Diseases of the spinal cord often have devastating consequences and imaging studies are indispensable for their diagnosis. The fundamental imaging technique to evaluate these diseases is magnetic resonance imaging of the spine. The diagnostic approach must be based on the clinical context, the time elapsed since the onset of symptoms and signs, and the imaging findings; for this reason, it sometimes necessary to broaden the study to include the brain. The first step in the diagnostic algorithm is to rule out spinal cord compression before evaluating other causes of myelopathy, which sometimes has multiple causes. This paper includes a broad review of the different diseases that can cause myelopathy, their imaging manifestations, their differential diagnoses, and diagnostic algorithms. Using an appropriate radiological approach will result in better management and prognosis of these patients.
Las enfermedades de la médula espinal tienen con frecuencia consecuencias devastadoras y su estudio radiológico es indispensable para su diagnóstico. La técnica de imagen fundamental para su valoración es la resonancia magnética espinal y el enfoque diagnóstico debe basarse en el contexto clínico, el tiempo de evolución y los hallazgos radiológicos, por lo que es necesario en algunos casos la ampliación del estudio a la región cerebral. El primer paso en el algoritmo diagnóstico debe ser excluir la compresión medular antes de valorar otras causas de mielopatía, entre las que se incluyen múltiples etiologías. Este artículo incluye una amplia revisión de las diferentes patologías que pueden producir mielopatías, sus manifestaciones radiológicas, diagnósticos diferenciales y algoritmos diagnósticos. Un adecuado enfoque por parte del radiólogo repercutirá en un mejor manejo y pronóstico de estos pacientes.
The approach to non-traumatic spinal disease is a common diagnostic challenge in clinical practice. Magnetic resonance imaging (MRI) is the first-line radiological examination in the study of most of these processes and should be performed in every patient with an acute/subacute or progressive spinal syndrome of non-traumatic origin.
Despite comprehensive imaging and laboratory testing, the origin of 16%–30% of myelopathies cannot be determined. This is due to the difficulty involved in taking histology samples and the fact that clinical and radiological presentation is sometimes unspecific.
Moreover, MRI may not show pathological findings in some patients with signs and symptoms of acute myelopathy. In these cases, clinicians should consider whether the symptoms might actually be due not to myelopathy but rather to impairment of the brain or peripheral nervous system (e.g. Guillain-Barré syndrome). Some cases merit consideration of a repeat MRI of the spine after a certain period of time, since MRI may show no abnormalities early in the course of some diseases. In addition, in some patients with a normal MRI of the spine, an intercurrent process such as trivial trauma or viral infection may decompensate pre-existing undiagnosed long-evolving myelopathy (e.g. Friedreich’s ataxia) and cause a pseudoacute clinical presentation.1
The radiologist plays an essential role in narrowing down the differential diagnosis and guiding the proper management of these patients. In many cases, early treatment may result in complete or partial resolution of symptoms. To this end, approaches from two different and complementary perspectives must be taken: according to the clinical picture and suspected aetiology and according to the distribution and uptake of lesions on MRI.2 This review examines these two diagnostic approaches and covers a broad spectrum of non-traumatic spinal disease originating in either the spinal cord or the meninges.
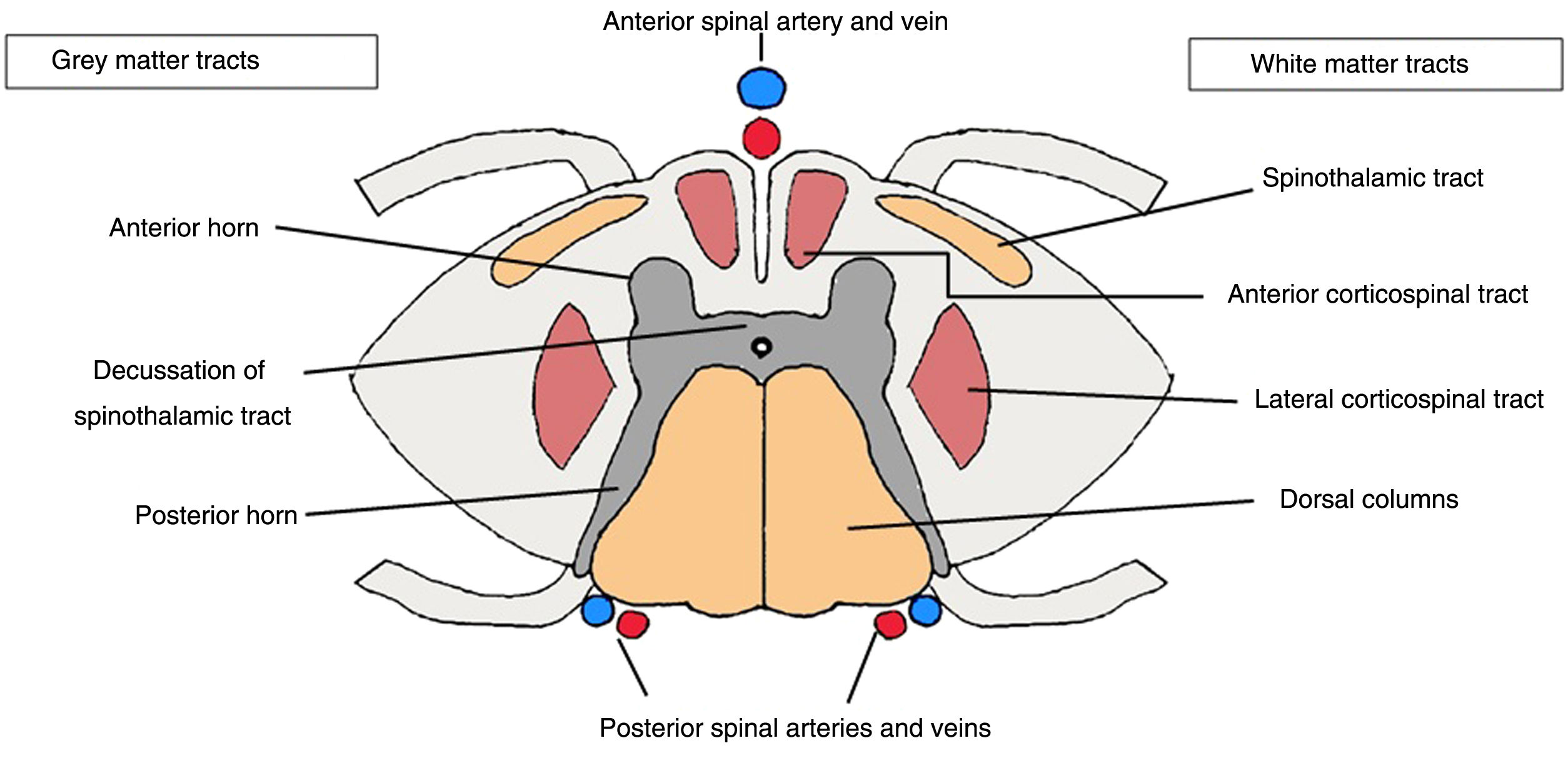
Anatomical reviewKnowledge of spinal anatomy is essential in assessing myelopathies, as certain diseases are associated with a characteristic topographic distribution responsible for a typical clinical presentation.
The spine, like the brain, is covered with three layers of meninges: the dura mater, the arachnoid mater and the pia mater. The spine contains white matter in the ascending and descending myelinated fibres, including: the anterior spinothalamic tract, with thermalgesic sensory fibres; the gracile and cuneate fasciculi (tract of Goll and tract of Burdach, respectively), in the posterior columns with the ascending fibres for vibratory and proprioceptive sensitivity; and the descending corticospinal tracts, with two components — the lateral component, responsible for fine motor control, and the anterior component, which controls voluntary movement. The central grey matter is comprised of anterior horns, containing motor neurons that synapse with the descending corticospinal tract, and posterior horns, comprised of sensory neurons that synapse with the ascending sensory fibres3 (Fig. 1).
Magnetic resonance imaging (MRI)Assessment of the spinal cord by MRI is complicated by the fact that the spinal cord is a small mobile structure in proximity to structures likely to cause artefacts such as the heart and great vessels. The usual protocols in the spinal cord include a combination of T1- and T2-weighted sequences on the axial and sagittal planes. The most commonly used T1-weighted sagittal sequences are spin echo (SE) and fast spin echo (FSE) sequences.4
Other commonly used sagittal sequences are short tau inversion recovery (STIR) sequences which, due to their additive strengthening of T1- and T2-weighted sequences, offer greater sensitivity and contrast in spinal cord lesions compared to conventional T2-weighted sequences.5,6
On the axial plane, one of the most commonly used sequences for evaluating both demyelinating lesions and post-traumatic damage is the gradient echo (GE) T2-weighted sequence.4,7 However, these sequences, as they lack refocusing pulses like SE sequences, are more susceptible to magnetic field heterogeneities. Different fat suppression techniques are also used on an axial plane (spectral presaturation with inversion recovery [SPIR], spectral attenuated inversion recovery [SPAIR], Dixon techniques and water excitation).4
Diagnostic approach according to clinical course and lesion topographyIn the clinical presentation, two points are key: the nature of the onset of symptoms and the clinical course8:
- •
Hyperacute onset (minutes to hours): characteristic of myelopathies of vascular origin.
- •
Acute or subacute onset (days to 2–6 weeks): includes infectious, autoimmune, inflammatory and metabolic diseases and vascular malformations.
- •
Chronic or progressive (more than 6 weeks): myelopathies of mechanical, metabolic (e.g. vitamin B12 deficiency), chronic infectious and tumoural origin.
Signs and symptoms, for their part, may be stable or fluctuating; the latter are characteristic of multiple sclerosis (MS).
