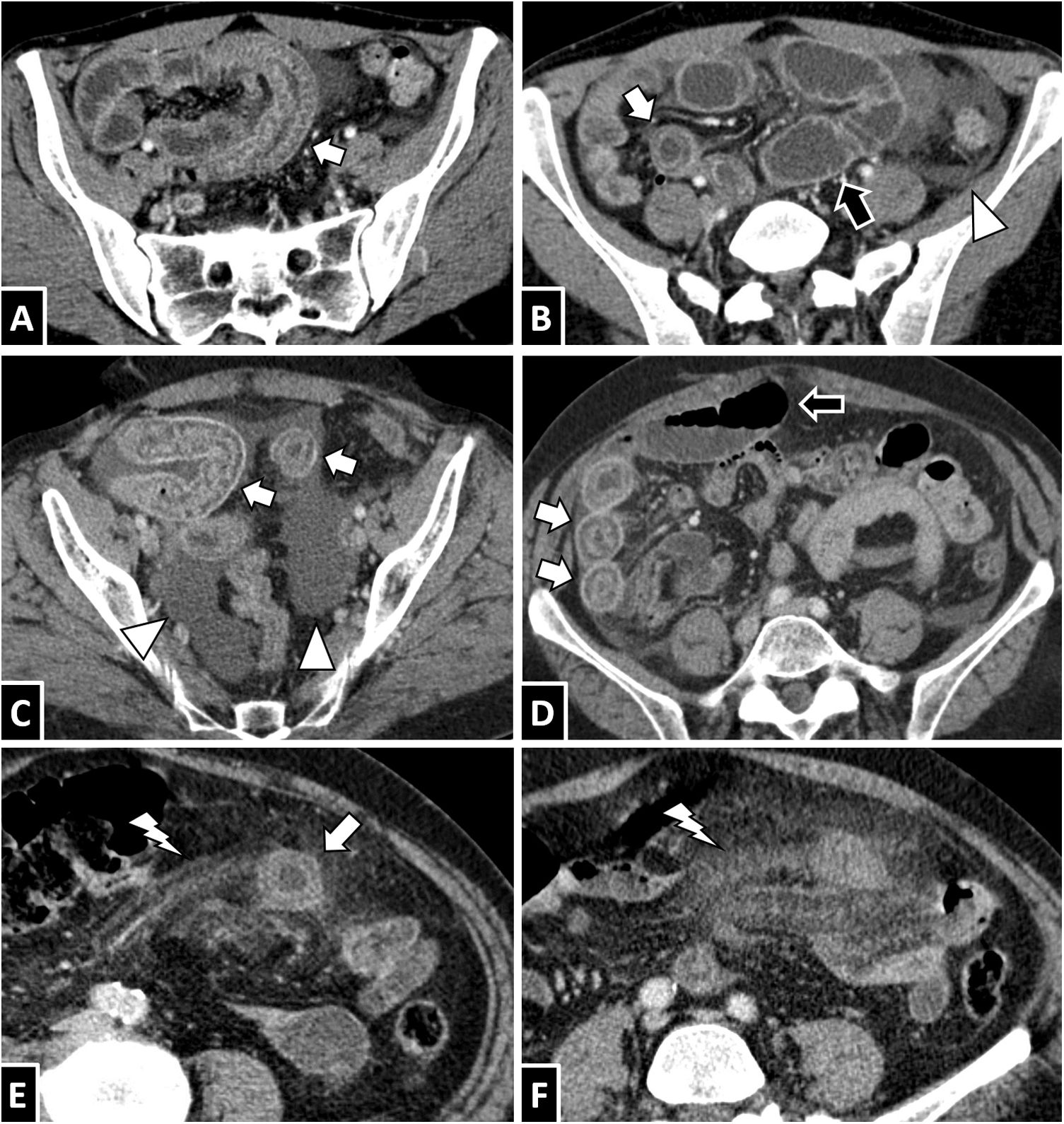
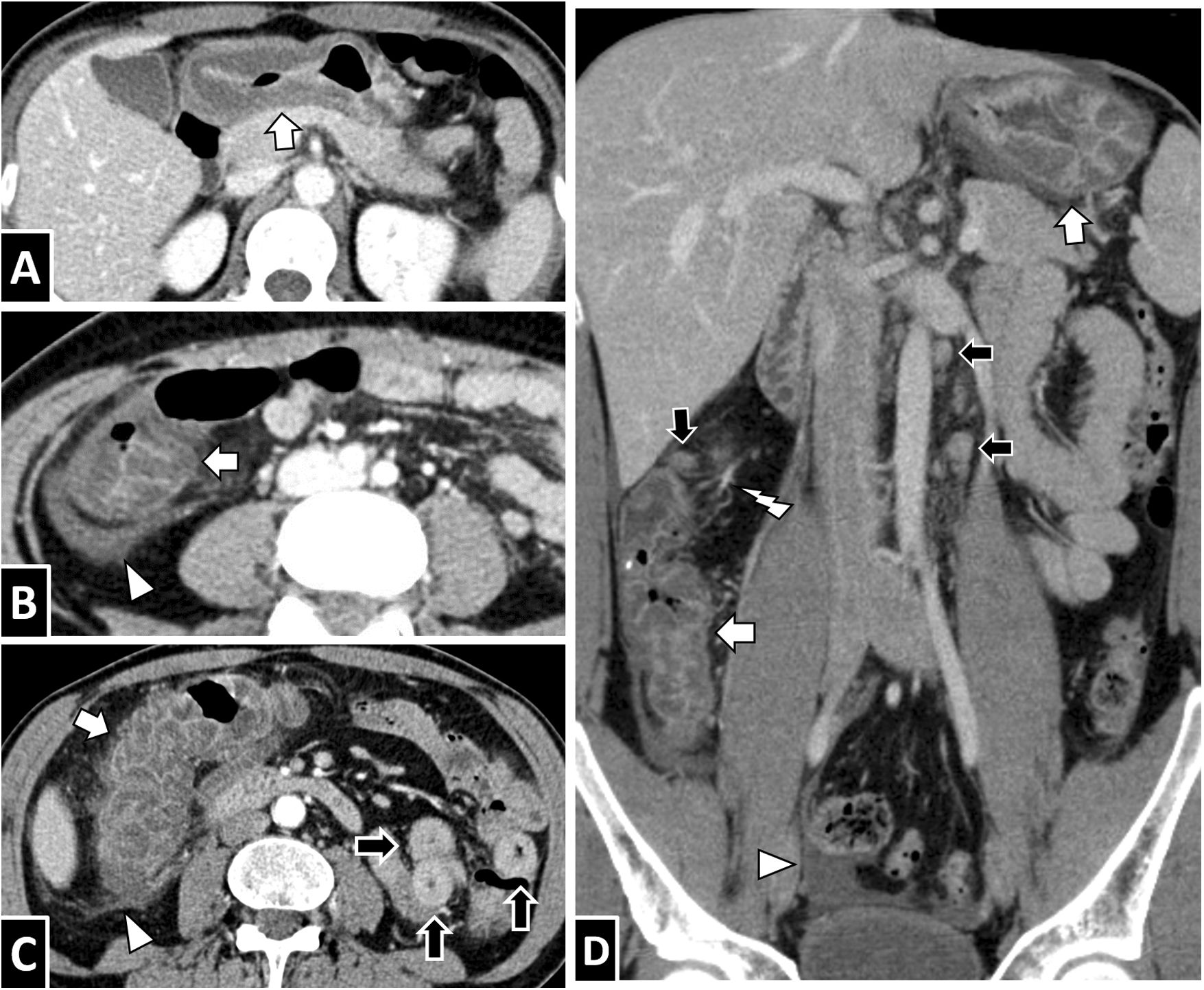
Anisakiasis with multisegmental involvement. A and B) A 52-year-old woman with cutaneous signs of an allergic reaction, severe abdominal pain and a history of ingestion of raw fish, with anisakiasis strongly suspected. A CT scan with intravenous contrast showed marked wall thickening and submucosal oedema, both in the gastric antrum and in the right colon (white arrows), as well as scant free fluid (white arrow tip). C) A 54-year-old man with suspected appendicitis. Significant thickening and submucosal oedema were seen in the right colon (white arrow) associated with segmental circumferential thickening in the ileum, with transmural hyperdensity (black arrows). D) A 43-year-old man with gastric and ileocaecal anisakiasis. A coronal reconstruction of an intravenous contrast-enhanced CT scan showed wall thickening and submucosal oedema of the affected segments (white arrows), mild ascites (arrow tip), lymphadenopathy (black arrows) and vascular hyperaemia (white lightning bolt). As these cases illustrate, involvement of multiple discontinuous segments of the gastrointestinal tract, sometimes with different characteristics, is not rare.
