Obstetric protocols dictate that the fetal cerebellum should always be assessed during sonograms during pregnancy. For various reasons, including technical limitations or inconclusive sonographic findings, suspicion of cerebellar abnormalities is one of the most common indications for prenatal magnetic resonance imaging (MRI). Although sonography is the imaging technique of choice to assess the cerebellum, MRI shows the anatomy of the posterior fossa and abnormalities in the development of the fetal cerebellum in greater detail and thus enables a more accurate prenatal diagnosis. We describe and illustrate the normal anatomy of the fetal cerebellum on MRI as well as the different diseases that can affect its development. Moreover, we review the most appropriate terminology to define developmental abnormalities, their differential diagnoses, and the role of MRI in the prenatal evaluation of the posterior fossa.
La valoración del cerebelo fetal debe realizarse siempre por protocolo en las ecografías realizadas durante la gestación. Diferentes motivos como limitaciones técnicas o hallazgos ecográficos poco concluyentes han convertido la sospecha de patología del cerebelo fetal en una de las indicaciones más frecuentes de resonancia magnética (RM) prenatal. Aunque la ecografía fetal es la técnica de imagen de elección para su valoración, la RM muestra con mayor detalle la anatomía de la fosa posterior y las anomalías del desarrollo del cerebelo fetal, lo que permite un diagnóstico prenatal más preciso. Describimos la anatomía normal del cerebelo fetal mediante RM, así como las diferentes patologías que afectan a su normal desarrollo, y revisamos la terminología más apropiada para definirla, su diagnóstico diferencial y el papel de la RM en la evaluación prenatal de la fosa posterior.
The cerebellum is one of the very first encephalic structures to grow and its development occurs two (2) years after the postnatal age.1 Perinatal development is especially vulnerable to multiple causes capable of inducing cerebellar dysfunction and have repercussions on the normal neurological development of the infant.2
The ultrasound, the first imaging modality for the assessment of fetal development, has technical limitations though, such as oligoamnios, fetal position, or maternal obesity that may lead to inconclusive ultrasound findings.1 Also the complexity of certain conditions of the posterior fossa, the possibility of associated intracranial abnormalities, or the need to plan a fetal intervention are reason enough to conduct prenatal MRIs.3,4
The ultrasound suspicion of posterior fossa malformations is not rare and, however, the description of some entities affecting the development of the cerebellum is confusing and there is no apparent consensus in the medical literature.3,5
The goal of this work is to describe the normal anatomy of the fetal posterior fossa through MRIs, as well as the different diseases that may affect the normal development of the fetal cerebellum, and review what is the most appropriate terminology to define it, its differential diagnosis, and the role that MRIs play in the prenatal assessment of the posterior fossa.
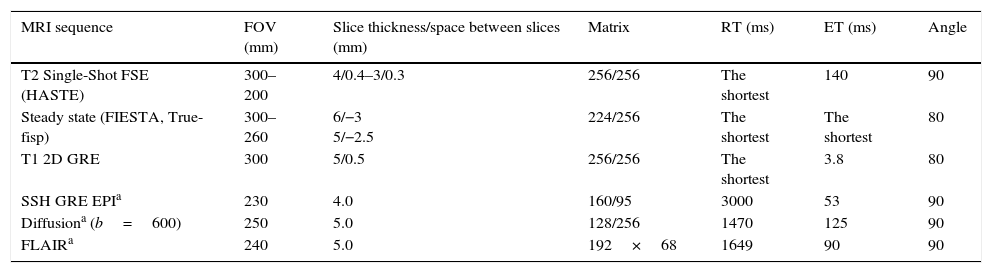
Protocol for conducting MRIs for the study of fetal cerebellumFrom the 20th week of pregnancy, the MRI is capable of characterizing the anatomy of the cerebellum in detail.6 Added to the acquisition of T2-weighted and steady state anatomical sequences, it is recommended to acquire other T1-weighted sequences or diffusion weighted imaging (DWI) for the detection of ischemia or cerebellar hemorrhages4 (Table 1).
