To review the radio-pathologic features of symptomatic breast cancers not detected at digital mammography (DM) and digital breast tomosynthesis (DBT).
Material and methodsRetrospective analysis of 169 lesions from symptomatic patients with breast cancer that were studied with DM, DBT, ultrasound (US) and magnetic resonance (MR). We identified occult lesions (true false negatives) in DM and DBT. Clinical data, density, US and MR findings were analyzed as well as histopathological results.
ResultsWe identified seven occult lesions in DM and DBT. 57 per cent (4/7) of the lesions were identified in high-density breasts (type c and d), and the rest of them in breasts of density type b. Six carcinomas were identified at US and MR (BI-RADS 4 masses); the remaining lesion was only identified at MR. The tumor size was larger than 3cm at MRI in 57 per cent of the lesions. All tumors were ductal infiltrating carcinomas, six of them with high stromal proportion. According to molecular classification, we found only one triple-negative breast cancer, the other lesions were luminal-type. We analyzed the tumor margins of two resected carcinomas that were not treated with neoadjuvant chemotherapy, both lesions presented margins that displaced the adjacent parenchyma without infiltrating it.
ConclusionOccult breast carcinomas in DM and DBT accounted for 4 per cent of lesions detected in patients with symptoms. They were mostly masses, all of them presented the diagnosis of infiltrating ductal carcinoma (with predominance of the luminal immunophenotype) and were detected in breasts of density type b, c and d.
Revisar las características radiopatológicas de carcinomas mamarios sintomáticos ocultos en mamografía digital (MD) y tomosíntesis (TS).
Material y métodosAnálisis retrospectivo de 169 lesiones provenientes de pacientes sintomáticas con diagnóstico histológico de cáncer de mama y que fueron estudiadas con MD, TS, ecografía y resonancia magnética (RM). Se identificaron las lesiones ocultas (falsos negativos verdaderos) en MD y TS. Se analizaron datos clínicos, de densidad, los hallazgos con ecografía y RM, y la histopatología de las lesiones.
ResultadosSe detectaron siete lesiones neoplásicas ocultas en MD y TS. El 57% (4/7) se presentó en mamas densas (tipo c y d), y las restantes en mamas de densidad b. Se identificaron seis de los carcinomas por ecografía y RM (masas BI-RADS 4); la lesión restante solo se visualizó en RM. En el 57% de las neoplasias, el tamaño medido con RM fue mayor de 3cm. Todas fueron carcinomas ductales infiltrantes, seis de ellos con alta proporción estromal. En cuanto a los subtipos moleculares, solo una fue triple negativo y las demás fueron de tipo luminal. Se analizaron los márgenes tumorales de dos carcinomas intervenidos sin quimioterapia previa, y ambos presentaban márgenes que desplazaban sin infiltrar el parénquima adyacente.
ConclusiónLos carcinomas ocultos en MD y TS representaron el 4% de las lesiones detectadas en pacientes sintomáticas, fueron mayoritariamente masas, todas tuvieron diagnóstico de carcinoma ductal infiltrante (con predominio del inmunofenotipo luminal) y se detectaron en mamas de densidad tipo b, c y d.
Breast tomosynthesis (TS) is an important contributor in the diagnostic process of breast cancer. Various studies have confirmed the advantages of using it together with mammograms in programs of population screening (improving the detection of cancer with fewer recitations1–4), and in the pathological setting (improving the sensitivity, characterization, and categorization of the lesions,5,6 with a lower percentage of findings classified BI-RADS 3,7 among other).
However, it is not a perfect modality and although it allows us to assess areas where possible lesions may be misdiagnosed in the digital mammograms (DM), at times, breast cancer can remain invisible in the DM and the TS. Since it is more and more widely used, we need to know more about the advantages and limitations of TS (information on true false negatives in both modalities in medical literature is scarce).
The goal of this work was to analyze the radiopathological characteristics of lesions with histologic diagnosis of breast cancer (detected in patients with breast symptoms) hidden in the DM and the TS.
