Breast tomosynthesis is a continually improving tool for diagnostic radiologists. This update about tomosynthesis reviews the advantages of the technique both in patients with suspected or known disease and in screening, as well as its limitations, of which the dose of radiation is the most important. The more recent advent of synthesised mammography, computer-assisted detection, and tomosynthesis-guided biopsy have helped to reduce the dose of radiation used and have improved the diagnostic performance of tomosynthesis, so they are also discussed in this review.
La tomosíntesis de la mama es una herramienta del diagnóstico radiológico que se encuentra en constante desarrollo. En esta actualización sobre tomosíntesis se hace una revisión de las ventajas de la técnica, tanto en mamografía diagnóstica como de cribado, así como de sus limitaciones, entre las que la dosis de radiación es la principal. La aparición más reciente de la mamografía sintetizada, la detección de lesiones asistida por ordenador y la biopsia guiada por tomosíntesis, ha contribuido a reducir la dosis de radiación empleada y a mejorar el rendimiento diagnóstico de la tomosíntesis, motivo por el cual también son objeto de esta revisión.
Digital mammography (DM) is the basic technique used for both screening and diagnosis of breast cancer and it is the first and only technique to have led to a decrease in mortality and morbidity rates.1,2 However, DM does have two important limitations: poor sensitivity because of overlapping of tissue in dense breasts preventing the detection of small lesions; and a limited specificity for images with superposition of normal mammary parenchyma, which can simulate abnormalities.3,4 Tomosynthesis overcomes these drawbacks and obtains a pseudo-three-dimensional image of the breast. This technique obtained European Conformity (CE) certification in 2008 and was approved by the United States Food and Drug Administration (FDA) in 2011.5,6
The aim of this article was, on the one hand, to outline the technical aspects which affect image quality and, on the other, to compare the advantages and limitations of tomosynthesis in the breast cancer diagnosis and screening process with the classic approach of conventional DM. We also analysed the impact of the more recent introduction of synthesised mammography, computer-aided detection (CAD) of lesions and tomosynthesis-guided biopsy.
The tomosynthesis systemThe aim of the tomosynthesis system is to create a pseudo-three-dimensional reconstruction of the breast from multiple 1mm-thick projections, all parallel to the detector, which can be obtained in any of the usual mammography views.5–7 It is not strictly a 3D image like volume rendering reconstructions of a computed tomography (CT) scan. Tomosynthesis images are presented consecutively as individual sections forming part of a larger set that sweeps the entire breast, so the term pseudo-three-dimensional is preferred.8
In digital mammography with tomosynthesis, the study is acquired by rotating the X-ray tube, which moves in an arc around the breast. The image created is determined by a series of technical factors, which vary according to the different manufacturers and which affect the final result. We discuss these factors below.
Sweep angleThe sweep angle may be wide (>15°) or narrow (≤15°). On the one hand, acquisition with a larger angular range increases the depth resolution and decreases tissue overlapping, but it can generate more blur and, by lengthening the scanning time, it can also increase the dose. On the other hand, too narrow an angle can mean an incomplete scan with the appearance of artefacts.5
Acquisition timeThis has to be short to avoid excessive compression time, as that can cause discomfort leading to lack of collaboration, which causes the appearance of slight movements and degrades the quality of the image.5
Tube movementThere are two acquisition modes. There is the “step-and-shoot” mode, where the tube stops at each exposure, avoiding motion blur but lengthening the acquisition time, and there is a “continuous mode”, which is faster, but potentially less clear.5
Tube structureThe combined use of tungsten tubes with rhodium and silver filters optimises dose and image quality.5
The quality of the detectorThe detector has to have a high-speed data transfer rate with a large enough size to be able to record the complete image of the breast.5
Reconstruction algorithmsReconstruction algorithms, like other algorithms classically used in CT, can be iterative (simultaneous iterative reconstruction techniques, or SIRT) or filtered back projection (FBP).5
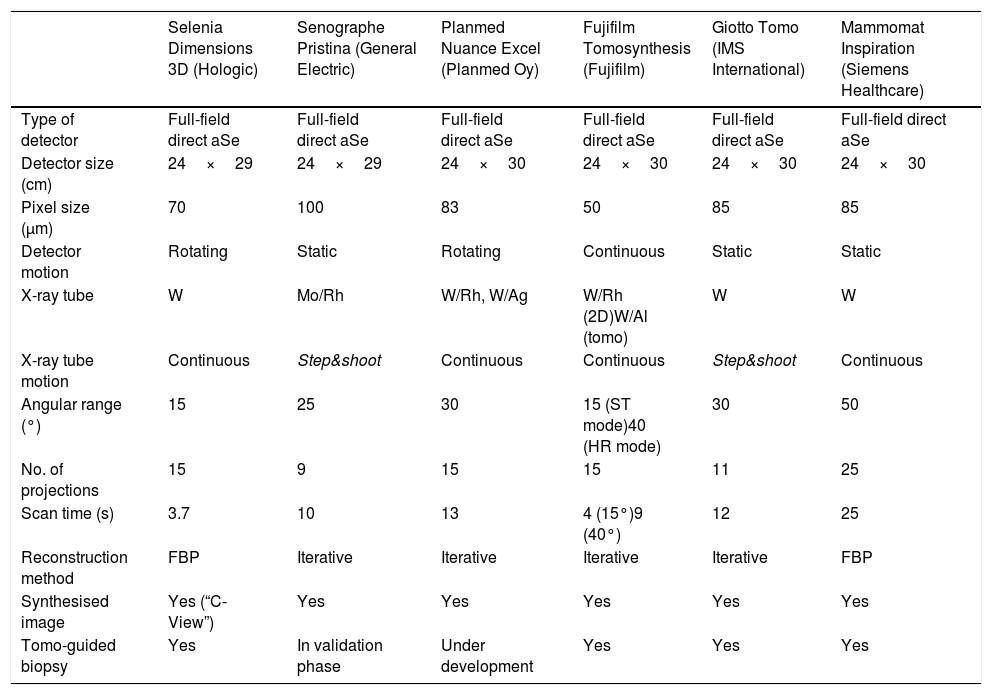
The main features and technical specifications of the systems with CE certification or FDA registration are summarised in Table 1.