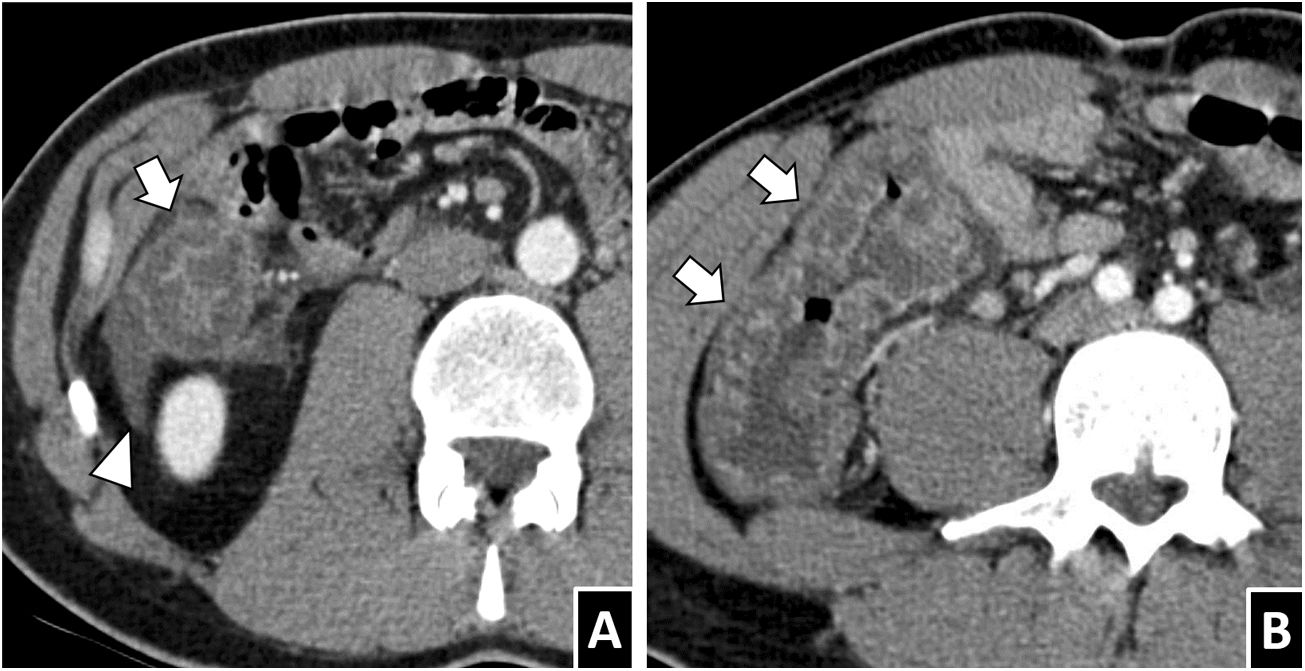
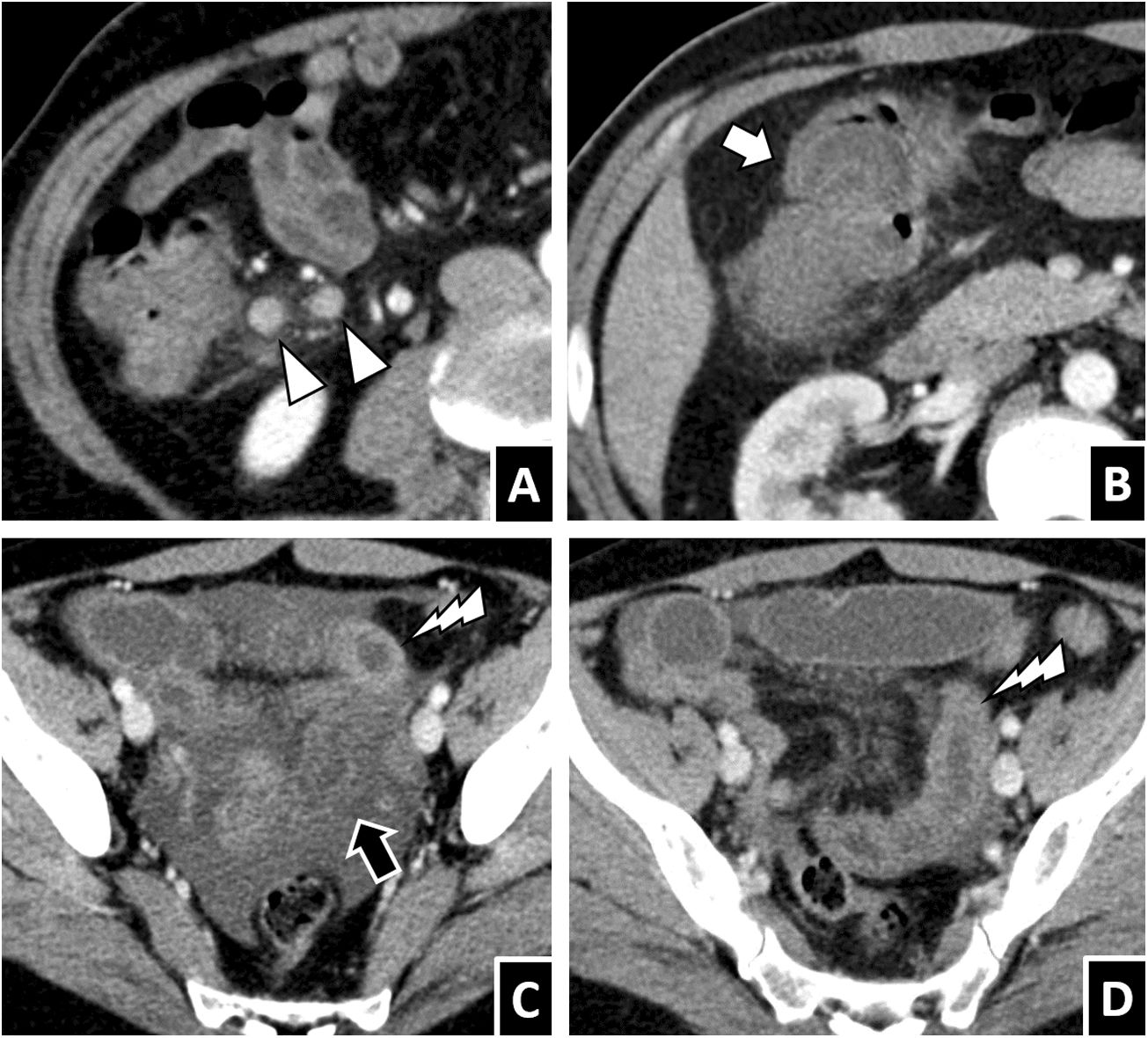
Less common findings. A) A 66-year-old woman with terminal ileitis due to Anisakis, with regional lymphadenopathy visible on CT (white arrow tips). Involvement of lymph nodes in anisakiasis varies across published series. B) A 46-year-old man with anisakiasis in the right colon who presented intestinal intussusception on CT (white arrow), one possible secondary complication. C and D) A 55-year-old man with severe abdominal pain and a definitive diagnosis of intestinal anisakiasis. A contrast-enhanced CT scan showed both segments of wall thickening with transmural hyperdensity (white lightning bolts) and significant submucosal oedema, in addition to ascites, oedema of fat and proximal dilation, all consistent with anisakiasis. One segment of submucosal oedema showed limited definition of the serosa (black arrow), in contrast with the hyperdense segments, raising the possibility of intestinal ischaemia (unconfirmed in this case).