Material and methodsIt is a retrospective study approved by our hospital ethics committee and focused on the descriptive analysis of hidden lesions in the DM and the TS with a histological diagnosis of breast cancer in patients with clinical suspicion. Out of the 387 female patients (both from the early detection program of our hospital and those with breast symptoms) with a diagnosis of breast cancer who were studied using DMs and TS as initial tests in the Breast Radiology Unit between Nov. 2011 and Dec. 2015, 160 patients with symptoms were selected (symptoms such as palpable nodules or indurations; changes in breast size or morphology; retraction or sinking of the nipple skin; nipple discharges, skin abnormalities; presence of axillary adenopathies; and even persistent mastodynia).
The patients were studied using the Selenia Dimensions system (Hologic, Bedford, MA, USA). The protocol used was the COMBO mode capable of acquiring 2D images and one tomosynthesis in one single compression; craniocaudal and mediolateral oblique projections were taken for every breast. As part of the diagnostic process, all cases underwent ultrasound scans with a 12MHz linear probe (Aplio MX, Toshiba Medical Systems, Tokyo, Japan); MRIs (RM de 1.5 T Avanto, Siemens, Erlangen, Germany); the acquisition of images included T1 and T2-weighted enhanced sequences, and diffusion and dynamic studies after the administrations of gadolinium; and percutaneous biopsies (low ultrasonography with a 14G tru-cut needle) as additional tests prior to surgical exeresis.
The images acquired and anonymized were part of a database that was reviewed by three (3) different radiologists with exclusive dedication (professional experience of 2–15 years) and a computed assisted detection system was used. Information such as age; clinical presentation; type of breast density; the BI-RADS category, and the number and type of lesions was gathered as