Protocol of fetal cerebellar 1.5T MRI.
| MRI sequence | FOV (mm) | Slice thickness/space between slices (mm) | Matrix | RT (ms) | ET (ms) | Angle |
|---|---|---|---|---|---|---|
| T2 Single-Shot FSE (HASTE) | 300–200 | 4/0.4–3/0.3 | 256/256 | The shortest | 140 | 90 |
| Steady state (FIESTA, True-fisp) | 300–260 | 6/−3 5/−2.5 | 224/256 | The shortest | The shortest | 80 |
| T1 2D GRE | 300 | 5/0.5 | 256/256 | The shortest | 3.8 | 80 |
| SSH GRE EPIa | 230 | 4.0 | 160/95 | 3000 | 53 | 90 |
| Diffusiona (b=600) | 250 | 5.0 | 128/256 | 1470 | 125 | 90 |
| FLAIRa | 240 | 5.0 | 192×68 | 1649 | 90 | 90 |
EPI: echo-planar imaging; FIESTA: fast imaging employing steady state acquisition; FSE: fast spin-echo; FLAIR: fluid liquid atenuation inversion recovery; GRE: gradient echo; HASTE: half Fourier adquisition turbo spin-echo.
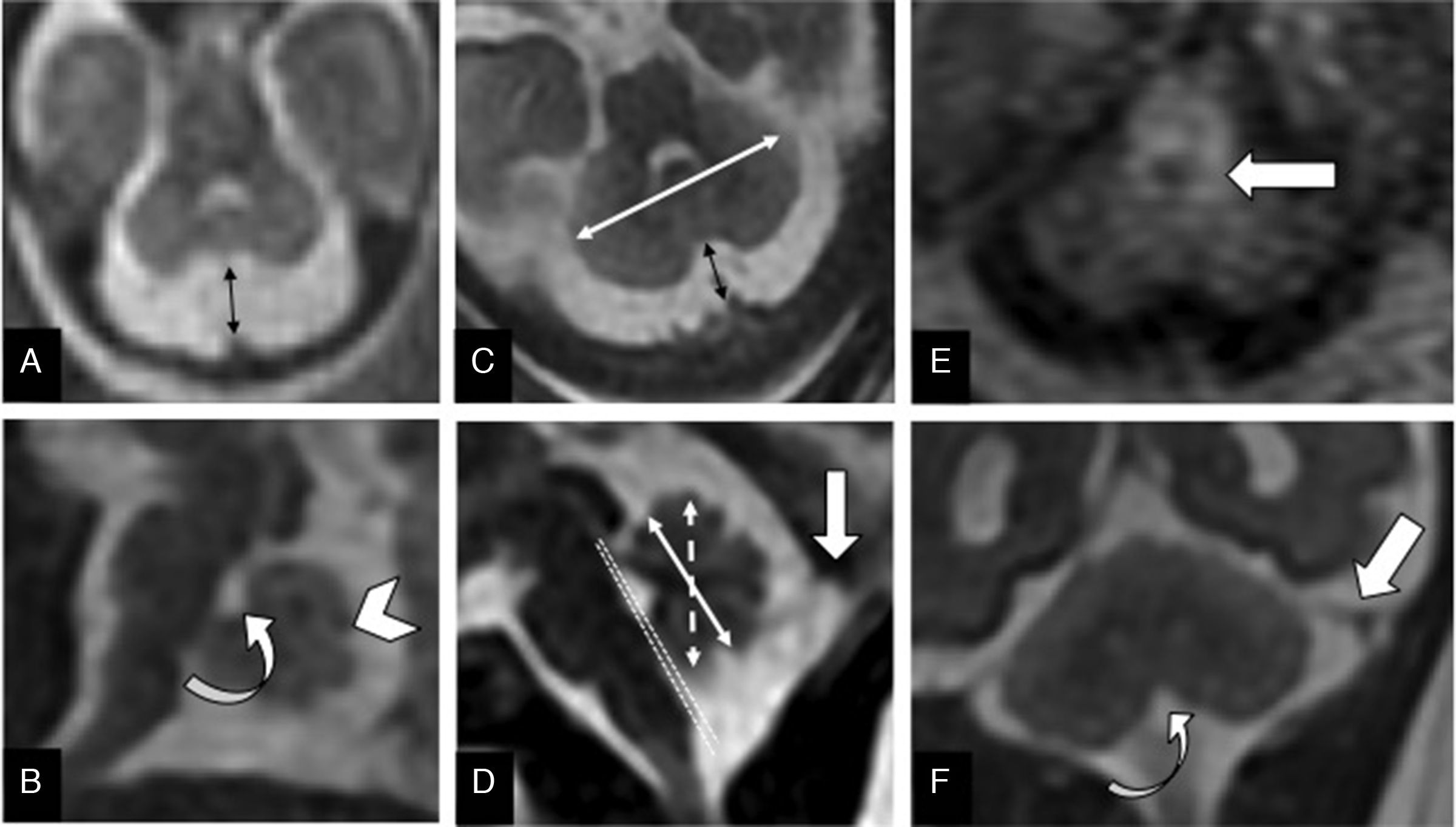
The systematic assessment of the morphology and biometry of the posterior fossa is essential and should always include7 (Fig. 1):
- •
Vermis: primary fissure that separates the anterior and posterior lobes of the vermis – the latter is twice as big; and secondary fissure. After week #21, the folia are visibles.1
- •
Cerebellar hemispheres: size asymmetries, and morphological anomalies.