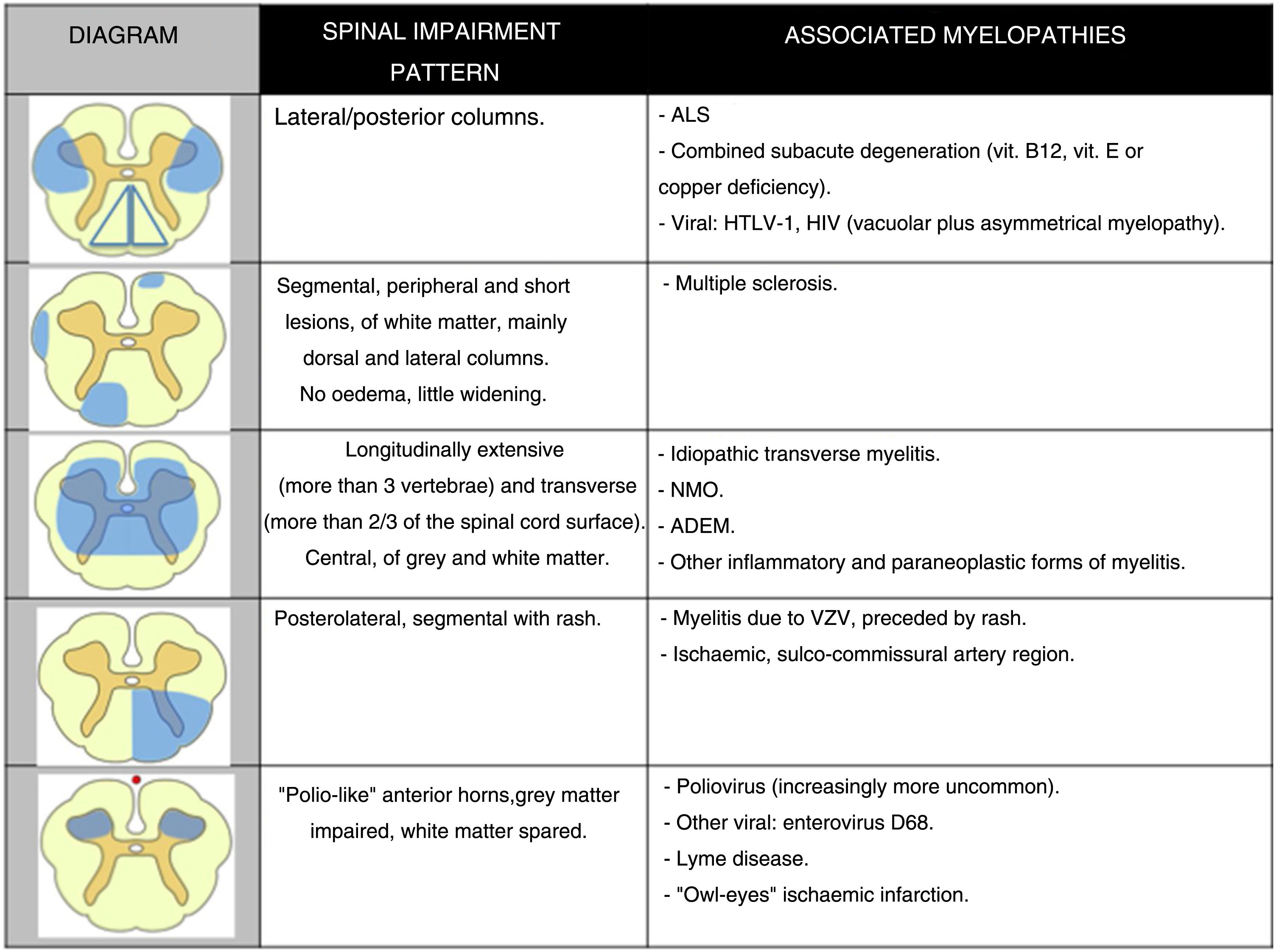
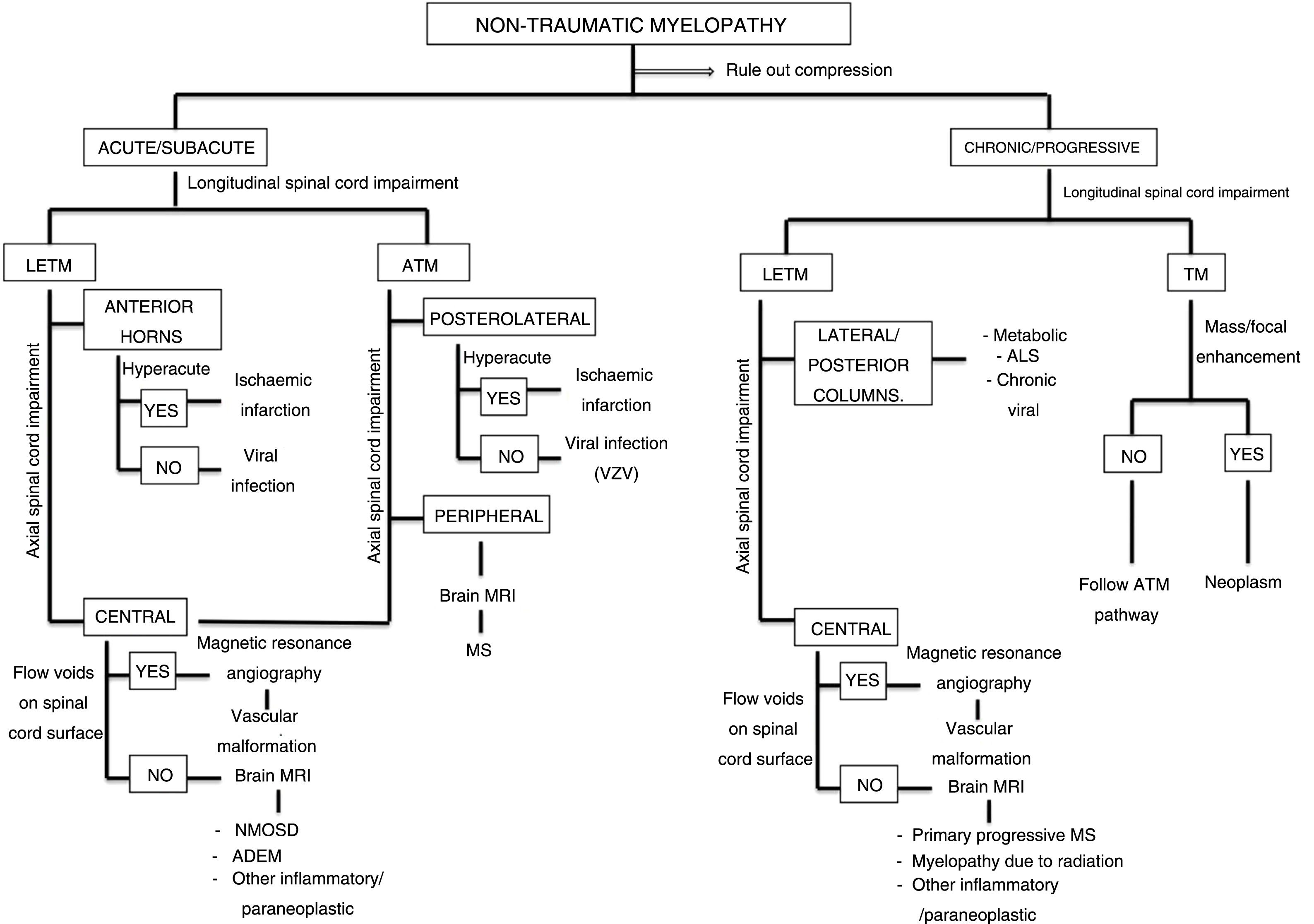
Patterns of topographical impairment of the spine on the axial plane may be divided into 5 groups (Fig. 2). These patterns, together with the longitudinal extension of spinal impairment, clinical correlation and results of other complementary tests, inform the diagnostic approach in a patient with non-traumatic myelopathy (Fig. 3).9
Algorithm for diagnostic approach to non-traumatic myelopathies.
ADEM: acute disseminated encephalomyelitis; ALS: amyotrophic lateral sclerosis; ATM: acute transverse myelopathy; LETM: longitudinally extensive transverse myelitis; MS: multiple sclerosis; NMOSD: neuromyelitis optica spectrum disorder; TM: transverse myelopathy.
This represents the most common cervical myelopathy in patients over 55 years of age.10 Osteophytes, disc degeneration and calcification of the posterior longitudinal ligament may compress the ventral portion of the spine, and impairment of the ligamentum flavum (yellow ligament) may affect the dorsal portion. Uncoarthrosis and facet joint osteoarthritis contribute to spinal canal stenosis and spinal compression. One of the most common presentations is progressive gait dysfunction, which may be accompanied by sensory and motor deficits in a radicular distribution.11
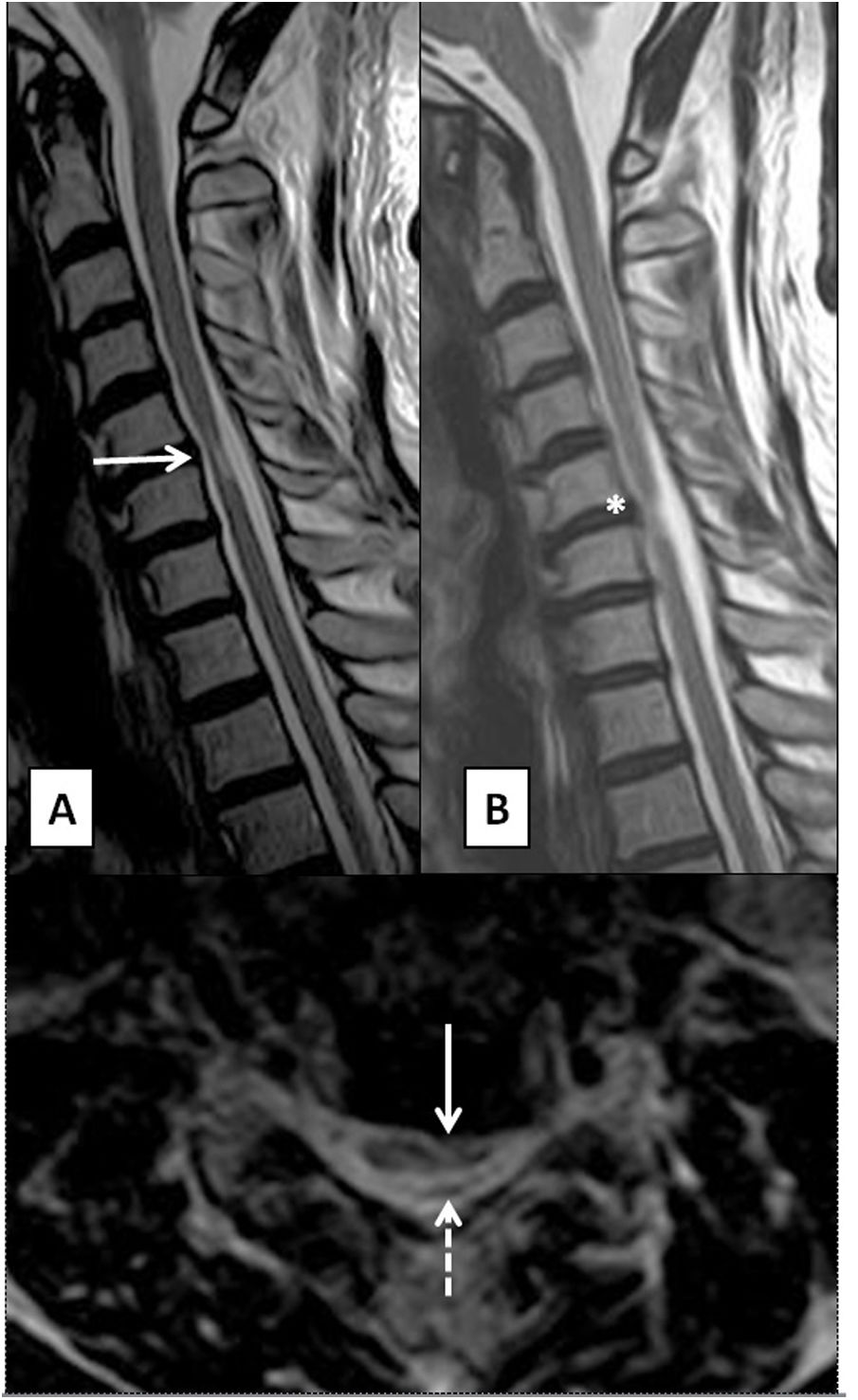
MRI is the diagnostic test of choice. Signs indicative of myelopathy are an anteroposterior spinal canal diameter less than 10 mm and intramedullary areas of T2 hypersignal with poorly defined margins in the acute phase, probably due to oedema and demyelination, which may be reversible following decompression. Well-defined T2 hypersignal, impairment of multiple segments and a weak T1-weighted signal due to necrosis and cavitation would indicate irreversible damage and a worse prognosis11 (Fig. 4). A classification of spinal impairment according to T2-weighted axial sequence findings has been proposed: type 1, featuring extensive, poorly defined impairment; type 2, featuring focal impairment with a “snake-eyes” or “owl-eyes” appearance and poorly defined margins; and type 3, featuring well-defined focal impairment. The type 2 pattern is correlated with a worse prognosis than the type 1 pattern.12 Reportedly, following administration of intravenous contrast, there is a characteristic ring-shaped pattern of enhancement on the axial plane, not involving grey matter, with a transverse, caudal distribution in the area of greatest stenosis.10
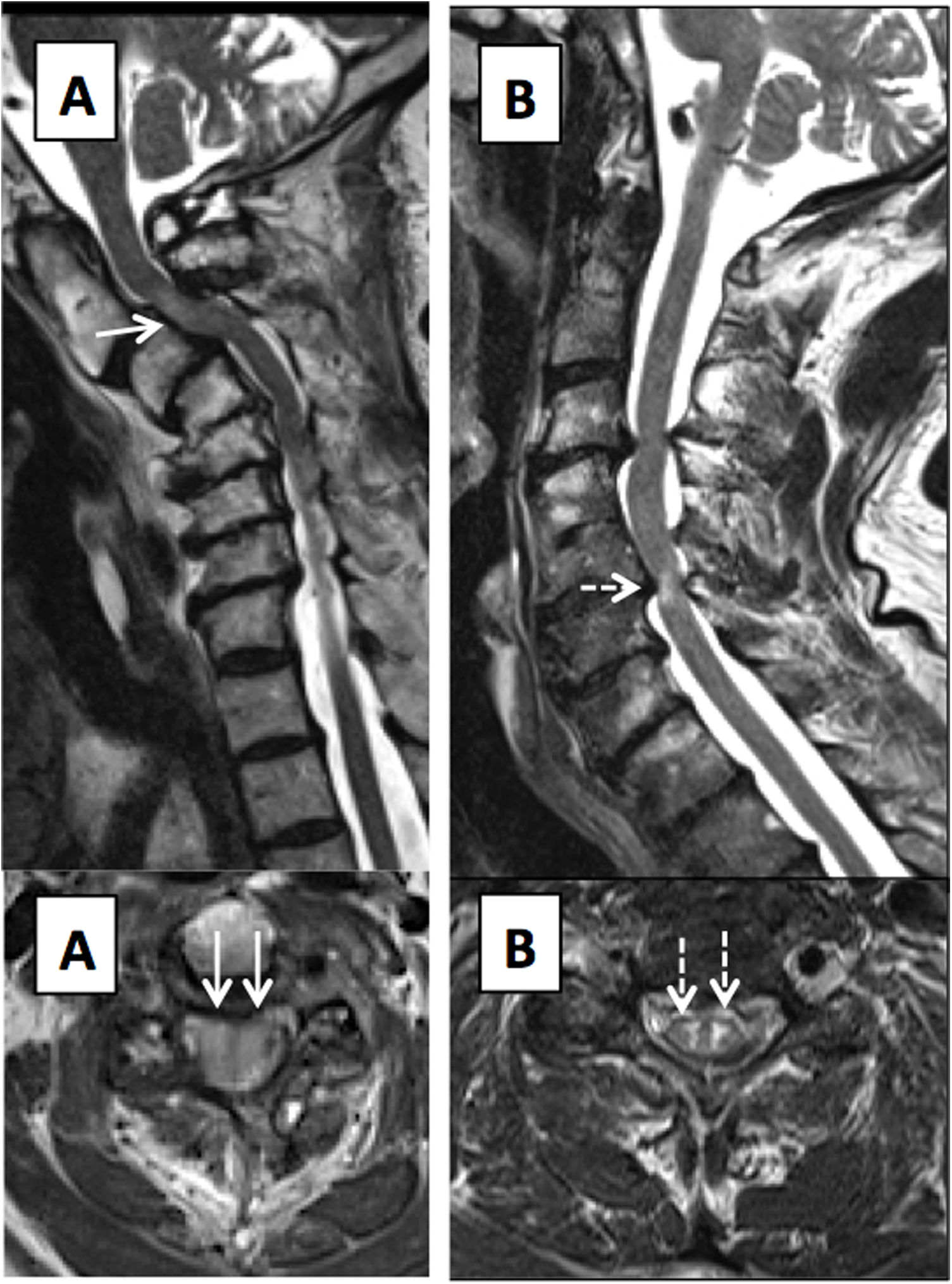
T2-weighted images on sagittal and axial planes of cervical spine. (A) Multisegmental degenerative disc disease in a patient with monoparesis of the right arm, showing spinal stenosis and a diffuse, poorly defined T2 hypersignal of the spinal cord, in relation to acute/subacute compressive myelopathy (solid arrows). (B) Patient with an area of compressive myelopathy, with foci of marked, well-defined T2 hypersignal in the central grey matter, causing an “owl-eyes” appearance (dashed arrows) and suggesting irreversible damage.
Dorsal myelopathy due to spondylosis is less common; the inferior segments are most often affected.
B. Non-degenerative mechanical myelopathy- •