Regular use of ultrasound in cases of acute abdominal pain should incline the clinician to consider a diagnosis of anisakiasis in the presence of consistent signs.6 Findings similar to those on CT have been reported (Figs. 10 and 11): segmental circumferential wall thickening, as well as, to a lesser extent, proximal intestinal dilation in the affected areas, mucosal/submucosal oedema and oedema of the valvulae conniventes, ascites, hypomotility of affected loops and wall hyperaemia on Doppler imaging.3,7,12,28,29 The wall usually maintains its ultrasound structure in layers, with thickening primarily due to oedema of Kerckring folds; this appears as enlargement and undulation of the hypoechogenic layer dependent on the muscularis mucosae, corresponding to the second layer from the intestinal lumen (Fig. 11).12,29
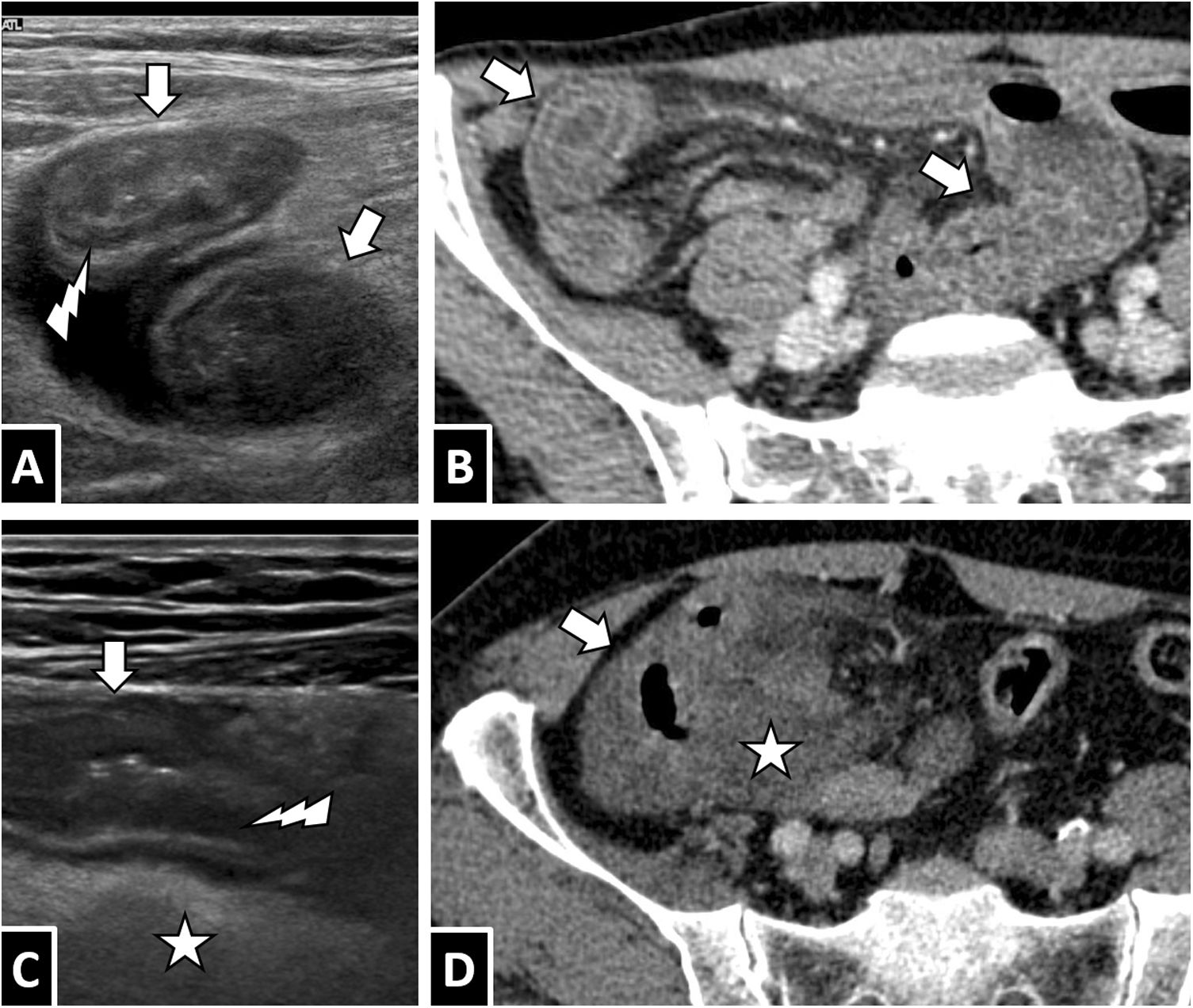
A 50-year-old man with severe abdominal pain and a diagnosis of gastritis due to Anisakis (previously shown in Fig. 2C). Correlation between findings on ultrasound (A, sagittal slice in the epigastric region) and contrast-enhanced CT (B). Both techniques showed marked wall thickening in the antral region with mucosal/submucosal oedema (arrows).
A and B) A 43-year-old man with abdominal pain and extensive ileitis. C and D) A 62-year-old man with terminal ileitis and right colitis. In both cases, the definitive diagnosis was intestinal anisakiasis. Correlation between ultrasound imaging with a high-frequency linear probe (A and C) and intravenous contrast-enhanced CT imaging (B and D): segments of intestinal wall thickening (white arrows) are seen with an intact layer structure and oedema primarily affecting the Kerckring folds (A and C, white lightning bolts), in addition to adjacent free fluid (arrow tips) and marked oedema of mesenteric fat (white star).