- •
Cerebellar biometry: transcerebellar diameter; anterior-posterior diameter; vermis height and surface. Its normal values adjusted for the gestational age have been published recently.8
- •
Fourth ventricle morphology: opened or communicated with the cisterna magna. Assess the position of the fastigium and the tegmento-vermian angle.
- •
Protuberance: normal protrusion of the pons, since there are cases of combined hypoplasia of the pons and the cerebellum.
- •
Insertion and orientation of the tentorium: considered normal when it rests at the level of the inion (internal occipital protuberance) and the musculature of the back of the neck.
- •
Cisterna magna: in the axial plane, it is the distance from the vermis to the internal occipital edge.
- •
Associated supra o infratentorial anomalies.
Normal fetal posterior fossa. (A) T2-weighted transverse image of a 20-week old fetus with normal cerebellum and cisterna magna (double arrow). (B) T2-weighted sagittal image of the posterior fossa of a 22-week old fetus showing the primary fissure (arrowhead) and the fastigium (curved arrow). (C) FIESTA transverse image of a 32-week old fetus. Normal trans-cerebellar diameter (white arrow) and cisterna magna (black arrow). (D) FIESTA sagittal image of a 32 week-old fetus showing normal vermis height (dotted double arrow) and anteroposterior diameter (continuous double arrow). The tegmento-vermian angle (dotted lines) is nearly zero. Normal implantation of the torcula (thick arrow). (E) T1-weighted transverse image of the adequate myelination of the dorsal mesencephalon and cerebellar peduncuncles (arrow) of a 32-week old fetus. (F) T2-weighted coronal image of a normal tentorium (straight arrow) and obex (curved arrow) of a 32-week old fetus.
In sum, the MRI assessment of the cerebellum is based on morphological and biometrical data. Its normality allows us to rule out most fetal cerebellar malformations, from asymptomatic benign conditions to anomalies that associate serious psychomotor retardation.9
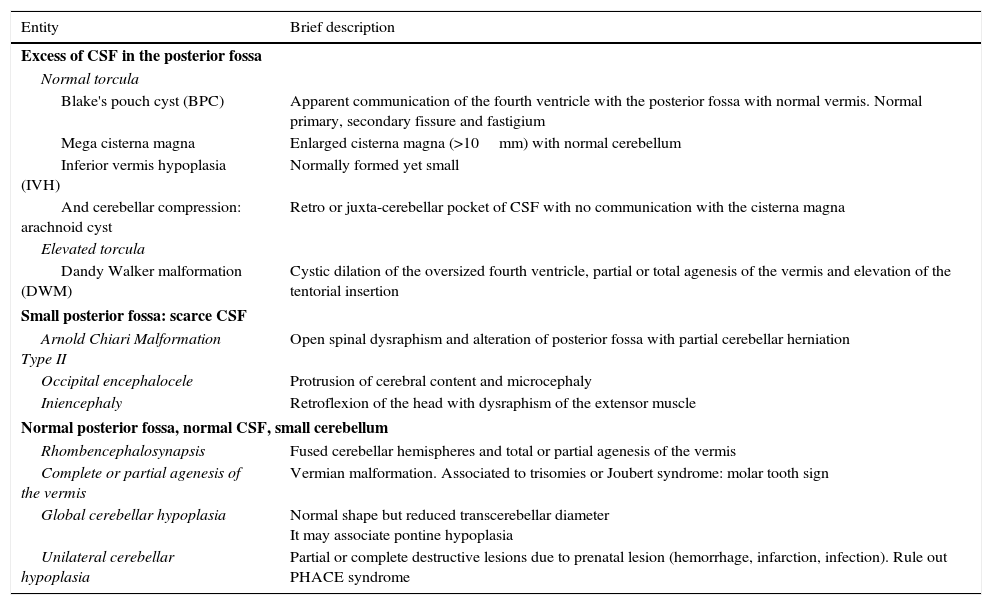