Hirayama disease or monomelic amyotrophy, is a rare disease, characteristically in young men, of typically insidious onset with unilateral and asymmetric impairment from C7 to T1. The aetiopathogenesis is unknown, but it is believed that insufficient growth of the dura mater leads to a less flexible dural sac incapable of accommodating movements, thereby causing anterior dural displacement in flexion which compresses the spinal cord and results in progressive damage to the anterior horn.13 MRI in flexion is the test of choice for demonstrating detachment of the posterior dura mater, although this may be a late finding or the patient may not be capable of assuming this posture. Several signs enabling a diagnostic approach identified in a neutral position have been reported: asymmetric spinal atrophy in the low cervical region, abnormal cervical curvature and non-attachment of the dural sac to the adjacent lamina14 (Fig. 5).
Figure 5.T2-weighted images on sagittal and axial planes of the cervical spine in a neutral position (A) and flexion (B) in a 47-year-old male patient with electromyographic signs of active denervation, with loss of motor units at C7, left side, consistent with Hirayama disease. Asymmetrical atrophy of the low cervical spine (arrows), anterior displacement of the spine in flexion (asterisk) and non-attachment of the dural sac to the posterior lamina (dashed arrow) are seen.
- •
Spinal meningeal cysts represent a rare cause of spinal cord compression. They are largely idiopathic, but may be associated with neural tube defects or acquired causes such as a complication of surgery, a complication of infection or bleeding.15–17 These cysts may be either intradural or extradural, and most of them are located in thoracic segments posterior to the spinal cord, compressing it in an anterior direction.15,16 Symptoms are related to compression of the spine or nerve roots. In some cases, bladder or intestinal dysfunction may be present. MRI permits delimitation of these extramedullary cystic lesions which present a thin wall and a signal similar to the cerebrospinal fluid (CSF) and may be associated with bone remodelling.16 A myelogram by MRI determines the connection to the subarachnoid space, which is useful in surgical treatment.
- •
Idiopathic spinal herniation through a dural defect affects the thoracic spine and may be associated with myelopathy and syringomyelia. It is a rare disease that usually presents as progressive spinal cord hemidissection (Brown-Séquard syndrome), given that the hernial orifice is usually ventrolateral.18 MRI is the technique of choice for its diagnosis, showing a fold and thinning of the spinal cord, accompanied by enlargement of the posterior subarachnoid space. The absence of posterior cysts may be confirmed with a myelogram by computed tomography (CT) or MRI including 2D cine phase-contrast sequences.19
- •
The arachnoid membranes are bands extending to the pial surface of the thoracic spine in the middle levels and are very difficult to identify on MRI. They cause flattening of the posterior contour of the spinal cord reminiscent of a scalpel, such that the corresponding radiological sign, which is pathognomonic, is called the “scalpel sign”.20 Their incidence is unknown; they represent a disease of an underestimated incidence with variable signs and symptoms: some patients have few symptoms whereas others present spinal cord compression. MRI may show an intramedullary T2 hypersignal secondary to myelopathy and syringomyelia, probably due to associated altered CSF flow dynamics.21
It is important to diagnose these diseases, since they cause myelopathies that are potentially reversible with early surgical treatment depending on the mechanism of compression (Fig. 6).