The finding of marked submucosal oedema with diffuse gastric wall thickening would suggest a differential diagnosis with multiple conditions.14,30 Acute gastritis and hypertrophic gastritis can cause similar imaging with a predominance in the antrum, but ascites and adjacent inflammatory changes are less common (Fig. 12A). Ménétrier disease causes significant thickening of folds, usually in the body and fundus, but usually does not affect perigastric tissue and has a more chronic clinical course. Ingestion of caustic substances also causes massive wall oedema with thickening of folds. Moreover, scirrhous carcinoma is associated with diffuse wall thickening that sometimes shows mucosal hyperdensity, but with a decreased pattern of folds and hypomotility (Fig. 12B).
Differential diagnosis examples. A) A 31-year-old man with gastritis and a penetrating ulcer in the antrum (arrow tip). Marked wall thickening with submucosal oedema (white arrow) can be seen. B) A 70-year-old man with signet ring cell gastric carcinoma and linitis plastica. There was diffuse wall thickening (white arrows), but with thinned folds, with neither submucosal oedema nor perigastric tissue abnormalities. C) A 45-year-old man with reactivation of ileal Crohn’s disease. In addition to wall thickening with submucosal oedema (black arrows), mild oedema of adjacent fat was seen with no appreciable ascites. Deposits of intramural fat measuring millimetres (white lightning bolts) suggestive of chronicity could be seen. D) A 75-year-old woman with radiation enteritis secondary to radiotherapy for rectal cancer. Several segments of circumferential wall thickening were seen in the pelvis with a “halo sign” (black arrows), as were vascular hyperaemia and a modest amount of adjacent fluid.