Technical specifications of the different systems currently available on the market.
| Selenia Dimensions 3D (Hologic) | Senographe Pristina (General Electric) | Planmed Nuance Excel (Planmed Oy) | Fujifilm Tomosynthesis (Fujifilm) | Giotto Tomo (IMS International) | Mammomat Inspiration (Siemens Healthcare) | |
|---|---|---|---|---|---|---|
| Type of detector | Full-field direct aSe | Full-field direct aSe | Full-field direct aSe | Full-field direct aSe | Full-field direct aSe | Full-field direct aSe |
| Detector size (cm) | 24×29 | 24×29 | 24×30 | 24×30 | 24×30 | 24×30 |
| Pixel size (μm) | 70 | 100 | 83 | 50 | 85 | 85 |
| Detector motion | Rotating | Static | Rotating | Continuous | Static | Static |
| X-ray tube | W | Mo/Rh | W/Rh, W/Ag | W/Rh (2D)W/Al (tomo) | W | W |
| X-ray tube motion | Continuous | Step&shoot | Continuous | Continuous | Step&shoot | Continuous |
| Angular range (°) | 15 | 25 | 30 | 15 (ST mode)40 (HR mode) | 30 | 50 |
| No. of projections | 15 | 9 | 15 | 15 | 11 | 25 |
| Scan time (s) | 3.7 | 10 | 13 | 4 (15°)9 (40°) | 12 | 25 |
| Reconstruction method | FBP | Iterative | Iterative | Iterative | Iterative | FBP |
| Synthesised image | Yes (“C-View”) | Yes | Yes | Yes | Yes | Yes |
| Tomo-guided biopsy | Yes | In validation phase | Under development | Yes | Yes | Yes |
FBP: filtered back projection.
Adapted and updated for Radiology according to the data provided by the manufacturers, and with the authorisation of the author of the original article.5
Conventional DM can be used in combination with tomosynthesis (“combo method”), separately acquiring the images from both studies. However, almost all makes have synthesised 2D (s2D) mammography, an application with which we can reconstruct the individual images of a tomosynthesis study, obtaining a 2D mammogram comparable to conventional DM. In this case, a complete tomosynthesis study is acquired, i.e. in craniocaudal (CC) and mediolateral oblique (MLO) views, and the 2D mammogram is created virtually for each view (CC and MLO). This avoids having to perform a conventional DM study with 2 views.
To obtain the virtual image, the multiple sections that make up a tomosynthesis study are post-processed according to a reconstruction algorithm where the voxels with high attenuation values are maintained, creating an image in a single 2D plane,9–14 similar to maximum intensity projection (MIP) reconstructions of a CT.
The main value of s2D mammography is from its use as a substitute technique for conventional DM in breast cancer screening with DM and tomosynthesis, with the consequent reduction in dose, notwithstanding the diagnostic capacity.15,16
The FDA has authorised the use of s2D mammography as an alternative to conventional DM since May 2013.17
The tomosynthesis studyExamination protocolA tomosynthesis system can acquire images in CC, MLO, mediolateral, spot compression and displaced views of implants, but magnified images cannot be produced.
A simple tomosynthesis examination (with CC and/or MLO projections) improves the detection and characterisation of lesions compared to a single DM examination.3,18–20 Initially it was suggested that tomosynthesis scans should be limited to the MLO view to limit the radiation dose. However, the differences in the visibility and detection of the lesions according to the view used have changed, and it is estimated that 7–9% of confirmed cancers are only observed in one of the two tomosynthesis views, meaning they could go unnoticed if the breast is not examined in that view. Moreover, 5–6% of cancers are only seen in the CC view and, in turn, if a cancer is only visible in one view, it is more likely that it will be in the CC projection.19,21,22
Therefore, if a synthesised image is not available, a complete tomosynthesis examination in the two views is advisable for diagnostic mammography. The protocol to be followed in each clinical scenario (diagnostic mammography or screening mammography), however, must be assessed at each centre.
Process for reading and reportingThe following are the most common findings detected with the use of tomosynthesis:
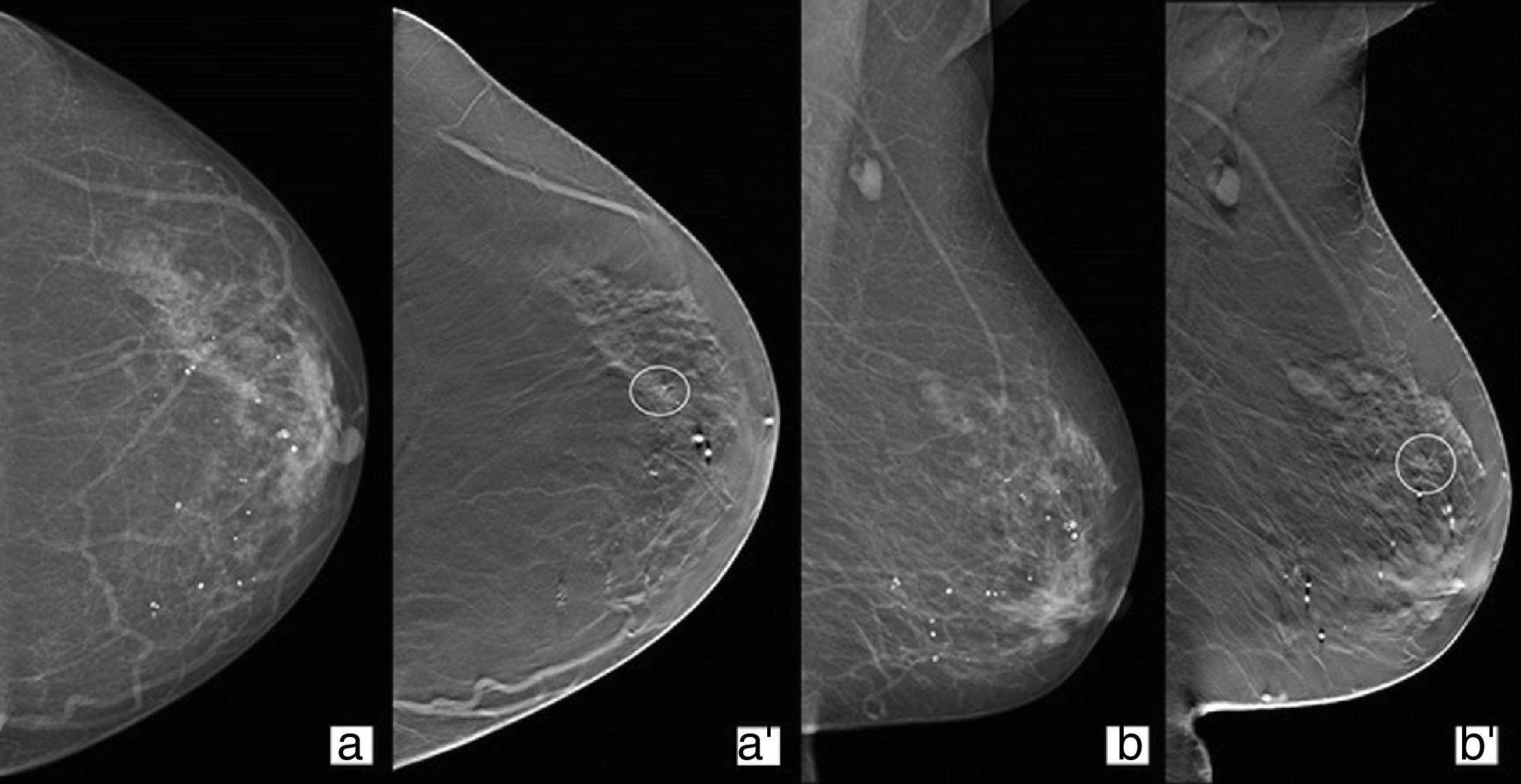
Architectural distortionBy frequency, this is the third most common type of finding detected in mammography studies. However, it is the most common of the lesions detected only in a tomosynthesis view. It is also the imaging descriptor most related to clinically negative breast cancer (Fig. 1).23
Follow-up of a 74-year-old woman with a history of right mastectomy in 2006 due to invasive carcinoma. Conventional 2D mammography craniocaudal (CC)/mediolateral oblique (MLO) views (a and b) showing a heterogeneously dense left breast (composition C) with no evidence of nodules or parenchymal distortions. Tomosynthesis CC/MLO views (a′ and b′), where a distortion of the parenchyma can be seen in the anterior segment time zone 2 of the upper outer quadrant with translation in ultrasound study. Result: invasive lobular carcinoma.