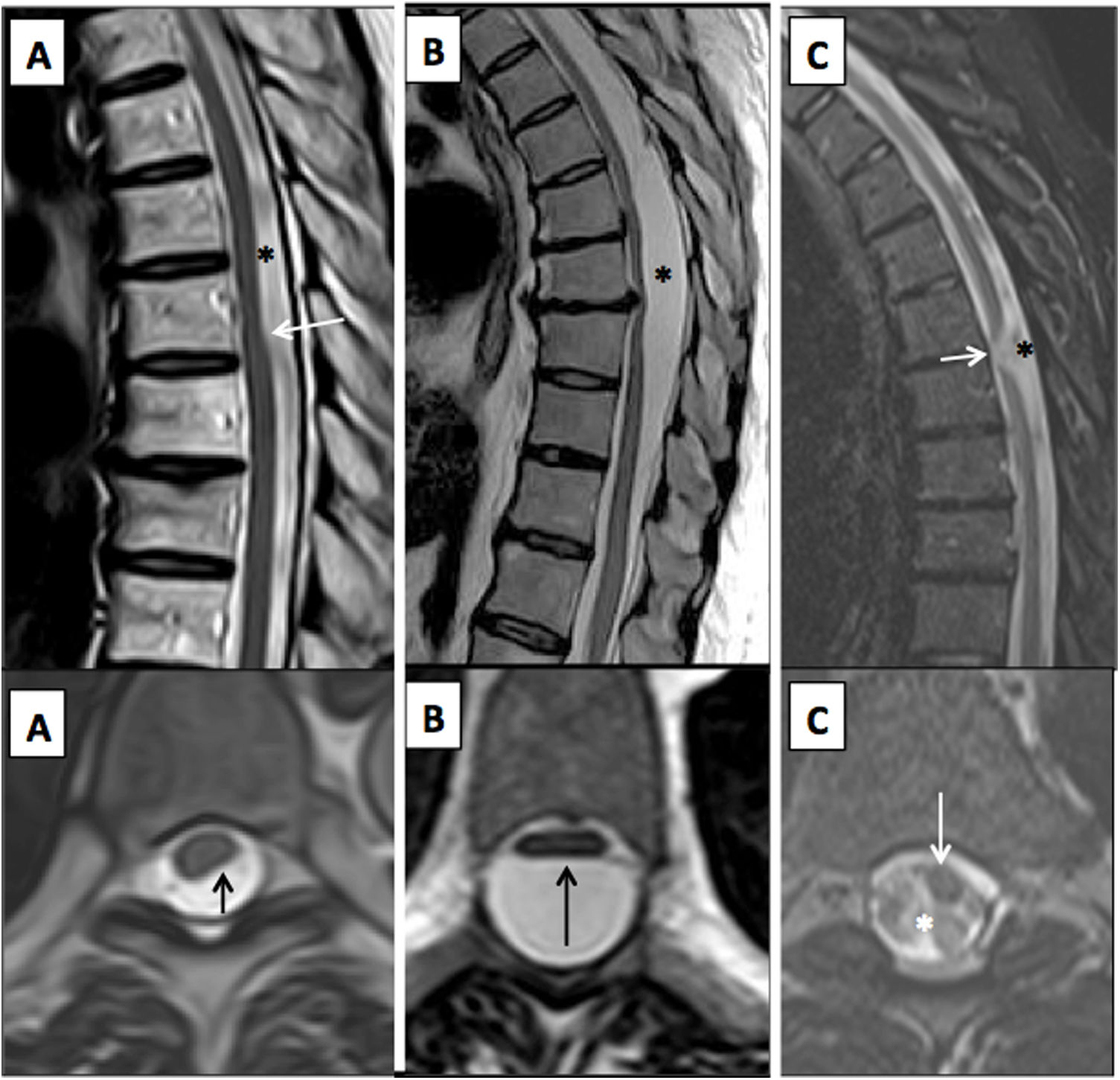
TSE and STIR T2-weighted images on the (superior) sagittal plane and T2-weighted images on the (inferior) axial plane of the thoracic spine in patients with mechanical myelopathy. A) Arachnoid band with posterior spinal cord compression, i.e. the “scalpel sign” (arrows), and a posterior CSF flow artefact indicating free circulation (asterisk). (B) Posterior intradural arachnoid cyst with spinal cord compression (arrow) and no CSF flow artefact inside (asterisk). (C) Idiopathic spinal cord herniation due to an anterior dural defect, with spinal cord angulation and deformity (arrows) and posterior increase in CSF associated with a larger flow artefact (asterisk).
To diagnose these, it is essential to consider epidemiological factors (immunosuppression, geographical considerations, etc.), time since onset, signs and symptoms and laboratory findings. MRI of the spinal cord is the imaging test of choice and an additional MRI of the brain should be performed to assess the patient for any impairment thereof.22
A. Viral aetiologyThis is the most common cause of infectious myelitis, although it is uncommon and usually underdiagnosed23 (Fig. 7).
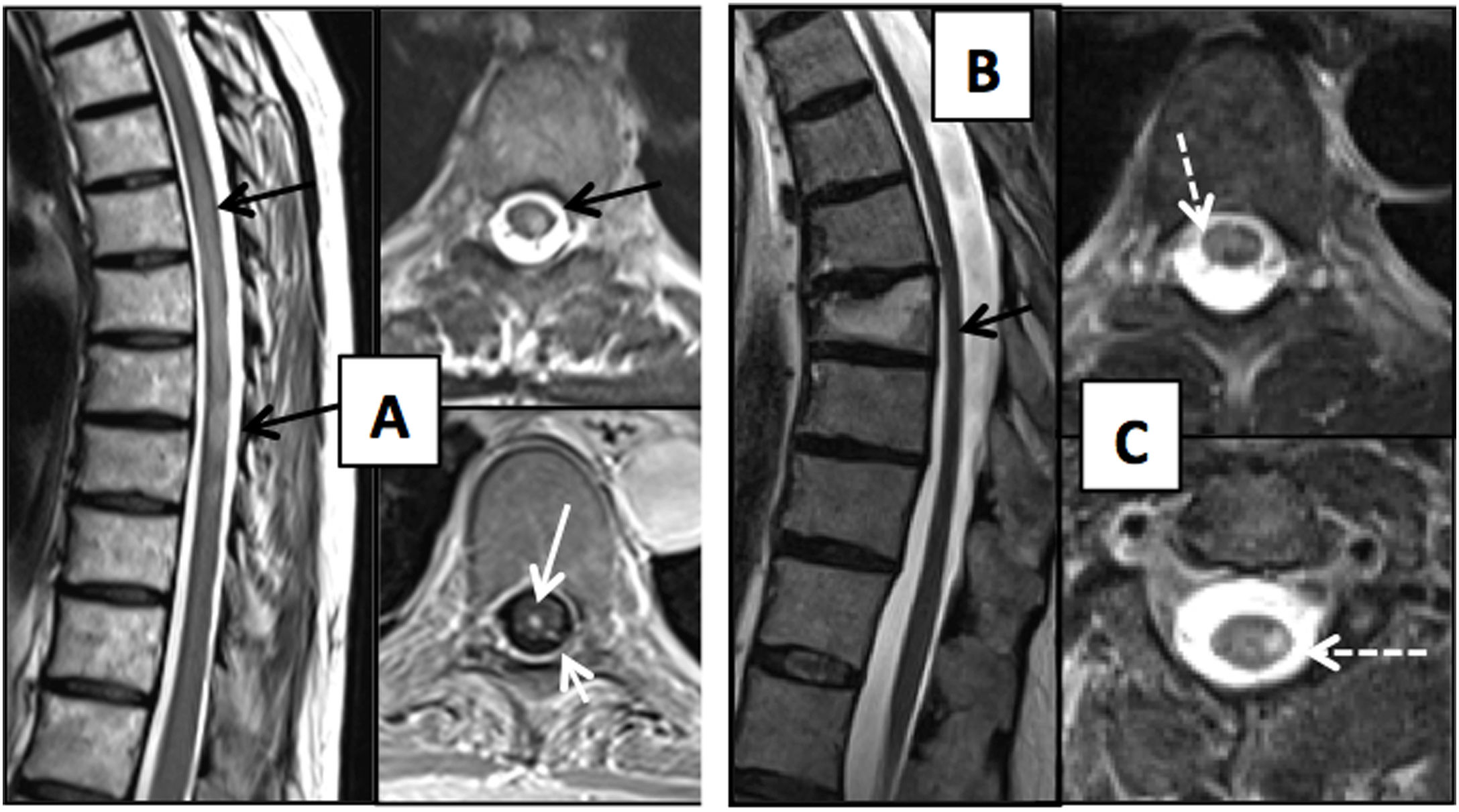
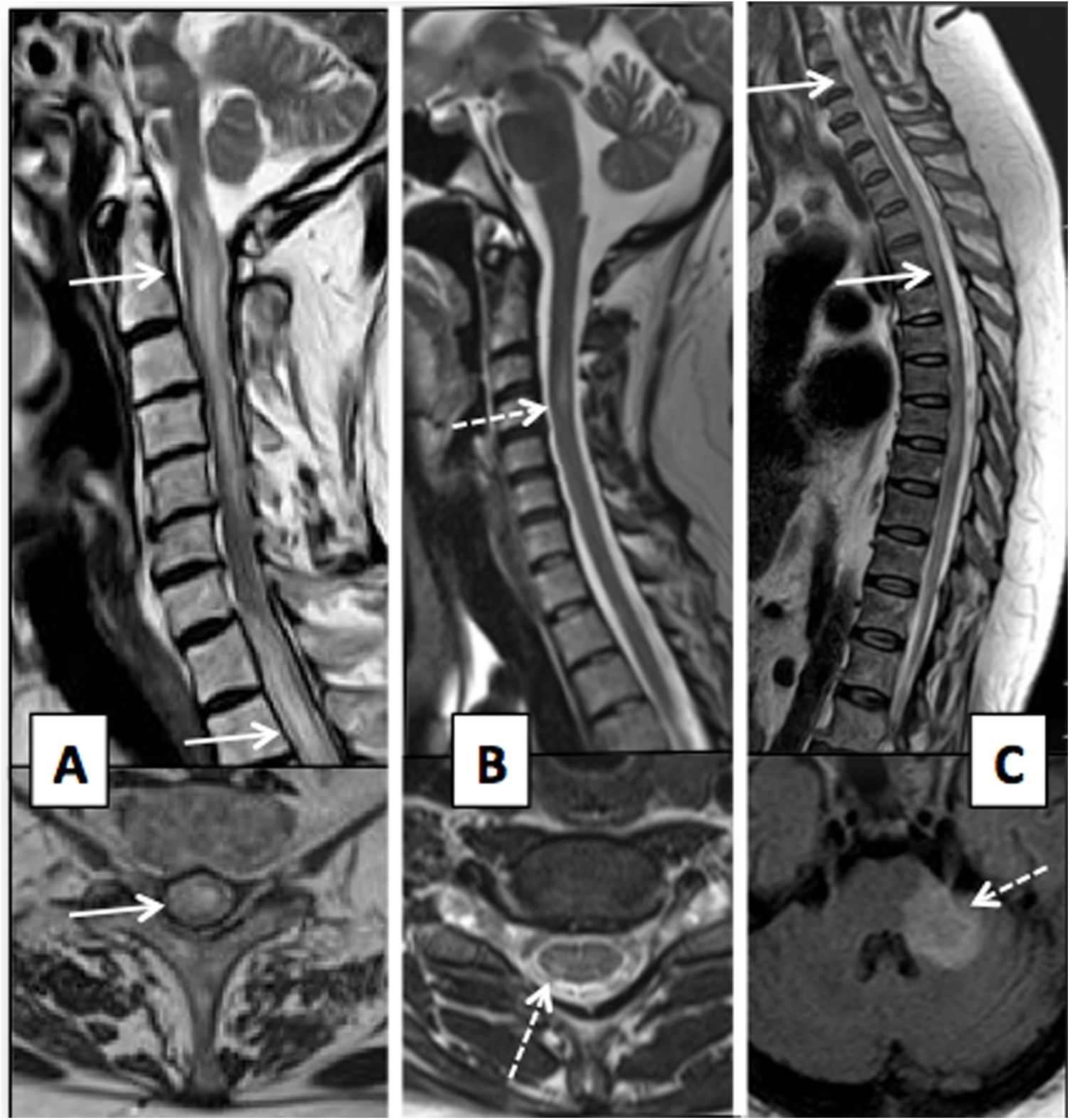
(A) Patient with thoracic spine impairment due to varicella-zoster virus, days after skin impairment. T2-weighted images on axial and sagittal planes and T1-weighted axial sequence with intravenous contrast. Extensive impairment of the posterior and left lateral spinal cord is seen with T2 hypersignal (black arrows) and enhancement with contrast measuring a millimetre of the central spinal cord and left dorsal roots (white arrows). Two patients with HIV (B and C) are shown; one of them (B) has mild spinal cord atrophy with a dorsal predominance (black arrow) and the other (C) has vacuolar myelopathy showing impairment of the lateral columns of the spinal cord on the T2-weighted image on the axial plane (dashed arrows).