well, and the lesions hidden in the DMs and TS were identified (true false negatives) having, as a reference point, the anatomopathological findings and the MRI findings (misreadings or technical mistakes were discarded). Eventually, the imaging findings of the lesions selected were consistent with their respective histopathological diagnoses.
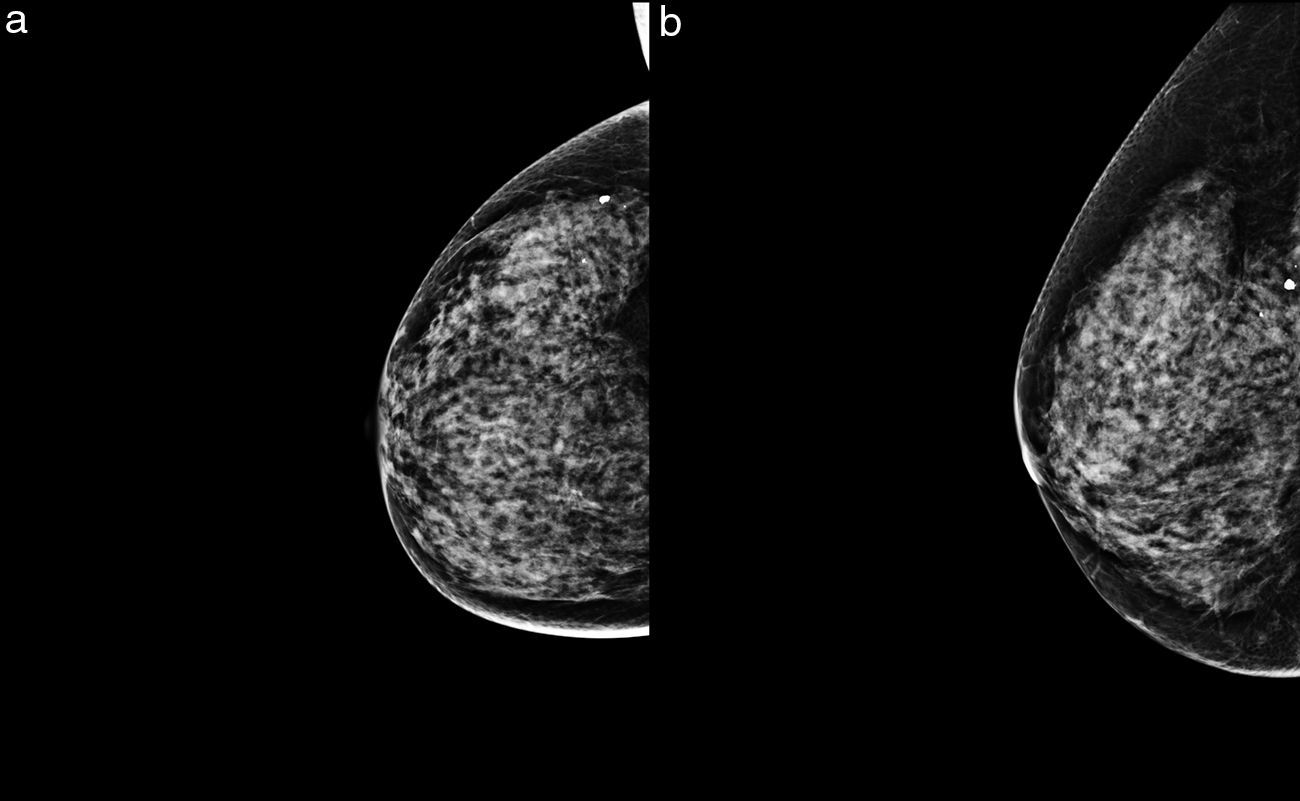
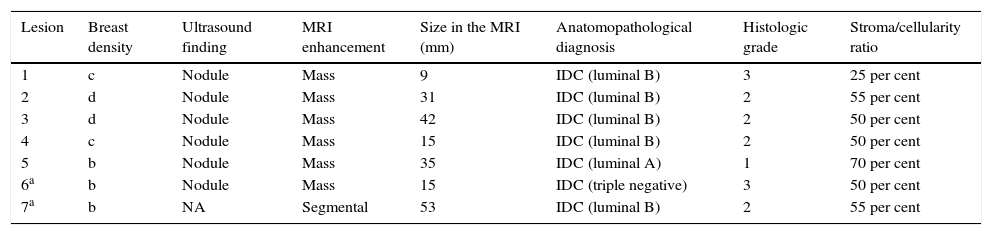
ResultsOverall, 169 neoplastic lesions were found in the symptomatic group, and out of all these lesions, seven (7) were selected (4 per cent) in which breast cancer was not visible through the DM or the TS (Fig. 1, Appendix B video 1–2). Two (2) lesions of our series were found in different quadrants of the same breast (multicentric affectation). The patients’ average age was 47 years old (range: 32–61 years old), and the associated symptoms were self-palpation of an area with greater consistency, or a nodule de novo. When it comes to breast density, four (4) lesions were found in high-density breasts (types c and d), and the rest in type b density breasts (Table 1).
Characteristics of BI-RADS 6 lesions hidden in the digital mammograms and tomosynthesis of our series.
| Lesion | Breast density | Ultrasound finding | MRI enhancement | Size in the MRI (mm) | Anatomopathological diagnosis | Histologic grade | Stroma/cellularity ratio |
|---|---|---|---|---|---|---|---|
| 1 | c | Nodule | Mass | 9 | IDC (luminal B) | 3 | 25 per cent |
| 2 | d | Nodule | Mass | 31 | IDC (luminal B) | 2 | 55 per cent |
| 3 | d | Nodule | Mass | 42 | IDC (luminal B) | 2 | 50 per cent |
| 4 | c | Nodule | Mass | 15 | IDC (luminal B) | 2 | 50 per cent |
| 5 | b | Nodule | Mass | 35 | IDC (luminal A) | 1 | 70 per cent |
| 6a | b | Nodule | Mass | 15 | IDC (triple negative) | 3 | 50 per cent |
| 7a | b | NA | Segmental | 53 | IDC (luminal B) | 2 | 55 per cent |
IDC: infiltrating ductal carcinoma; NA: non applicable.