Within the differential diagnosis of intestinal involvement,4,14 acute-phase Crohn’s disease may present similar findings and a similar location to anisakiasis, although the onset of ascites is uncommon; the presence of abnormalities associated with chronic stages, such as deposits and fibrofatty proliferation or signs of transmural involvement, facilitate the differentiation thereof (Fig. 12C). Infectious and inflammatory enteritis also occasionally produce the “target sign”, but with normally longer thickened segments and lower rates of ascites (Fig. 12D). Intestinal ischaemia is associated with wall thickening of variable density (there may be hyperdensity of the mucosa or submucosal haemorrhage), in addition to oedema of mesenteric fat and ascites. However, involvement is usually more extensive with segmental dilation. Vascular structures may be seen not to fill with contrast and pneumatosis may be observed in advanced cases.25 In eosinophilic gastroenteritis, segmental thickening with submucosal oedema usually appears, but with concentric luminal stenosis, dysmotility and more chronic signs and symptoms. B-cell lymphoma, whether gastric or intestinal, usually shows thickening of folds and wall thickening, but with soft-tissue density; it is usually associated with lymphadenopathy and sometimes associated with aneurysmal dilatation of the segment. Ascites is rare. Thickening associated with adenocarcinoma is usually more asymmetrical and irregular, affecting a short segment with an abrupt transition.19 Acute or tuberculous peritonitis would be possibilities to be considered in cases of extraintestinal involvement.4
Remember: due to non-specific signs, symptoms and laboratory abnormalities, these patients often do not present with prior suspicion of anisakiasis.
Remember: anisakiasis might be suspected in the presence of segmental circumferential wall thickening with submucosal oedema, free fluid, oedema of mesenteric fat and proximal dilation of loops, especially in a patient with a history of exposure to foods associated with a risk of anisakiasis.
TreatmentIn most cases, conservative treatment is pursued. Often, symptomatic management of pain, nothing by mouth and fluid therapy are sufficient. There is some debate as to the indication for proton-pump inhibitors.4,5,7,13 However, careful follow-up in intestinal involvement is recommended, since there are documented cases of failure of conservative treatment with the onset of ischaemia or intestinal perforation.13
Removal of larvae significantly improves symptoms; hence, in some cases, endoscopic removal is attempted if the segment is accessible.4,5,13 In such cases, it is recommended that the procedure not be delayed: while larvae may be visible up to six days following ingestion, the passage of time increases the possibility of them degenerating, being eliminated or crossing the wall, leading to an ectopic form.4,7
In general, surgical treatment is only necessary in cases associated with secondary complications, such as obstruction with a poor course, perforation or significant clinical decline.19,23,24,26 Although the use of anthelmintics such as albendazole has been documented in at least one case, they do not have a defined role at present.4,5
ConclusionsAnisakiasis has a rising incidence in Western countries. Given the low initial clinical suspicion in a significant number of cases, the variable latency period of symptoms and the routine use of imaging tests in acute abdomen, imaging tests may play a role in its assessment. In the presence of consistent radiological findings, such as segmental wall thickening with submucosal oedema, ascites and inflammatory changes in fat, this possibility should be considered in the differential diagnosis.
Authorship- 1
Responsible for the integrity of the study: RFP.
- 2
Study concept: RFP, MUG and MPB.
- 3
Study design: RFP, MUG and MPB.
- 4
Data analysis and interpretation: not applicable.
- 5
Statistical processing: not applicable.
- 6
Literature search: RFP, MUG and MPB.
- 7
Drafting of the article: RFP.
- 8
Critical review of the manuscript with intellectually significant contributions: MUG and MPB.
- 9
Approval of the final version: RFP, MUG and MPB.
This study received no specific grants from public agencies, the commercial sector or non-profit organisations.
Conflict of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Fornell Pérez R, Urizar Gorosarri M, Pérez Bea M. Anisakiasis: manifestaciones radiológicas. Radiología. 2022;64:245–255.