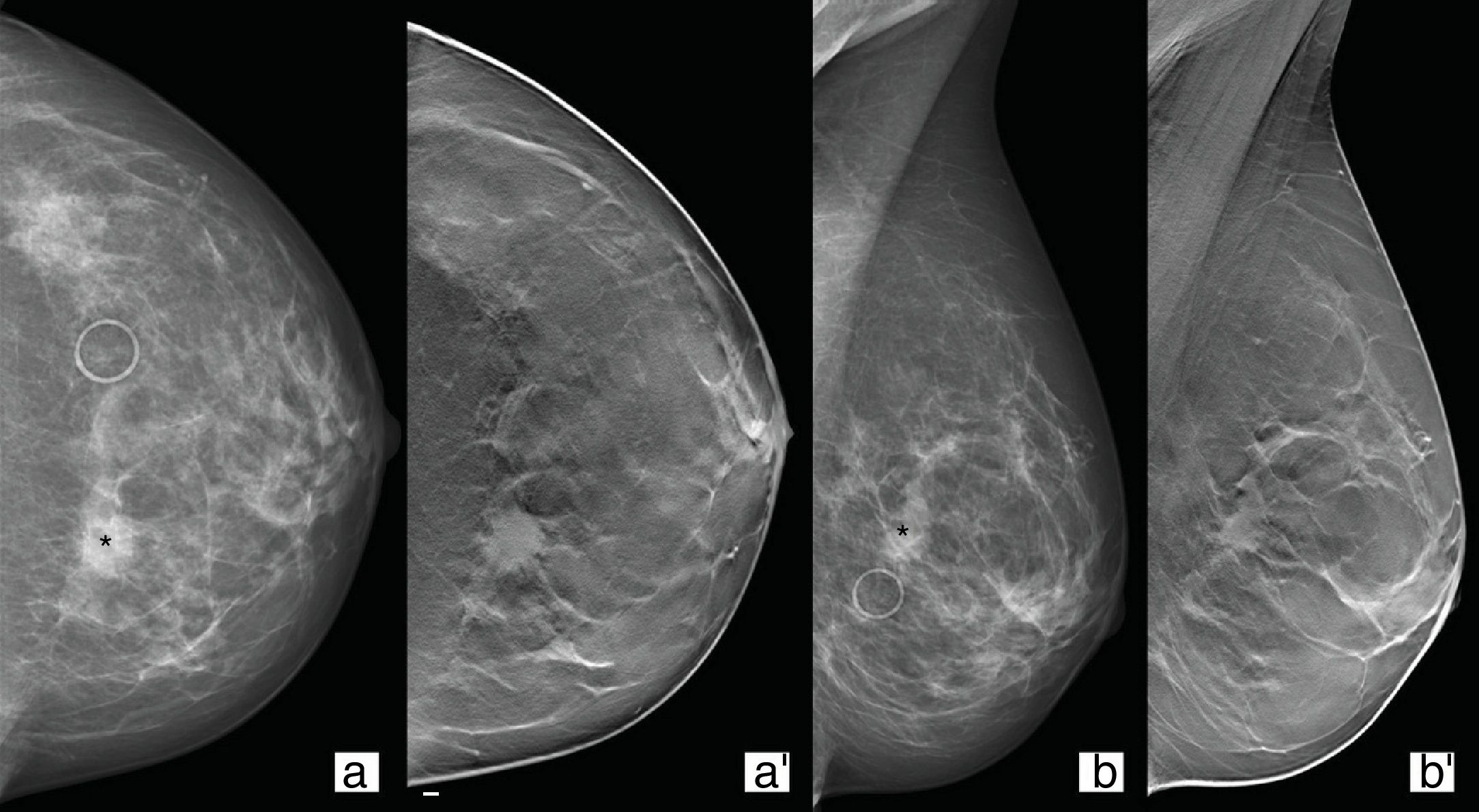
Tomosynthesis is useful for confirming and characterising an increase in density in a mammography study as a “true” focal asymmetry, ruling it out as overlap or reclassifying it as a nodule (Fig. 2).24
49-Year-old patient from screening programme with focal asymmetry (asterisk) in left breast (a and b). Tomosynthesis CC/MLO views (a′ and b′) where an oval-shaped nodule with spiculated borders can be seen underlying the asymmetry for which the patient was referred. Result: invasive ductal carcinoma.