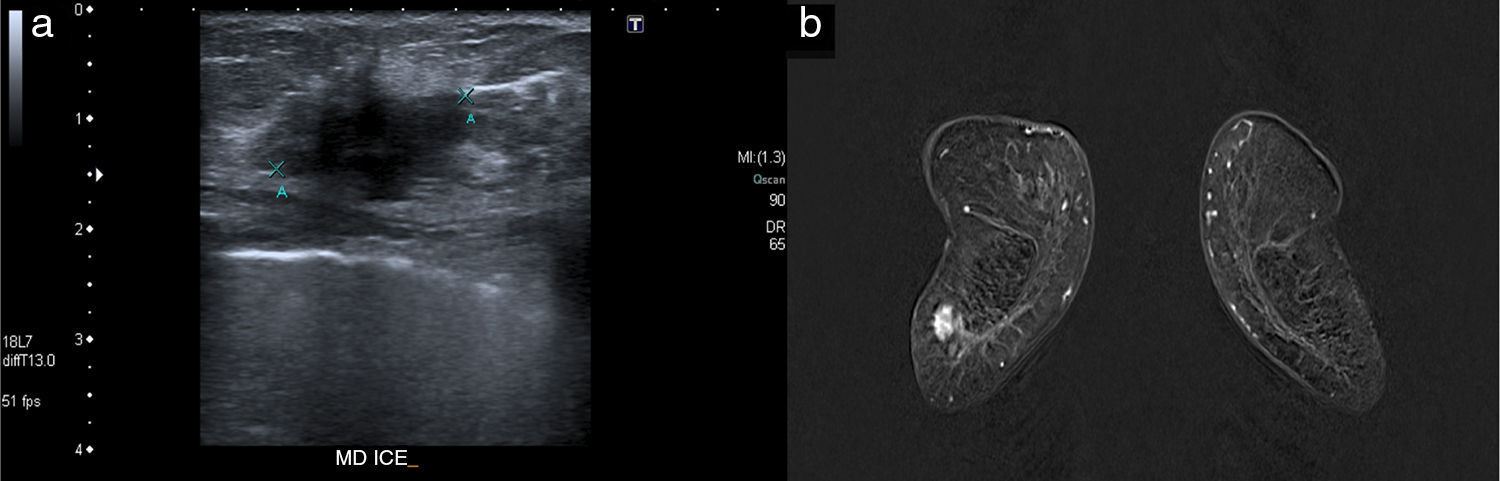
When it comes to the remaining imaging modalities, six (6) carcinomas were seen in the ultrasound scan (looking as hypoechogenic masses of irregular BI-RADS 4 morphology), and in the MRI (mass enhancement with type 2 uptake curves, and average ADC map of 0.68×10−3mm2/s [they varied from 0.61 to 0.78×10−3mm2/s]) (Fig. 2). Only one (1) lesion was not detected in the ultrasound scan, but it was identified in the MRI as segmental enhancement (this case was the aforementioned multicenter affectation). In the MRI tumor size was close to 1cm in three (3) cases (43 per cent), and >3cm in the remaining cases (57 per cent). In two (2) small lesions, conservative surgery was performed, and the size of the final piece had a minimal variation (1–2mm) with respect to the size measure through the MRI; the rest of the group received neoadjuvant therapy prior to exeresis.
All neoplasms were infiltrating ductal carcinomas (IDC), four (4) of them were grade 2 tumors according to the Nottingham grading system (moderately differentiated) and two (2) were grade 3 tumors (poorly differentiated). All biopsies (except for one lesion) confirmed high percentages of tumor stroma with respect to neoplastic cellularity (55–70 per cent). With respect to the molecular subtypes surrogate to immunohistochemical markers, only one (1) of the cases was triple negative and the rest were luminal category tumors (5 were luminal B like tumors, and 1 was a luminal A like tumor).