Pathological development of the fetal posterior fossaTable 2 schematically shows the most common disease that affects the development of the fetal cerebellum and that we can distinguish based on whether there is more or less cerebrospinal fluid (CSF) in the posterior fossa, or other data such as the tentorium insertion or cerebellar compression. If we have a normal size posterior fossa, we can distinguish the cases of cerebellar hypoplasia based on whether the whole cerebellum is affected, just the vermis or the cerebellar hemispheres, with or without associated pontine hypoplasia.
List and brief description of the different entities affecting the cerebellum and the posterior fossa.
| Entity | Brief description |
|---|---|
| Excess of CSF in the posterior fossa | |
| Normal torcula | |
| Blake's pouch cyst (BPC) | Apparent communication of the fourth ventricle with the posterior fossa with normal vermis. Normal primary, secondary fissure and fastigium |
| Mega cisterna magna | Enlarged cisterna magna (>10mm) with normal cerebellum |
| Inferior vermis hypoplasia (IVH) | Normally formed yet small |
| And cerebellar compression: arachnoid cyst | Retro or juxta-cerebellar pocket of CSF with no communication with the cisterna magna |
| Elevated torcula | |
| Dandy Walker malformation (DWM) | Cystic dilation of the oversized fourth ventricle, partial or total agenesis of the vermis and elevation of the tentorial insertion |
| Small posterior fossa: scarce CSF | |
| Arnold Chiari Malformation Type II | Open spinal dysraphism and alteration of posterior fossa with partial cerebellar herniation |
| Occipital encephalocele | Protrusion of cerebral content and microcephaly |
| Iniencephaly | Retroflexion of the head with dysraphism of the extensor muscle |
| Normal posterior fossa, normal CSF, small cerebellum | |
| Rhombencephalosynapsis | Fused cerebellar hemispheres and total or partial agenesis of the vermis |
| Complete or partial agenesis of the vermis | Vermian malformation. Associated to trisomies or Joubert syndrome: molar tooth sign |
| Global cerebellar hypoplasia | Normal shape but reduced transcerebellar diameter It may associate pontine hypoplasia |
| Unilateral cerebellar hypoplasia | Partial or complete destructive lesions due to prenatal lesion (hemorrhage, infarction, infection). Rule out PHACE syndrome |
PHACE: Posterior fossa anomalies, Hemangioma, Cardiac or aortic coarctation, Eye abnormalities; CSF: cerebrospinal fluid.