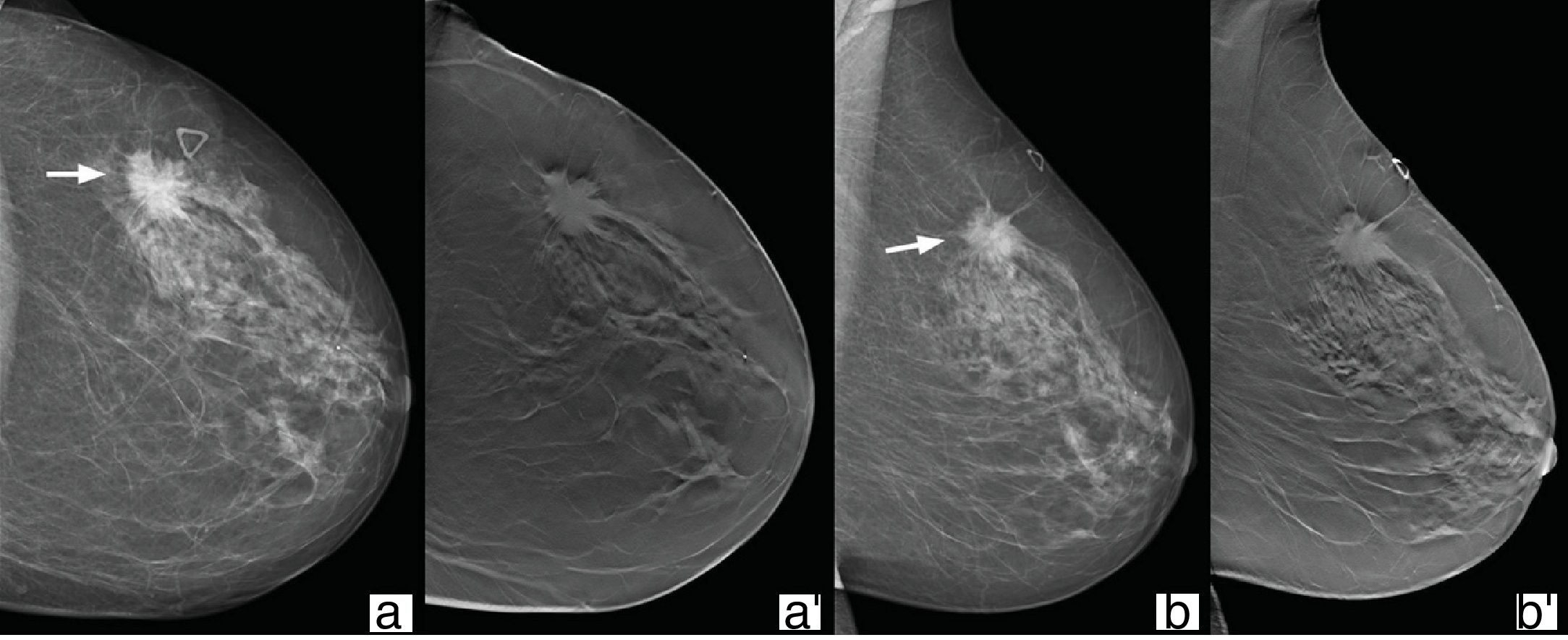
Tomosynthesis provides better characterisation of the form, margins and density of nodules. Although hyperdensity is a characteristic of malignant processes, it does not occur with the use of tomosynthesis, as the overlapping of the tissues is avoided (Fig. 3). Similarly, the presence of intralesional fat is not characteristic of a benign lesion in nodules visualised with tomosynthesis, as cancers can incorporate neighbouring adipose tissue as they grow. Careful assessment is therefore essential in the case of nodules containing non-encapsulated fat.25
72-Year-old woman referred with palpable nodule in left breast. In the upper outer quadrant, posterior segment time zone 2 of the left breast, we can see a nodule with irregular shape and borders (arrows), hyperdense with respect to the breast parenchyma in conventional 2D mammography (a and b) which in the tomosynthesis study (a′ and b′) is isodense. Result: invasive ductal carcinoma.
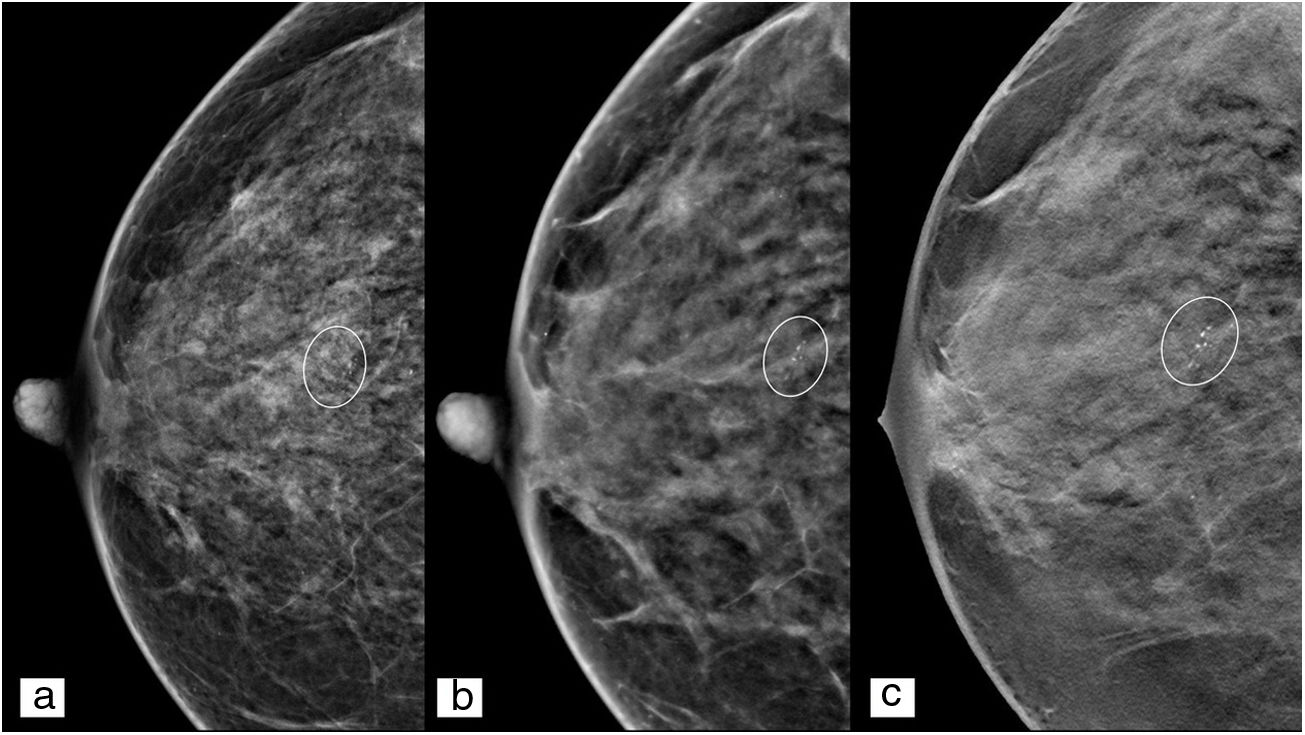
The use of a magnified projection is recommended for characterisation of microcalcifications, as a limited number of microcalcifications will be detectable in each individual tomosynthesis image. Tomosynthesis is helpful for distinguishing calcifications inside a nodule or to distinguish those in the skin from those within the tissue.26
It should be pointed out that s2D mammography has a higher contrast resolution than conventional DM, as a consequence of the reconstruction algorithms used9–14 (Fig. 4), and this leads to calcifications becoming visible which would otherwise be undetectable, and even to false positives resulting from misinterpretation of dense structures.27
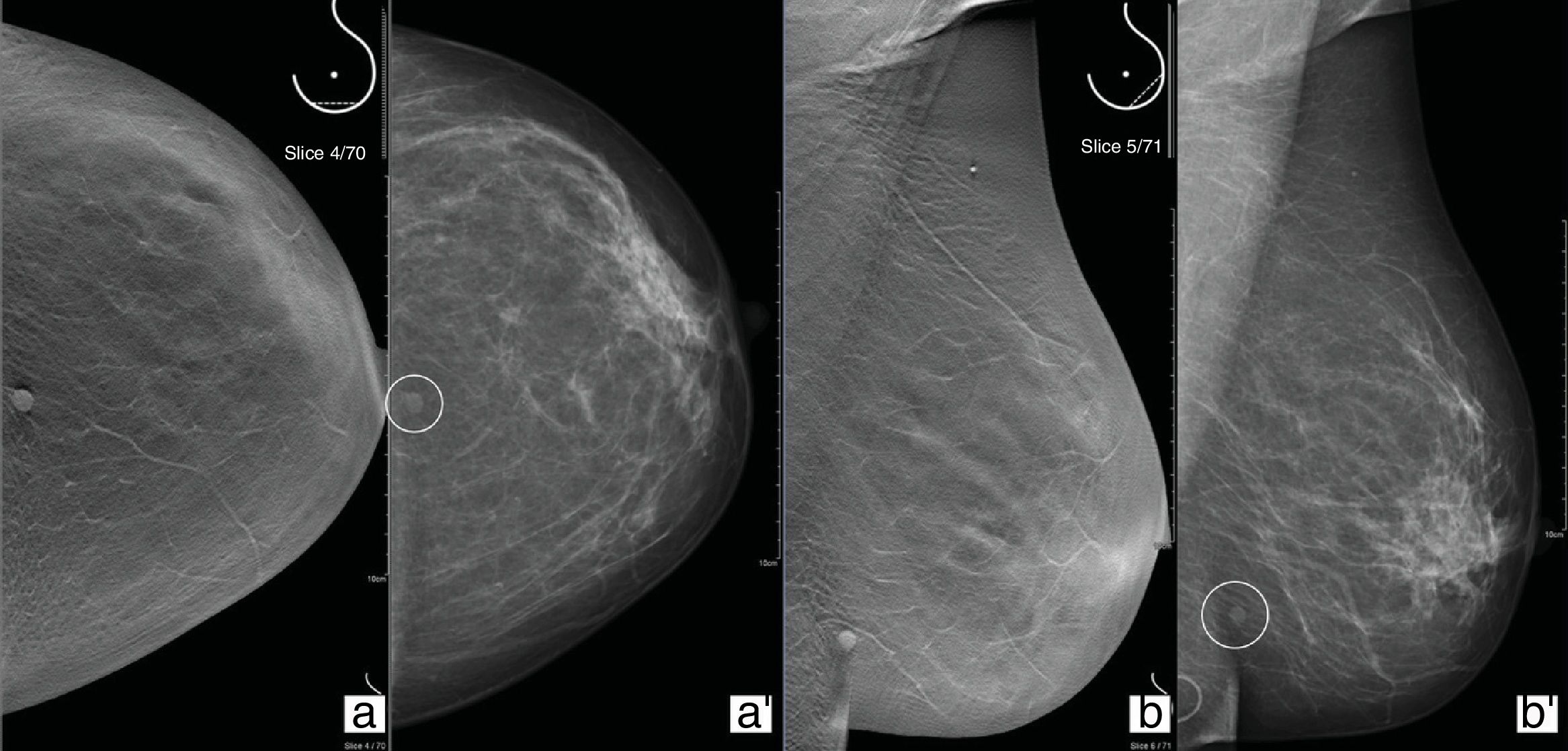
Effects on reportingAs tomosynthesis is a complementary tool in the diagnostic process, there is not a specific report separate from the DM report. Using the Breast Imaging Reporting and Data System (BI-RADS) is recommended for compiling a structured report. In the case of lesions only visualised with tomosynthesis, it is useful to specify the projection and the slice number where the findings were identified, as each individual tomosynthesis image is assigned a number corresponding to the distance in millimetres from the detector (Fig. 5).
52-Year-old patient from the screening programme with posterior central nodule in the left breast (a and b). Tomosynthesis CC/MLO views (a′ and b′). In left breast, lower-central quadrants, posterior segment of time zone 6, a round-shaped nodule with defined borders (circle) can be seen located in subcutaneous cellular tissue (first slices of the set of images), compatible with epidermal inclusion cyst in an ultrasound study, and corresponding with the finding for which the patient was referred.
There is also no specific medical order required for the study, as it is the radiologist who determines whether or not to perform tomosynthesis based on the patient, and in line with the centre's protocols.