- •
Herpesviruses. Varicella-zoster virus (VZV) is among the most common causes of infectious myelitis.22 It is caused by reactivation of the virus, latent in the dorsal root ganglia, which reaches the spinal cord through the dorsal root, affecting the horns and the posterior columns. Clinically, it is characterised by asymmetrical weakness and abnormal thermalgesic sensitivity, following a skin rash. MRI shows T2 signal hyperintensity and enhancement of the horns and posterior columns on the same side as the skin lesions, sometimes accompanied by thickening and enhancement of the affected root.23 Within this group, herpes simplex virus type 2, Epstein–Barr virus and cytomegalovirus (CMV) can also cause myelitis.
- •
Enteroviruses. These present a special tropism for the motor neurons of the anterior horns of the spinal cord, causing acute flaccid paralysis. This group includes poliovirus, Coxsackie virus and enteroviruses (EVs) D68 and 71. Myelitis due to poliovirus causes a unilateral or bilateral signal abnormality in the anterior horns of the spinal cord that persists in the chronic phase. Its incidence has decreased greatly thanks to vaccination. EVs D68 and 71 are emerging pathogens that have caused epidemics in the paediatric population, including in countries such as Spain. EV 71 generally affects infants and only affects the central nervous system (CNS) in some cases, causing rhombencephalitis with or without myelitis and even isolated myelitis. EV D68 affects somewhat older children, especially those with asthma. MRI shows a pattern of impairment similar to myelitis due to poliovirus.24
- •
Human T-lymphotropic virus 1 (HTLV-1) is endemic in sub-Saharan Africa, Japan, the Caribbean and some parts of South America. The corresponding mechanism of spinal damage is an immune response to infected lymphocytes; the thoracic spine is particularly vulnerable. The most common finding is spinal atrophy, in the chronic phase, although early phases may feature oedema and spinal cord expansion, with longitudinally extensive T2 hyperintensity in the lateral columns. MRI of the brain also usually shows white-matter lesions.22,23
- •
Human immunodeficiency virus (HIV). Vacuolar myelopathy is the most characteristic form of spinal impairment in patients with HIV infection due to the direct effect of the virus on the white matter. The most common finding is atrophy of the spine (the thoracic and possibly the cervical spine), followed by signal abnormalities with no enhancement in white matter tracts without a defined pattern or with a posterior predominance. It requires differential diagnosis with opportunistic infections such as VZV, CMV and deficiency disorders.25 Primary HIV infection may cause acute transverse myelitis, although this is very uncommon.26 Concomitant infection with HIV and HTLV-1 is believed to increase the risk of myelopathy.27
Bacterial infections of the spine are usually limited to the bone, intervertebral discs and subarachnoid, subdural or epidural spaces, and very rarely directly affect the spinal cord.28
PyogenicPyogenic infections of the spine may occur through haematogenous spread (the most common cause), direct inoculation (iatrogenic) or contiguity. The most common causal pathogen is Staphylococcus aureus. The infection spreads towards the spinal canal, leading to epidural or subdural abscesses, which cause myelopathy due to direct compression or vascular compromise. MRI with contrast distinguishes a non-epidural abscess, which shows uniform enhancement and requires conservative treatment, from an epidural abscess, which may restrict diffusion on diffusion sequences, presents peripheral enhancement and usually requires emergency surgery.29–31
Leptomeningitis is due to different pathogens depending on age group; the most common pathogens in adults are streptococci and staphylococci. Exudate is produced in the subarachnoid space; this induces myelitis and vasculitis and may cause oedema, infarction and necrosis. In the acute phase, leptomeningeal enhancement is identified. This may exhibit a linear or (nodular or patchy) focal pattern and is indistinguishable from leptomeningeal carcinomatosis. Advanced phases show evidence of arachnoiditis (loss of definition between the spinal cord and CSF, thickening of meninges and nerve roots, adhesions and loculation). Syringomyelia may occur as a result of altered CSF dynamics.29–31
Neuroborreliosis (Lyme disease)This is caused by spirochetes belonging to the genus Borrelia which are transmitted to humans by ticks from the genus Ixodes. It may cause inflammatory central spinal cord lesions, with variable longitudinal extension. A syndrome similar to poliomyelitis, with anterior horn impairment, has also been reported.22
TuberculousThe most common cause of myelopathy in patients with tuberculosis is vertebral infection or Pott’s disease, with a predominance in the thoracic spine. Usually, it starts with spondylitis anterior to the intervertebral disc and subligamentous extension that is sometimes discontinuous. It causes large paravertebral abscesses which may spread towards the epidural space and cause myelopathy. In tuberculous arachnoiditis, imaging findings are similar to pyogenic disease.32
C. Fungal aetiologyThis occurs in diabetic and immunosuppressed patients. It has imaging characteristics similar to pyogenic and tuberculous infection.30
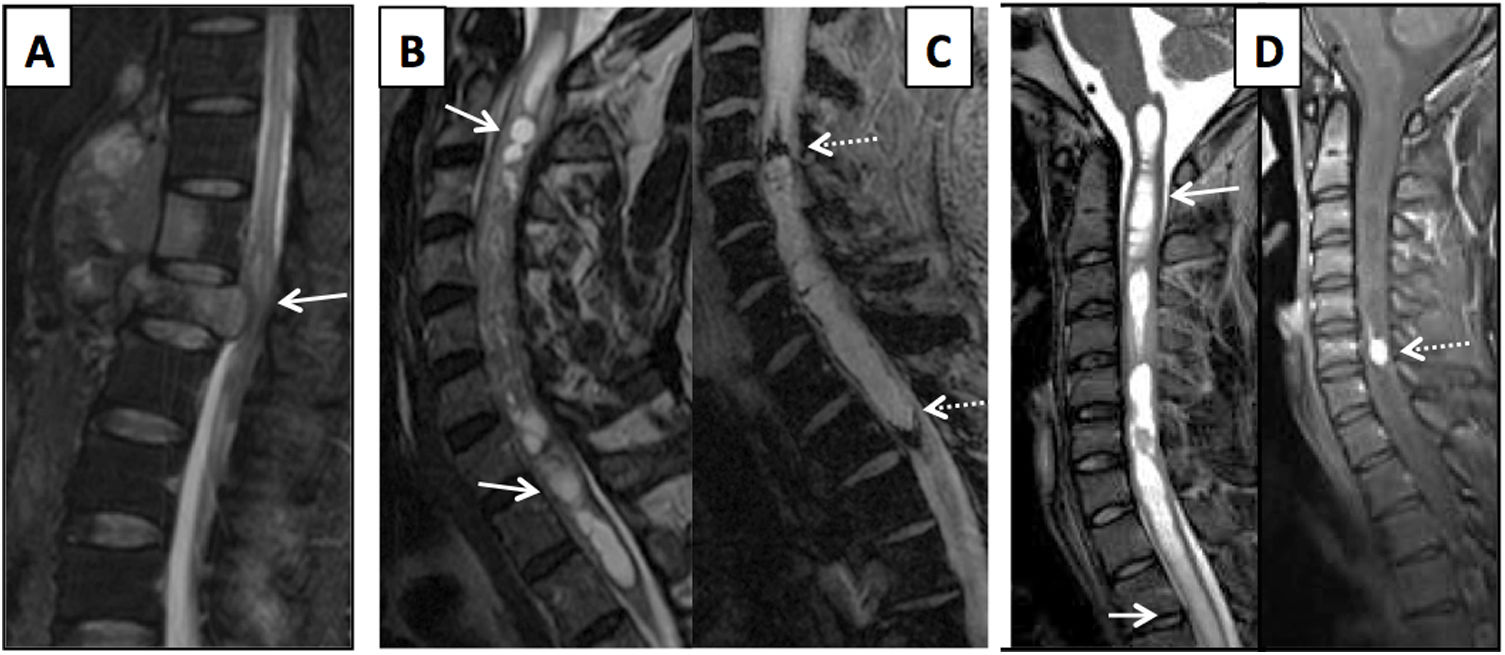
D. Parasitic aetiologyThe most common parasitic spinal infection is cysticercosis, though hydatid disease remains endemic in some rural areas of Spain. Hydatid disease is acquired by ingesting food contaminated with the eggs of the parasite (Echinococcus granulosus). Hydatid cysts have a capsule that is hypointense on T1- and T2-weighted sequences and hyperintense contents on T2-weighted sequences. They are not associated with underlying oedema and they barely enhance with contrast. Impairment is usually extradural (of the vertebra or posterior vertebral elements); intradural impairment is rare, and intramedullary impairment is extremely rare33 (Fig. 8).