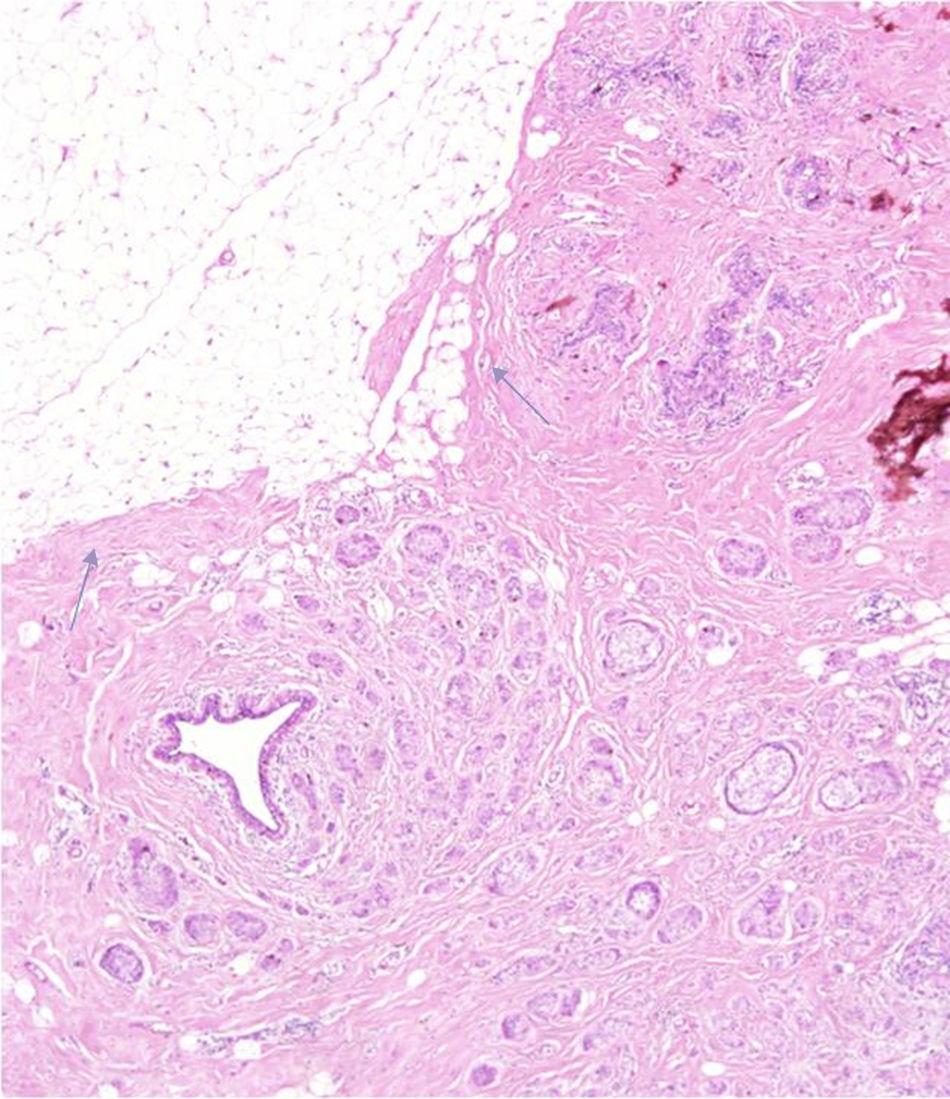
Finally, the tumor margins of the two (2) lesions were intervened without prior chemotherapy. Both were moving masses that did not infiltrate the adjacent breast parenchyma (Fig. 3). Lesions were surrounded by fibrous tissue, or benign proliferative changes (sclerosing adenosis) without direct contact with neighboring fat.