Advantages and limitations of tomosynthesisAdvantagesDetection rateThe combined use of tomosynthesis and DM has been shown to produce a 30–50% increase in the rate of detection of additional cancers,3,4,22 with this figure reaching 90% in recent studies.28 Unlike in screening with DM only, these results can be superimposed in all categories of tissue composition, even for dense breasts (BI-RADS categories C and D).29 We should stress that breast density is not only an independent risk factor for the development of breast cancer. The density also affects the ability to detect lesions with conventional DM.30–33 With that in mind, in agreement with the American Society of Breast Surgeons, more than half of US states require that all women undergoing a mammography study be informed of their breast density,34 so that this data can be taken into account when deciding on the need for complementary study with another technique, e.g. tomosynthesis, ultrasound, contrast-enhanced mammography, magnetic resonance imaging (MRI) and/or positron emission tomography (PET). However, in relation to the synthesised image, it should be noted that no clinically significant differences have been found between s2D mammography and DM in the subjective density assignment for the same patient using the BI-RADS categories.35,36 A clinically significant difference is understood as when, for the same breast, the density assignment with DM corresponds to a non-dense category (BI-RADS A or B), while with s2D mammography it corresponds to a dense breast (BI-RADS C or D) or vice versa. However, there are slight variations between s2D mammography and conventional DM, with a slight tendency to assign a higher density in studies acquired with s2D mammography (occurs in approximately 12.5% of cases) deriving from the higher contrast resolution of s2D images compared to conventional DM.35
Despite the subjective differences discussed, in terms of sensitivity, specificity, recall rate, cancer detection rate and positive predictive value, the results of screening using s2D mammography and tomosynthesis are not inferior to those of DM combined with tomosynthesis15,27,37 and use of this combination as a substitute tool for conventional 2D DM is therefore acceptable.15,16
With tomosynthesis, there is a higher proportion of detection of invasive carcinomas than carcinomas in situ,38 as the visualisation of non-calcified lesions is improved and also, possibly, because radiologists pay more attention to the tomosynthesis images (which have a lower specificity in the case of microcalcifications) than to the 2D study. However, recent studies have shown that the detection rate is similar, as the use of the synthesised image improves the detection of microcalcifications with respect to individual tomosynthesis images,39 and microcalcifications are the most common finding in carcinoma in situ.