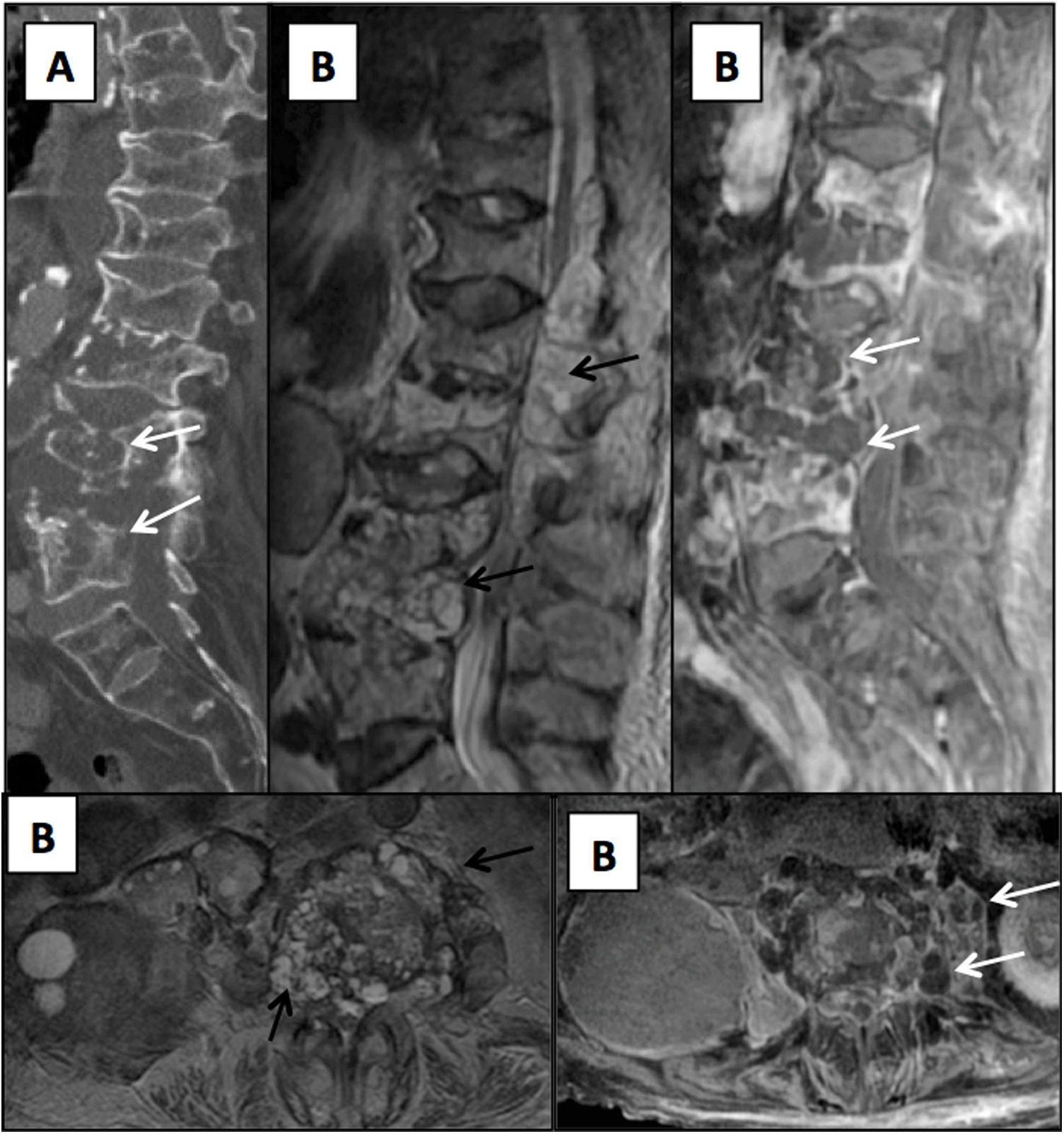
Elderly patient with a history of hydatid disease and insidious lumbar pain coursing for several months. (A) Sagittal reconstruction from a lumbar computed tomography scan showing lithic lesions in the lumbar vertebrae (arrows). (B) Magnetic resonance imaging of the lumbar spine with T2-weighted images on the axial and sagittal planes and T1-weighted images on the axial and sagittal planes with intravenous contrast. Multiloculated cystic lesions hyperintense on T2-weighted sequences are seen with impairment of the vertebrae and the epidural and paravertebral spaces (black arrows) which, following administration of contrast, show slight peripheral enhancement (white arrows).
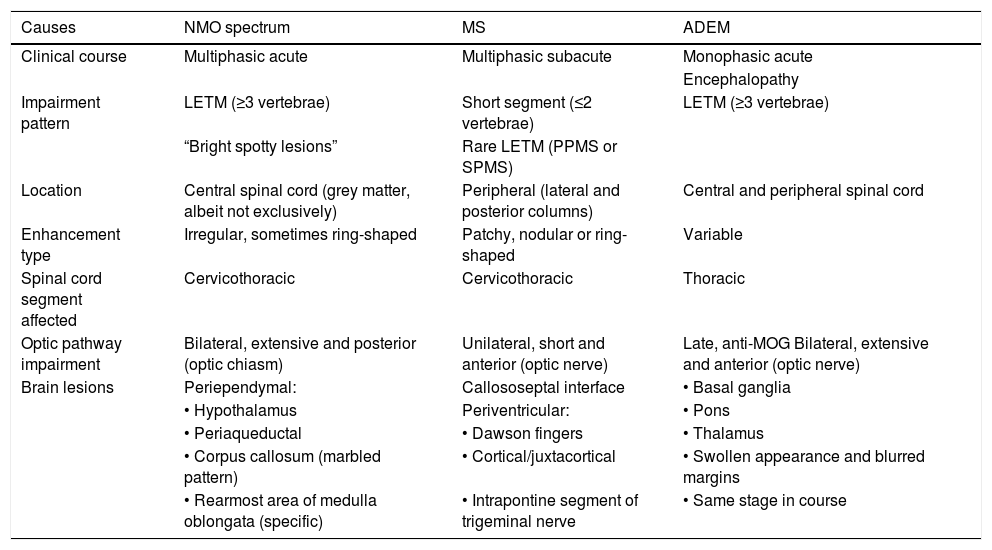
Inflammatory and demyelinating diseases affecting the spinal cord may be primary CNS diseases or diseases secondary to a systemic process. They present different patterns of impairment, both on the axial plane and in longitudinal extension. The term acute transverse myelitis (ATM) encompasses a group of diseases that cause acute focal inflammation of the spinal cord. Its incidence is low (1–8 cases per million inhabitants per year); however, it may cause serious neurological sequelae in a third of patients.34 In 2002, the Transverse Myelitis Consortium Working Group proposed a set of criteria for distinguishing idiopathic ATM from ATM of known origin.35 The inclusion criteria are: sensory, motor or autonomic dysfunction that can be attributed to the spinal cord; bilateral (though not necessarily symmetrical) symptoms; clearly defined sensory level; exclusion of compressive origin by imaging techniques; spinal cord inflammation (pleocytosis or elevated IgG index in CSF or enhancement with gadolinium on MRI) and onset of symptoms between 4 and 21 days. The exclusion criteria are: a history of spinal cord radiation in the past 10 years; clinical distribution deficit consistent with thrombosis of the anterior spinal cord; flow voids on the spinal cord surface consistent with an arteriovenous malformation; clinical or serological evidence of connective tissue disease; clinical or laboratory indicators of infection; findings suggestive of MS in an MRI of the head; and history or apparent signs and symptoms of optic neuritis. The diagnosis of idiopathic ATM requires fulfilment of all the inclusion criteria and none of the exclusion criteria. A diagnosis of ATM of known origin requires fulfilment of all the inclusion criteria and one exclusion criterion. Idiopathic ATM is the most common form of acute myelitis; however, this proportion is likely to decrease with the development of imaging, immunology and microbiology techniques.1 The term longitudinally extensive transverse myelitis (LETM) refers to a spinal lesion with craniocaudal extension of three or more vertebrae.28 The most common aetiology is neuromyelitis optica spectrum disorder (NMOSD).36 However, other diseases may also manifest as LETM, such as acute disseminated encephalomyelitis, connective tissue diseases, vasculitis, infectious diseases, MS, neoplasms, paraneoplastic processes, vascular malformations, infarctions and even nutritional deficiencies (Fig. 9). When the cause is not inflammatory, the term transverse myelopathy35 or longitudinally extensive transverse myelopathy should be used.
Inflammatory myelopathy. (A) Patient with transverse myelitis in a context of granulomatosis with polyangiitis (Wegener’s disease). Hypersignal on a T2-weighted image extending longitudinally from the bulbomedullary junction to T6 with extensive impairment on the transverse plane (arrows). (B) Patient with multiple sclerosis with cervical spine impairment. Lesions are seen to be hyperintense on T2-weighted images, longitudinally short on the sagittal image (dashed arrow) and in a peripheral distribution on the axial plane (dashed arrow). (C) Magnetic resonance imaging of the spinal cord and brain with T2-weighted and T2-weighted FLAIR images in a patient with acute-onset myelopathy (solid arrows), plus signs and symptoms of encephalopathy and evidence of brain lesions (dashed arrow). The patient was diagnosed with acute disseminated encephalomyelitis.
The characteristics of the main inflammatory myelopathies are summarised in Table 1.
Differential diagnosis of inflammatory myelopathy.
| Causes | NMO spectrum | MS | ADEM |
|---|---|---|---|
| Clinical course | Multiphasic acute | Multiphasic subacute | Monophasic acute |
| Encephalopathy | |||
| Impairment pattern | LETM (≥3 vertebrae) | Short segment (≤2 vertebrae) | LETM (≥3 vertebrae) |
| “Bright spotty lesions” | Rare LETM (PPMS or SPMS) | ||
| Location | Central spinal cord (grey matter, albeit not exclusively) | Peripheral (lateral and posterior columns) | Central and peripheral spinal cord |
| Enhancement type | Irregular, sometimes ring-shaped | Patchy, nodular or ring-shaped | Variable |
| Spinal cord segment affected | Cervicothoracic | Cervicothoracic | Thoracic |
| Optic pathway impairment | Bilateral, extensive and posterior (optic chiasm) | Unilateral, short and anterior (optic nerve) | Late, anti-MOG Bilateral, extensive and anterior (optic nerve) |
| Brain lesions | Periependymal: | Callososeptal interface | • Basal ganglia |
| • Hypothalamus | Periventricular: | • Pons | |
| • Periaqueductal | • Dawson fingers | • Thalamus | |
| • Corpus callosum (marbled pattern) | • Cortical/juxtacortical | • Swollen appearance and blurred margins | |
| • Rearmost area of medulla oblongata (specific) | • Intrapontine segment of trigeminal nerve | • Same stage in course |
MS: multiple sclerosis. ADEM: acute disseminated encephalomyelitis; LETM: longitudinally extensive transverse myelitis; NMO: neuromyelitis optica; PPMS: primary progressive MS; SPMS: secondary progressive multiple sclerosis.
This is a primary demyelinating disorder of the CNS characterised by neurological deficits in different places, spaced out over time. It is more common in women (2:1), with a peak incidence between 20 and 40 years of age. The most common form is the relapsing-remitting form, which is characterised by exacerbations followed by partial or complete recovery.37 There are three patterns of impairment of the spine31:
- 1.
Partial or incomplete transverse myelitis: the most common pattern, characterised by peripheral focal lesions occupying less than half of the area of the spine on the axial plane and primarily affecting the posterior and lateral columns. Their length usually does not exceed that of two vertebrae. They do not respect boundaries between grey and white matter and they usually do not deform the spinal column, save for some lesions which may have a swollen appearance in an acute phase (Fig. 9).
- 2.
LETM: This is rare, but somewhat more common in primary and secondary progressive forms.
- 3.
Focal or diffuse atrophy: in the chronic phase of the disease.