DiscussionThe TS is a modality that, when combined with mammograms, contributes to the detection and characterization of breast cancer. However, this modality has some limitations, and some lesions still do not differentiate breast parenchyma from the rest. According to the medical literature, the rate of false negatives in conventional mammograms goes from 8 to 10 per cent up to 35 per cent,8,9 and some of the causes may be high breast density; a poor technique; perception errors; or incorrect interpretations, among others.10 One of the great contributions of TS is that it helps reduce the effect of structure overlapping, improves visualization and reveals new findings.4 Yet despite all these advantages, in our series we found a small percentage (4 per cent) of false negative lesions in the DM and the TS in the group of symptomatic patients after ruling out all possible interpretation errors, and technical mistakes due to our study inclusion criteria.
When it comes to breast density, 57 per cent of the lesions occurred in high-density breasts, and the remaining ones in low-density breasts (type b). This was probably due to the fact that there are several factors that may have influenced tumor visualization, such as the presence of a fat plane separating the lesion from the neighboring parenchyma11; the location of the tumor in an area of higher density with respect to the rest of the parenchyma that eclipses the lesion12; or the presence of other signs of suspicion associated with the lesions, such as distortions or microcalcifications, that are necessary for their identification.11,13 Not a single carcinoma showed other signs suggestive of malignancy, and 86 per cent of the masses were undistinguishable from breast tissue in the DMs and TS, with BI-RADS 4 semiological data in the ultrasound scan and the MRI. In our own experience, small size would not be a characteristic of false negatives in DMs and TS with associated clinical presentations, since the size of most of these false negatives (57 per cent) was ≥3cm, and all cases (except for one lesion only detected through an MRI) were clinically evident.
With respect to the anatomopathological findings, all neoplasms of our series were infiltrating ductal carcinomas (most hidden carcinomas were poorly or moderately differentiated). With respect to the molecular classification, only one (1) lesion was categorized as a triple negative carcinoma and the rest were luminal neoplasms (most luminal B tumors), something that we also saw in our series.14 This finding would indicate that, when it comes to immunophenotyping, neoplasms misdiagnosed in the DM and the TS would have worse prognosis.15
Our study has some limitations such as the low number of hidden lesions, which makes it hard to draw definitive conclusions, the group selected for the analysis (symptomatic neoplasms where the findings reported could only be compared to this group), and the type of study (retrospective, without case follow-up).
In sum, false negative symptomatic carcinomas in the DM and TS of our small series were basically masses; all lesions were diagnosed as infiltrating ductal carcinomas (predominantly immunophenotype luminal B tumors); and they were identified in breasts with densities types b, c, and d. We need further studies to specifically analyze the impact of false negatives in diagnostic and screening programs of breast cancer that use digital mammograms and TS, with a special attention to their possible causes.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Authors- 1.
Manager of the integrity of the study: PMAA, CRC.
- 2.
Study idea: PMAA, CRC, JRM, MPSCGC, LMCH.
- 3.
Study design: PMAA, CRC, JRM.
- 4.
Data mining: PMAA, JRM, MPSCGC, LMCH.
- 5.
Data analysis and interpretation: PMAA, CRC, JRM.
- 6.
Statistical analysis: PMAA, CRC, JRM.
- 7.
Reference: PMAA, MPSCGC, LMCH.
- 8.
Writing: PMAA, CRC, JRM.
- 9.
Critical review of the manuscript with intellectually relevant remarks: PMAA, CRC, JRM, MPSCGC, LMCH.
- 10.
Approval of final version: PMAA, CRC, JRM, MPSCGC, LMCH.
The authors declare no conflict of interests associated with this article whatsoever.
The following are the supplementary data to this article:
Video 1–2. Tomosynthesis in craniocaudal and lateral projections of the patient from Fig. 1; there are no suspicious signs or other significant findings in either of the two (2) cases.
Please cite this article as: Aguilar Angulo PM, Romero Castellano C, Ruiz Martín J, Sánchez-Camacho González-Carrato MP, Cruz Hernández LM. Caracterización de cánceres de mama sintomáticos invisibles en mamografía digital y tomosíntesis: correlación radiopatológica. Radiología. 2017;59:511–515.