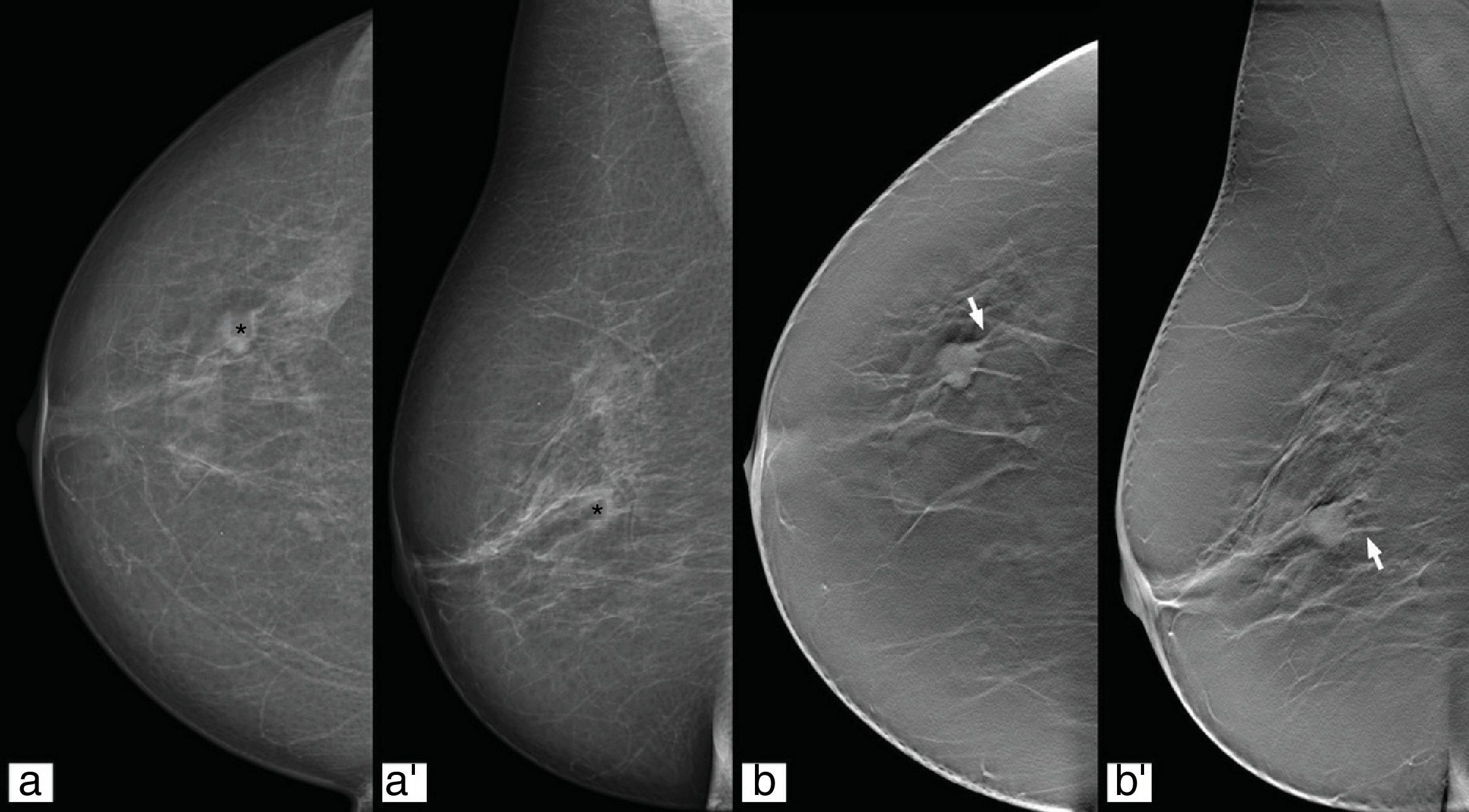
Characterisation of the lesionsThe improvement in the characterisation of lesions with the use of tomosynthesis has shown that fewer benign lesions are categorised as BI-RADS categories 3, 4 and 5, and more malignant lesions are classified as BI-RADS categories 4 and 5 (Fig. 6).40,41
In the group of probably benign lesions (BI-RADS category 3) there was no significant change in the case of focal asymmetries, but there was a reduction in the follow-up of these findings through being able to clarify in many cases whether or not they were dealing with a “true” lesion.42
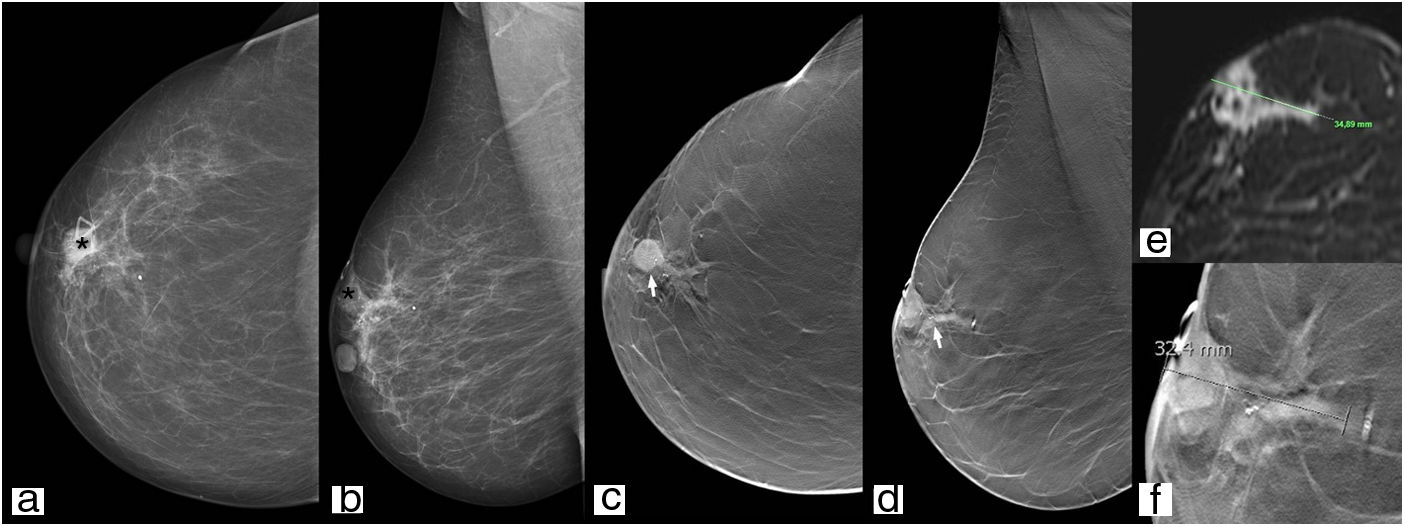
Tumour stagingTomosynthesis is superior to DM in determining the size of the lesion (the nucleus of the nodule must be measured and not the spicules visualised in the individual tomosynthesis image) (Fig. 7) and the number of lesions (multifocality/multicentricity), and in assessment of the contralateral breast; in the case of a new diagnosis of breast cancer there is a 2–3% probability of cancer in the contralateral breast, and not all centres routinely perform staging a MRI (Fig. 8).43
81-Year-old woman referred with palpable nodule. In the right breast, retroareolar region (asterisk), a round hyperdense nodule with partially hidden borders can be seen with microcalcifications inside (a and b) which, in the tomosynthesis study, is oval-shaped with non-defined borders (arrow) (c, d and f), and similar dimensions to those of the magnetic resonance imaging study for staging (e).