This disorder is typically seen in children and young adults. It is due to an autoimmune response to myelin basic protein following infection or vaccination (although the causal relationship has never been established), leading to widespread demyelination and inflammation of the brain, spinal cord and optic nerves and co-occurring with encephalopathy.38 Usually, it follows a monophasic course, although 10% of cases follow a multiphasic course, characterised by the presence of a second episode separated from the first by at least 3 months.39 Spinal lesions occur in 11%–28% of patients and are extensive, with a LETM pattern and variable enhancement31 (Fig. 9).
Neuromyelitis optica spectrum disorderThis term, coined by Wingerchuk et al. in 2015, encompasses a mixed group of demyelinating disorders of the CNS (previously known as neuromyelitis optica or Devic’s disease) linked to anti-aquaporin-4 (AQP4) IgG antibodies. AQP4 is a transmembrane water channel located in the feet of astrocytes and is highly expressed in the optic nerves, spinal cord and diencephalon. Although these antibodies have high specificity for the diagnosis of the disease and are an essential diagnostic criterion, there is a group of IgG-AQP4—seronegative patients with typical signs and symptoms of neuromyelitis optica.40,41 A minority of these seronegative patients have anti-MOG antibodies, and there is debate as to whether they are different diseases. NMOSD is uncommon. Mean age at diagnosis is 39 and there is a marked predominance in women (9:1).31,42 The most specific finding on imaging is T2 signal hyperintensity in the central spinal cord with a LETM pattern and spinal cord expansion. Some patients may not meet LETM criteria if the MRI is performed in very early stages or in late stages, as the lesions “fragment” over the course of the disorder and may have a discontinuous appearance.40 “Bright spotty lesions” are typical and show a stronger signal than CSF on T2-weighted sequences, possibly reflecting deeper damage with necrotic and microcystic changes. Enhancement is variable; in general, it is patchy or ring-shaped. In advanced stages there may be spinal cord atrophy.
Other inflammatory myelopathiesSarcoidosis is a systemic disease characterised by non-caseating granulomatous inflammation, which typically affects the lungs, lymph nodes and skin. Involvement of the CNS is very rare (25% of all patients), and spinal impairment is extremely rare (<1%). The most common finding is leptomeningeal and pachymeningeal enhancement, but other possible findings are intramedullary granulomas that enhance, associated with spinal cord expansion.43
Lupus myelopathy is very rare. The most common finding is centrally located T2 signal hyperintensity, in a long segment with variable uptake.31
Myelopathy of neoplastic originTumours may cause myelopathy due to spinal cord compression from any spinal compartment (Fig. 10). Bone metastases are the most common extradural tumours. Primary bone tumours and lymphoma can also cause compressive myelopathy.31 Extramedullary intradural tumours include nerve sheath tumours (schwannomas and neurofibromas), meningiomas, metastases, myxopapillary ependymomas (the most common tumours of the filum terminale) and paragangliomas.44
Neoplastic impairment in different patients. (A) STIR sequence on the sagittal plane showing bone metastases of lung carcinoma with myelopathy due to spinal cord compression (arrow). (B) and (C) TSE T2-weighted and T2-weighted images* in patients with spinal cord ependymomas showing these lesions’ characteristic heterogeneity, with cysts (solid arrows) and bleeding (dashed arrows) on the margins of the tumour (D). Patient with von Hippel-Lindau syndrome showing a nodular tumour with intense enhancement in the posterior region of the cervical spine (dashed arrow), associated with extensive syringomyelia (solid arrows), corresponding to a hemangioblastoma.
The most common intramedullary tumours are ependymomas, followed by astrocytomas. Together, they account for 95% of intramedullary tumours:
- •
Ependymomas: these originate in the ependyma; hence, they are initially located in the central spine. They are generally well-defined heterogeneous tumours that are often associated with bleeding and, on their margins, oedema and cysts. Enhancement is usually intense and heterogeneous, or ring-shaped.28
- •
Astrocytomas: 75% of intramedullary astrocytomas are low-grade. Unlike ependymomas, they are expansive lesions with eccentric growth and poorly defined margins.45 Bleeding is less common. A third do not enhance; those that do exhibit partial heterogeneous enhancement.28 They affect younger patients than ependymomas do.37
- •
Haemangioblastomas: these are the third most common type of intramedullary neoplasm. They are benign mesenchymal tumours of vascular origin. A patient may present a single or multiple haemangioblastomas; the latter case is generally associated with von Hippel-Lindau syndrome. These lesions enhance very intensely, are well-delimited and have a subpial location in the thoracic spine.46 They may be associated with peritumoural cysts, syringomyelic cavities and peritumoural oedema.
- •
Metastases: these are rare and occur in a context of widespread metastatic disease. Usually, they are small lesions (<1.5 cm), with enhancement and extensive oedema, located in posterolateral regions.47
This uncommon condition is estimated to account for just 1.2% of CNS infarctions.48 The associated causes are many; they are often linked to vascular or thoracic surgical procedures, aortic disease or vascular malformations.48–50
Signs and symptoms and MRI findings depend on the vessel that is damaged (Fig. 2); the anterior spinal artery is the most commonly affected vessel.48 Signs and symptoms tend to develop quickly and commence with pain in a radicular distribution,50 triggered following a Valsalva manoeuvre or movement.48
MRI shows no abnormalities in more than 50% of cases in the first 24 h.50 The inferior thoracic spine and the lumbar spine are most often affected.51 Diffusion-weighted imaging may show restriction approximately 8 h from the onset of signs and symptoms with pseudonormalisation after a week.50 Enhancement is common between five days and 3 weeks following presentation50 (Fig. 11). Infarctions associated with vertebrae constitute a finding that supports a diagnosis of medullary infarction.52
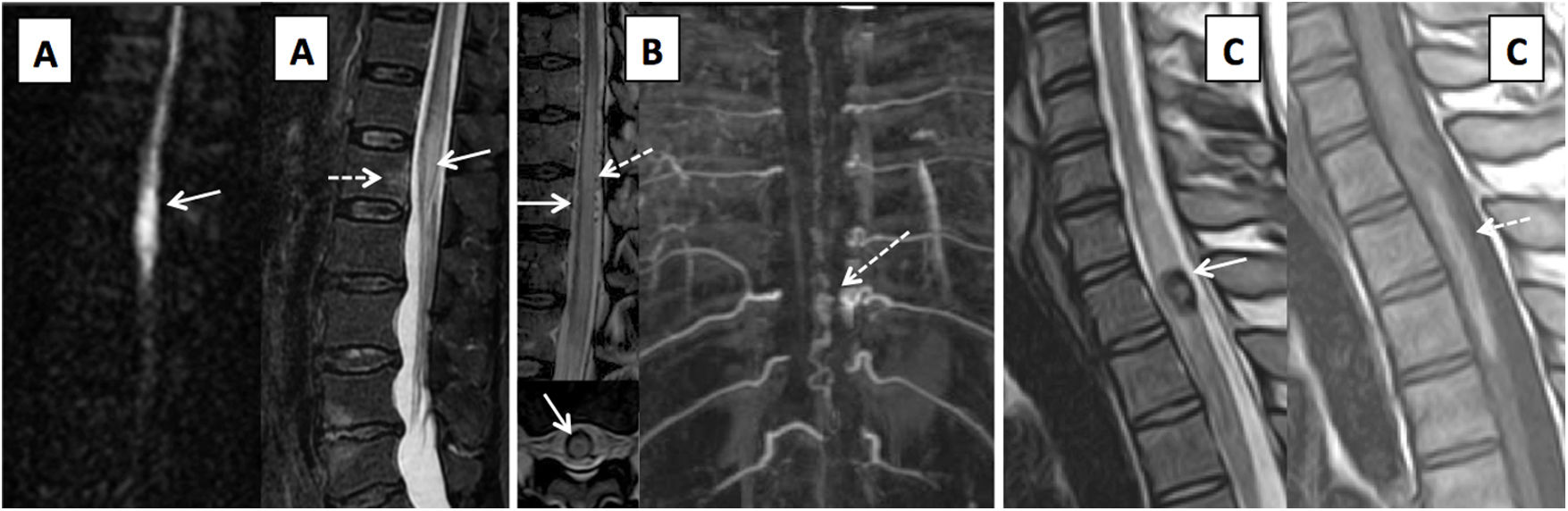
Impairment of the spinal cord vasculature. (A) Diffusion- and T2-weighted images centred in the medullary cone, showing an infarction of the cone with restricted diffusion in the acute phase and T2 hypersignal (solid arrows). Associated bone infarction (dashed arrow). (B) Type I dural arteriovenous fistula, with central spinal cord hypersignal on T2-weighted sequences (arrow) and dilated, tortuous perimedullary vessels confirmed on magnetic resonance angiography, in conjunction with the location of the fistula area (dashed arrows). (C) Patient with cavernous malformation of the spinal cord with a hypointense halo on TSE T2-weighted images (arrow), associated with spinal cord bleeding, hyperintense on the T1-weighted sequence (dashed arrow).
Cavernous malformations (CMs) of the spinal cord are uncommon, accounting for 5% of CMs in the CNS and 5%–12% of spinal vascular malformations.53,54 Most of these lesions are symptomatic.53 Imaging findings are similar to CMs in the CNS; lesions are well-defined, with a reticulated centre due to blood following a different course, a peripheral ring with T2 hypointensity due to haemosiderin deposits and no enhancement following contrast administration55 (Fig. 11).