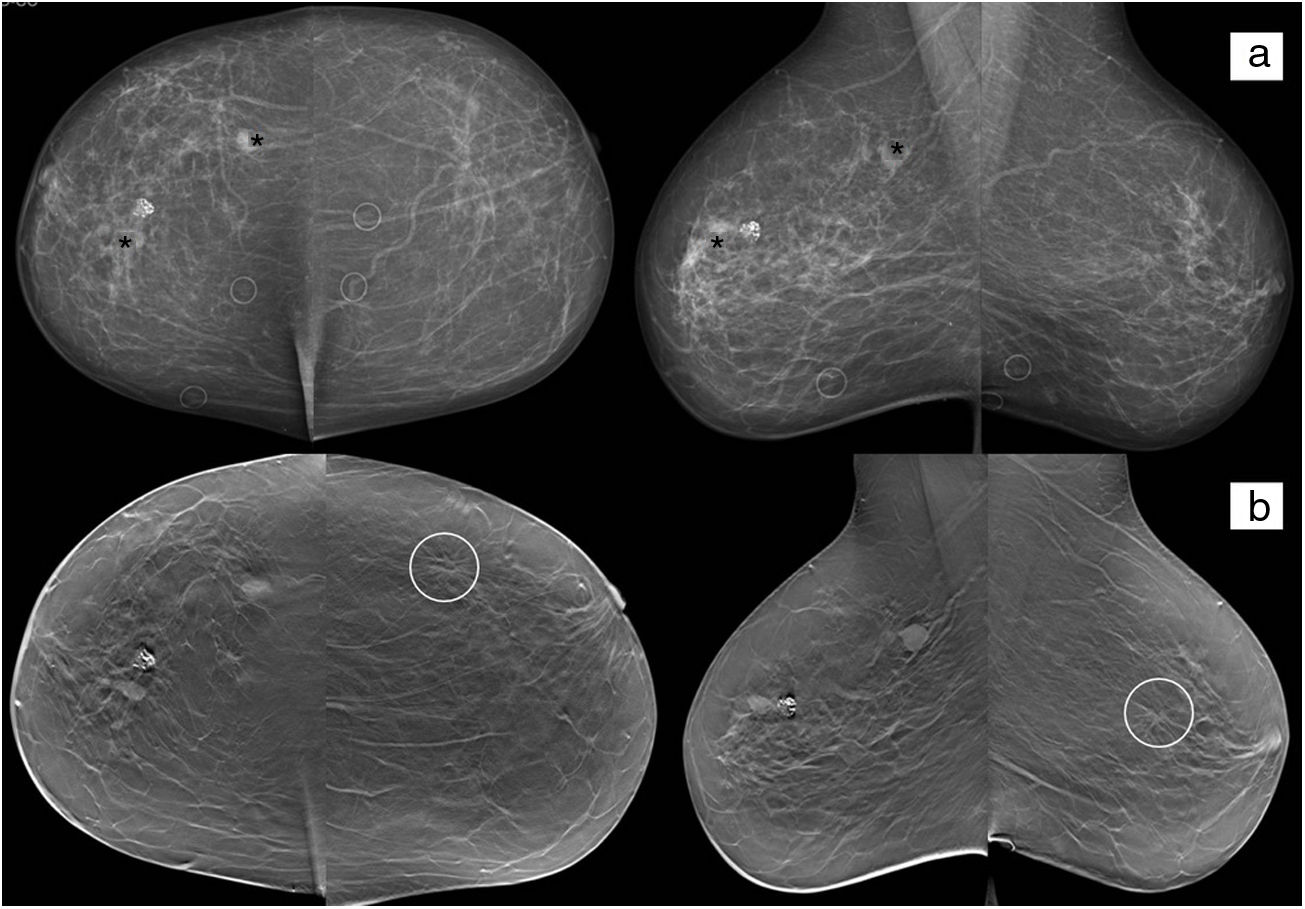
72-Year-old woman referred for investigation of paraneoplastic syndrome and complex renal cyst. There are three nodules in the right breast (asterisks), one of which is densely calcified. The nodules marked with asterisks have a confirmed histological diagnosis of invasive ductal carcinoma. The densely calcified nodule is a fibroadenoma and was also confirmed. In the left breast, a distortion of the parenchyma in the upper outer quadrant (circle) can be seen in the tomosynthesis study (b). Biopsy confirmed the suspicion of bilateral involvement.
The improvement in locating and characterising lesions with the use of tomosynthesis reduces the recall rate in noncalcified lesions by an estimated 15–40%, and therefore saves additional costs and anxiety for the patients.44 However, large population studies have produced conflicting results. We do have to consider the fact that there are significant differences in the patient inclusion requirements in these population studies, not only from one country to another, but even within the same country. Nevertheless, the finding that the recall rate is more stable with tomosynthesis than with DM is consistent.
Radiologist performance and workflowDiagnostic performance is improved in terms of greater sensitivity in the detection of lesions and better negative predictive value using a tomosynthesis view only compared to the use of DM; this benefit is greater for radiologists with less experience in mammography.45
In terms of workflows, tomosynthesis effectively replaces the use of the spot projections used to characterise or confirm the existence of lesions, thereby decreasing the movement of patients in and out of the room, while also reducing their anxiety levels.20,46,47
LimitationsRadiation doseThe mean glandular dose per projection of a tomosynthesis study is from 1.7 to 2.2mGy,48,49 which is one to one and a half times the dose of standard DM.50
The combined use of tomosynthesis and DM (“combo method”), being a dual acquisition that obtains the images of both studies separately, doubles the radiation dose. However, this remains within the limit of 3.0mGy per projection set out in the FDA regulations, the Mammography Quality Standards Act.51
Consequently, the key point for the use of tomosynthesis to become more generalised is to design a method that reduces the dose used in this type of examination.
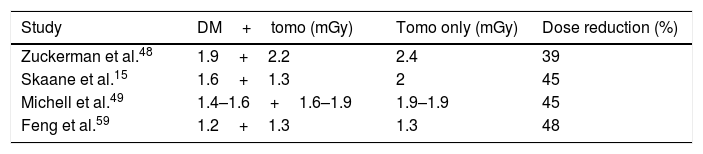
It has to be borne in mind that the 2D component is still a very important part of the study of the breast, both in screening and in diagnostic studies of symptomatic patients, as it is used for the assessment of possible mammary asymmetries, comparative study with previous mammograms and identification of microcalcifications. With the introduction of s2D mammography and the possible phasing out of the 2D DM component, patients will receive only the radiation corresponding to the tomosynthesis study. This represents a reduction of 39–45% in the dose compared to the combined DM and tomosynthesis study, similar to the dose of a conventional 2D DM study (Table 2).15,52–54,49
Reading timeThe number of images in a tomosynthesis examination depends on the thickness of the breast and that of the reconstruction slice. For example, in the study of a breast with a tissue thickness of 5cm, using a slice thickness of 1mm, 100 images are obtained, leading to an increase in the reading time of 35–70% compared to a conventional DM study. The wide range depends mainly on the DM reading speed. Experience can therefore be a point in favour, in order to achieve shorter reading times. One important point is that, in the case of a positive finding, the increase in reading time is due to the multiple images and not to taking longer to interpret the findings.55
Acquisition timeThe acquisition time of conventional DM is approximately 4s per projection, while a dual acquisition of tomosynthesis and DM takes 26% longer.56
Learning curveThere is a learning curve for both the technician and the radiologist who interprets the tomosynthesis. However, it is not much different from training with other new applications or equipment. According to the FDA's Mammography Quality Standards Act and Program, staff must receive 8h of initial training before independently using any new form of mammography. The number of recalls may increase initially, but usually stabilises at six months and then starts to fall.57
Specific systems for managing and storing imagesThe use of tomosynthesis involves larger data files and so specific systems for managing and storing images are required. The size of an individual tomosynthesis image is from 3 to 4.5MB. The combined use of tomosynthesis and DM produces approximately 1800MB, much larger than the 80MB of a set of DM images from four views.6,58
Lesions not detected by tomosynthesisFalse-negative results have been reported in symptomatic patients in small cases series. These were found to be luminal neoplasms (mainly type B) and so had a worse prognosis. As not all cancers can be detected by tomosynthesis, the level of individual risk of breast cancer and the clinical symptoms should always dictate the route to be followed in these patients, and a multimodal study should be performed if warranted.59,60
Tomosynthesis in population screeningThe only technique which has been demonstrated to reduce mortality rates in screening is mammography. However, the additional cancers detected by tomosynthesis in population studies such as Oslo61 and Malmo18 corresponded mainly to invasive cancers, but of lesser grade and size, and luminal type A phenotypes, without it being possible to determine whether these results represented earlier diagnosis or overdiagnosis. More studies analysing tumour development are needed to understand the impact of the detection and treatment of slow-growing lesions, as they are part of overdiagnosis and one of the limitations of the incorporation of tomosynthesis in screening programmes.62
The European Commission recommends a prudent use of tomosynthesis in screening due the low level of evidence. It is therefore necessary to carry out randomised trials, but that means studies lasting over 10 or 20 years.63
One of the limitations of tomosynthesis is, as already mentioned, the increase in reading time and in time spent performing the examination; an important aspect in screening programmes, due to the high number of women participating in these campaigns. However, European guidelines recommend double reading in screening studies, and there are results to suggest that the combined use of tomosynthesis with synthesised imaging and CAD could reduce the need for double reading.63–65
Moreover, the cost-effectiveness ratio should be considered in any population screening programme. The need for a greater image storage capacity in the picture archiving and communication system (PACS) has to be taken into account, as this makes the introduction of tomosynthesis more expensive in screening systems.
Interval cancers (those that develop between a normal screening mammogram and the next round of screening), which are associated with poorer tumour characteristics and survival rates and therefore reflect the effectiveness of the programme, have not been sufficiently evaluated. The most recent studies show no significant differences in detection with 2D DM compared to tomosynthesis.66,67
Advances in tomosynthesis: computer-assisted lesion detection and tomosynthesis-guided biopsyComputer-assisted lesion detectionOne way to improve the diagnostic performance of tomosynthesis is the application of a computer-aided detection (CAD) system in the reading process.
CAD has been used over the years to improve the detection and interpretation of imaging findings in a range of different radiological modalities, not only mammography. It consists of an application which analyses the images using mathematical algorithms and assigns marks that highlight the findings. These are systems designed to reduce oversights during a first reading and, consequently, the number of false negatives.68
There are two applications on the market which allow this technology to be applied in tomosynthesis studies (iCAD Inc., Nashua, NH, USA and GE Healthcare, Buc, France).69,70 In both cases, the programme identifies the findings in each individual tomosynthesis image and they are then merged and presented in an improved 2D synthesised image which is better than standard s2D mammography images.
The diagnostic performance of any synthesised 2D virtual image depends a lot on the mathematical algorithms used to generate it. However, in terms of diagnostic performance and according to the latest research, synthesised 2D mammography generated with CAD systems has greater specificity than conventional 2D DM (57.35% vs. 17.64%) and higher sensitivity than s2D mammography (94.1% vs. 52.94%).71
Tomosynthesis-guided biopsyOf the total number of breast lesions with recommendation for biopsy, only a small percentage are only visible in tomosynthesis, being hidden in conventional 2D DM (approximately 7%, mostly in dense breasts). However, as many as 53% of these lesions end up with a confirmed final diagnosis of malignancy.23 Based on these results, any suspected radiological findings in a tomosynthesis study should be categorised as BI-RADS 4C. This highlights the importance of the histological analysis of these lesions and the need for development of a tomosynthesis-guided biopsy technique as an accurate and efficient sampling tool, especially when there is no representation of the findings in other imaging modalities such as ultrasound or MRI.72
The prone biopsy table stereotactic-guided vacuum-assisted biopsy (VAB) is considered the standard technique of reference for the sampling of lesions with representation only in mammography studies.73
Unlike stereotactic biopsy, tomosynthesis-guided biopsy allows the use of the full size of the detector and facilitates the anatomical location of the lesions with respect to the rest of the breast. It also enables re-identification of low density lesions, such as noncalcified masses, which are often difficult to visualise in stereotactic systems after a diagnostic study with DM. As a result of all this, there is a reduction in the execution times, and particularly in the planning of the test, compared to the standard method of stereotactic-guided biopsy (13 vs. 29min).72
Based on the above, tomosynthesis-guided biopsy is not only useful for guiding the procedure in visible lesions using this technique, but it can even replace stereotactic biopsy as a sampling method in lesions with representation in two-dimensional DM studies.72
ConclusionsThe main benefits supporting the widespread use of tomosynthesis are the improvement in the detection rate of breast cancer, the characterisation of the lesions, better tumour staging and a reduced recall rate.
The main limitation is the radiation dose. The introduction of the synthesised image as a substitute technique for conventional DM brings all the benefits of tomosynthesis with a reduction in radiation dose.
The use of tomosynthesis in screening still requires long-term studies before it can be considered for routine application.
The availability of tomosynthesis-guided CAD and BAV will help to improve diagnostic performance.
Authorship- 1.
Responsible for the study as a whole: AMF and DMF.
- 2.
Study conception: AMF and DMF.
- 3.
Study design: AMF and DMF.
- 4.
Data acquisition: AMF and DMF.
- 5.
Analysis and interpretation of the data: AMF and DMF.
- 6.
Statistical processing: AMF and DMF.
- 7.
Literature search: AMF and DMF.
- 8.
Drafting of the manuscript: AMF and DMF.
- 9.
Critical review of the manuscript with relevant intellectual contributions: AMF and DMF.
- 10.
Approval of the final version: AMF and DMF.
The authors declare that they have no conflicts of interest.
Please cite this article as: Rocha García AM, Mera Fernández D. Tomosíntesis de la mama: estado actual. Radiología. 2019;61:274–285.