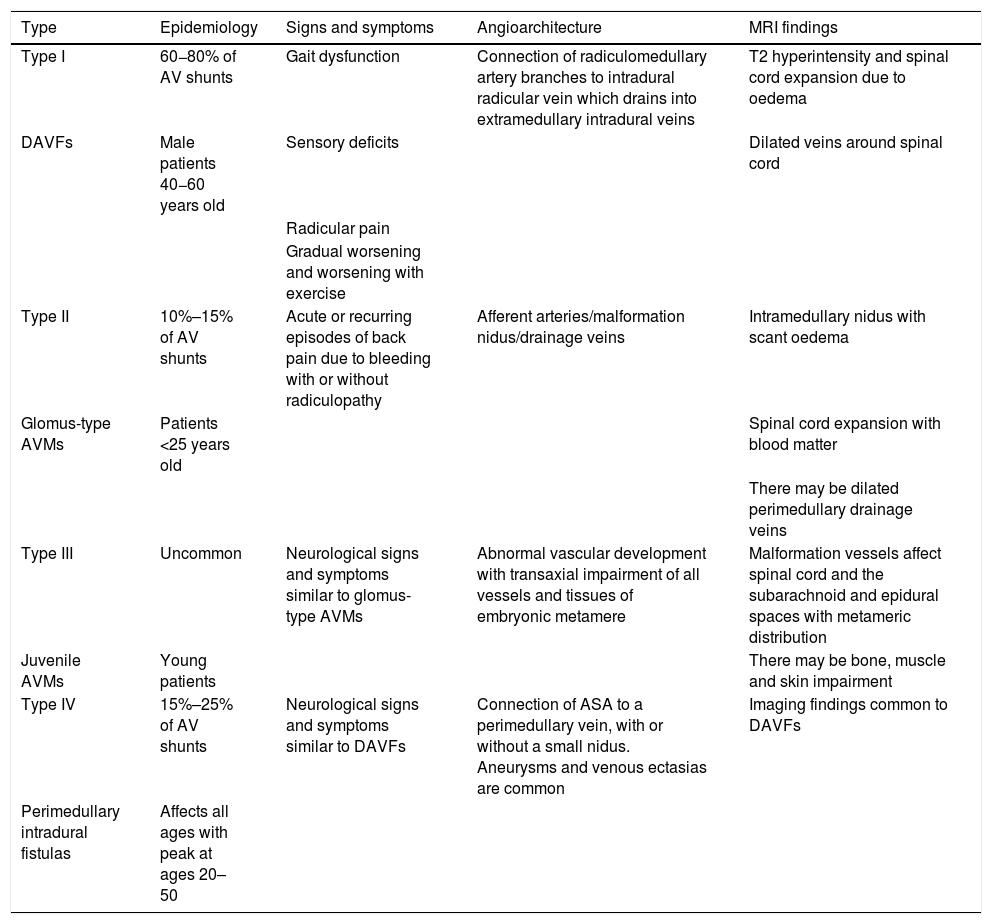
C. Spinal vascular malformationsDifferent classifications of spinal vascular malformations have been proposed. The most commonly used classification is probably that of Anson-Spetzler, which is based on angioarchitecture and enhancement pattern48,56 (Table 2) (Fig. 11).
Anson-Spetzler classification of spinal vascular malformations.
| Type | Epidemiology | Signs and symptoms | Angioarchitecture | MRI findings |
|---|---|---|---|---|
| Type I | 60−80% of AV shunts | Gait dysfunction | Connection of radiculomedullary artery branches to intradural radicular vein which drains into extramedullary intradural veins | T2 hyperintensity and spinal cord expansion due to oedema |
| DAVFs | Male patients 40−60 years old | Sensory deficits | Dilated veins around spinal cord | |
| Radicular pain | ||||
| Gradual worsening and worsening with exercise | ||||
| Type II | 10%–15% of AV shunts | Acute or recurring episodes of back pain due to bleeding with or without radiculopathy | Afferent arteries/malformation nidus/drainage veins | Intramedullary nidus with scant oedema |
| Glomus-type AVMs | Patients <25 years old | Spinal cord expansion with blood matter | ||
| There may be dilated perimedullary drainage veins | ||||
| Type III | Uncommon | Neurological signs and symptoms similar to glomus-type AVMs | Abnormal vascular development with transaxial impairment of all vessels and tissues of embryonic metamere | Malformation vessels affect spinal cord and the subarachnoid and epidural spaces with metameric distribution |
| Juvenile AVMs | Young patients | There may be bone, muscle and skin impairment | ||
| Type IV | 15%–25% of AV shunts | Neurological signs and symptoms similar to DAVFs | Connection of ASA to a perimedullary vein, with or without a small nidus. Aneurysms and venous ectasias are common | Imaging findings common to DAVFs |
| Perimedullary intradural fistulas | Affects all ages with peak at ages 20–50 |
ASA: anterior spinal artery; AV: arteriovenous; AVMs: arteriovenous malformations; DAVFs: dural arteriovenous fistulas.
Myelopathy due to vitamin B12 deficiency, subacute combined degeneration, is a rare cause of demyelination of the dorsal columns of the spinal cord57,58 with a worse prognosis in patients over 50 years of age.
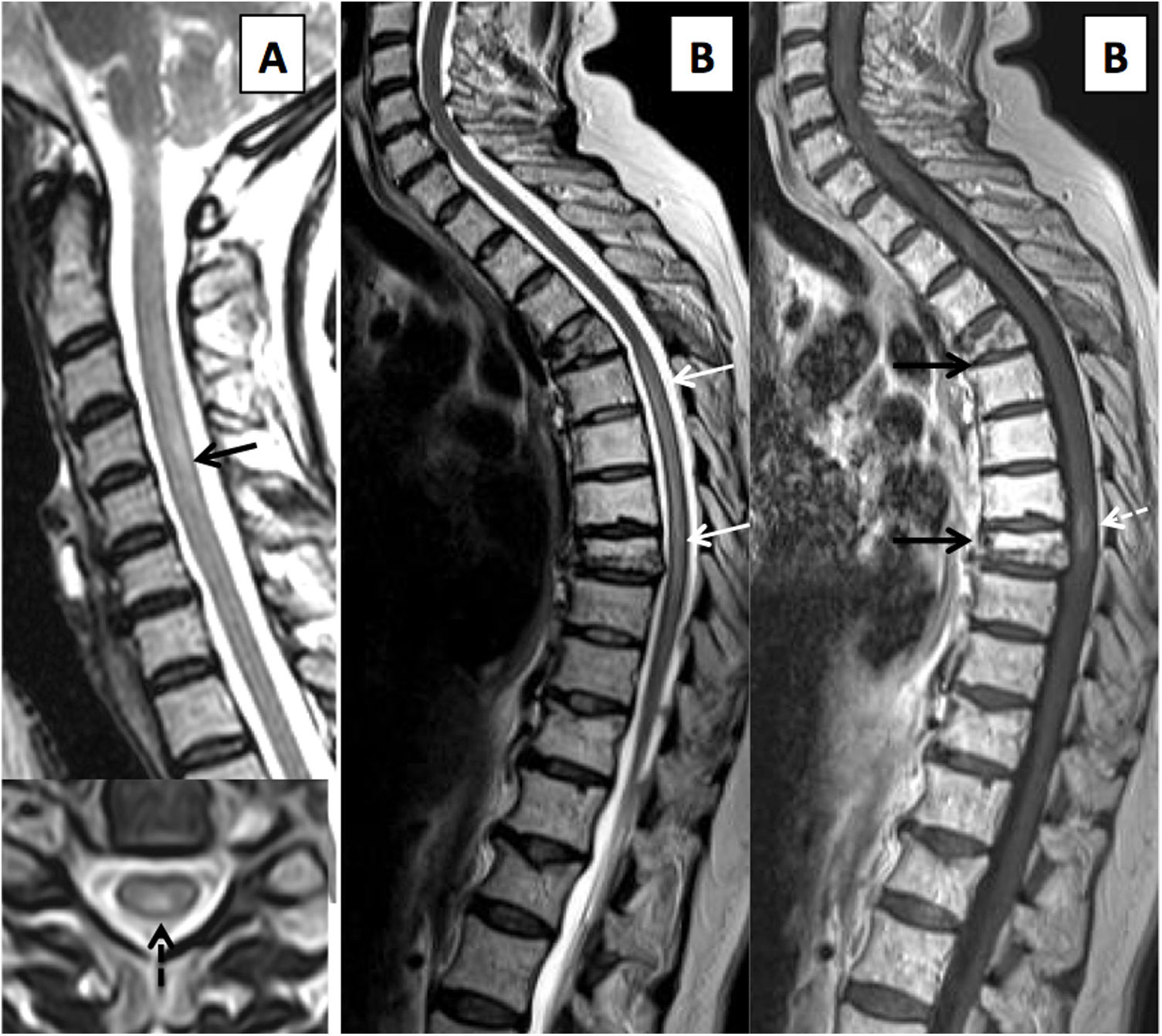
MRI identifies T2 hyperintensity affecting the dorsal columns, predominantly in the inferior cervical and superior thoracic regions57 (Fig. 12). Lesions show variable extension, and a patchy pattern of small multifocal lesions has also been reported.58 Following treatment, imaging findings may resolve completely in a small percentage of patients.57,58
Metabolic/toxic myelopathy. (A) Sagittal and axial T2-weighted images showing cervical myelopathy in a patient with vitamin B12 deficiency, presenting extensive myelopathy (solid arrows) in a symmetrical dorsal distribution (dashed arrows). (B) A woman who 12 months after completion of radiotherapy for multiple myeloma presents gait dysfunction of gradual onset. She has extensive thoracic myelitis with T2 hypersignal (solid white arrows), with a focus of post-contrast enhancement (white dashed arrow), plus diffuse fatty degeneration of the bone marrow and osteoporotic fractures in the radiated area (black arrows).
This entity is clinically and radiologically indistinguishable from copper deficiency myelopathy.59
B. Radiation myelopathyThis is a late, devastating effect of radiotherapy treatment. It is uncommon, as the spinal cord is a critical dose-limiting organ. The risk of permanent damage is 0.03%–0.2% when the total dose does not exceed 45–50 Gy in daily fractions of 1.8–2 Gy.60 However, there is still uncertainty as to spinal tolerance to stereotactic body radiotherapy, which is used increasingly more.60
Two different clinical entities are distinguished:
- 1.
Early delayed damage: this occurs following a latency period of 2–4 months and is characterised by Lhermitte's sign. MRI shows no abnormalities, and signs and symptoms largely resolve entirely.61
Late delayed damage: damage is typically irreversible and appears 18 months following treatment.59 This disease represents a diagnosis of exclusion with variable signs and symptoms.60,61 MRI shows areas of T1 hypointensity with T2 hyperintensity and usually patchy areas of uptake which histologically represent areas of demyelination, oedema and necrosis (Fig. 12). Over time, it may develop into spinal cord atrophy.60–62
ConclusionNon-traumatic myelopathies represent a diagnostic challenge. Proper imaging techniques and systematic analysis of radiological findings, together with clinical data, may aid in making a diagnosis as precise and as early as possible. This point is crucial to the reversibility of certain clinical conditions when properly treated in initial stages. MRI also permits the optimisation of management and follow-up of patients with myelopathy and sometimes contributes prognostic information.
Authorship- 1.
Responsible for study integrity: IHH.
- 2.
Study concept: IHH and ÀRC.
- 3.
Study design: IHH and ÀRC.
- 4.
Data acquisition: IHH, COG and ÀRC.
- 5.
Data analysis and interpretation: IHH, COG and ÀRC.
- 6.
Statistical processing: N/A.
- 7.
Literature search: IHH, IGM and JGVL.
- 8.
Drafting of the article: IHH, IGM and JGVL.
- 9.
Critical review of the manuscript with intellectually significant contributions: IHH, IGM, JGVL, COG and ÀRC.
- 10.
Approval of the final version: IHH, IGM, JGVL, COG and ÀRC.
The authors declare that they have no conflicts of interest.
We would like to thank Dr Julia Romero Martínez for her contributions to the preparation of this article.
Please cite this article as: Herrera Herrera I, Garrido Morro I, Guzmán de Villoria Lebiedziejewski J, Ordoñez González C, Rovira À. Enfoque clínico-radiológico de la mielopatía no traumática. Radiología. 2020;62:464–480.