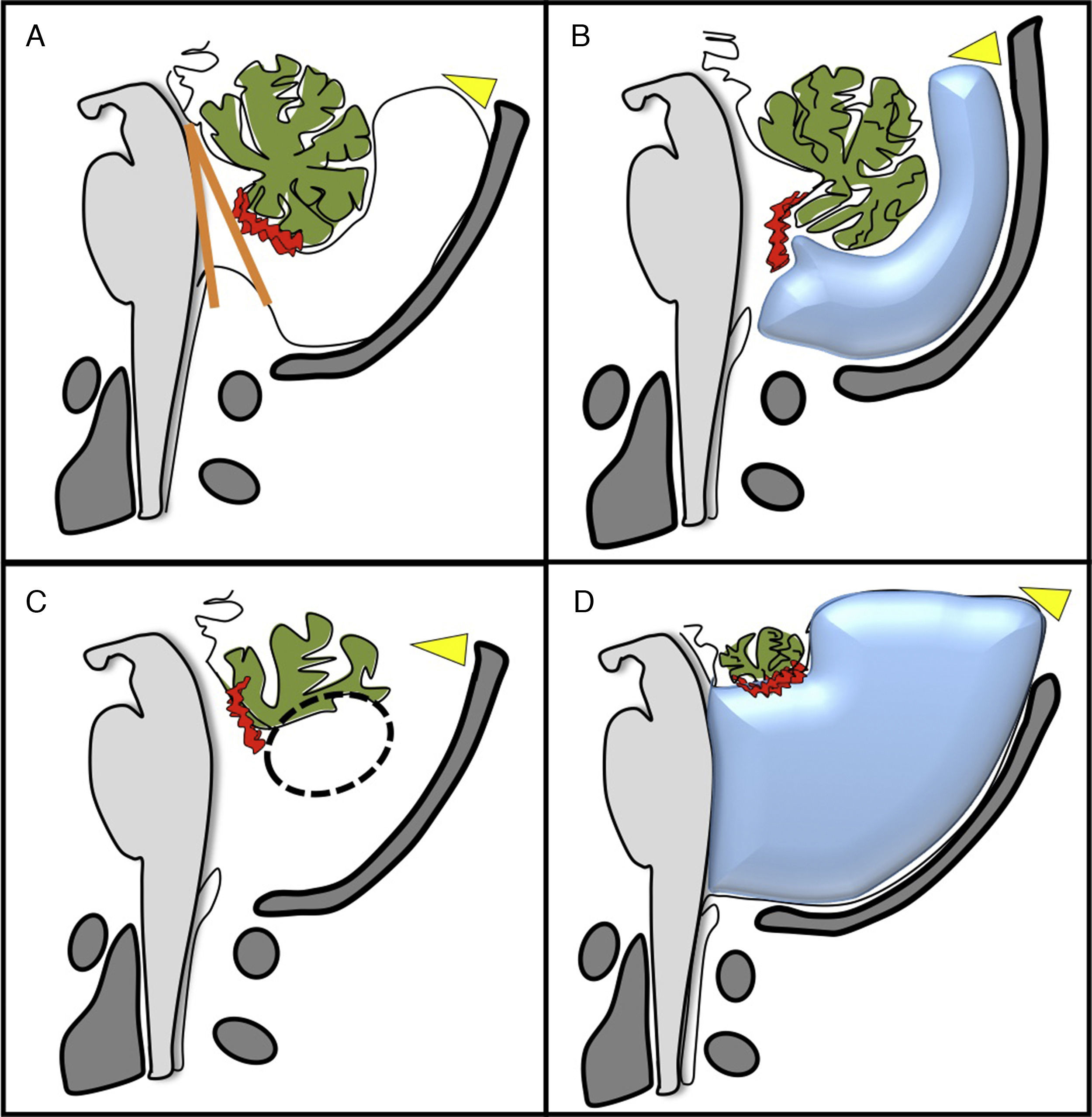
The so-called cystic malformations of the posterior fossa have been considered as a spectrum of malformation called Dandy–Walker malformation (Fig. 2). Although widely used in the scientific literature, this terminology is confusing and terms such as Dandy–Walker variant are preferably avoided.1,5,9,10 It is important to obtain the biometry of the cerebellum because even though the most common conditions such as Blake's pouch cyst (BPC), vermian hypoplasia (VH), or the Dandy–Walker malformation (DWM) may look similar in the images, the vermian biometry is different.10,11
Image of the four most important types of posterior fossa cystic malformations that exist. (A) Blake's pouch cyst causing vermian malrotation with an enlarged tegmento-vermian angle (in orage), with normal cerebellar vermis. (B) Mega cisterna magna: enlargement of the posterior fossa subarachnoid space with normal cerebellum. (C) Hypoplasia of the inferior vermis (circumference). (D) Dandy–Walker malformation: cystic dilation of the fourth ventricle, partial agenesis with anti-clockwise rotation of the vermis and elevation of the torcula (yellow arrowhead).
When there is an excess of CSF in the posterior fossa, we need to determine the position of the tentorium and whether or not there is cerebellar compression:
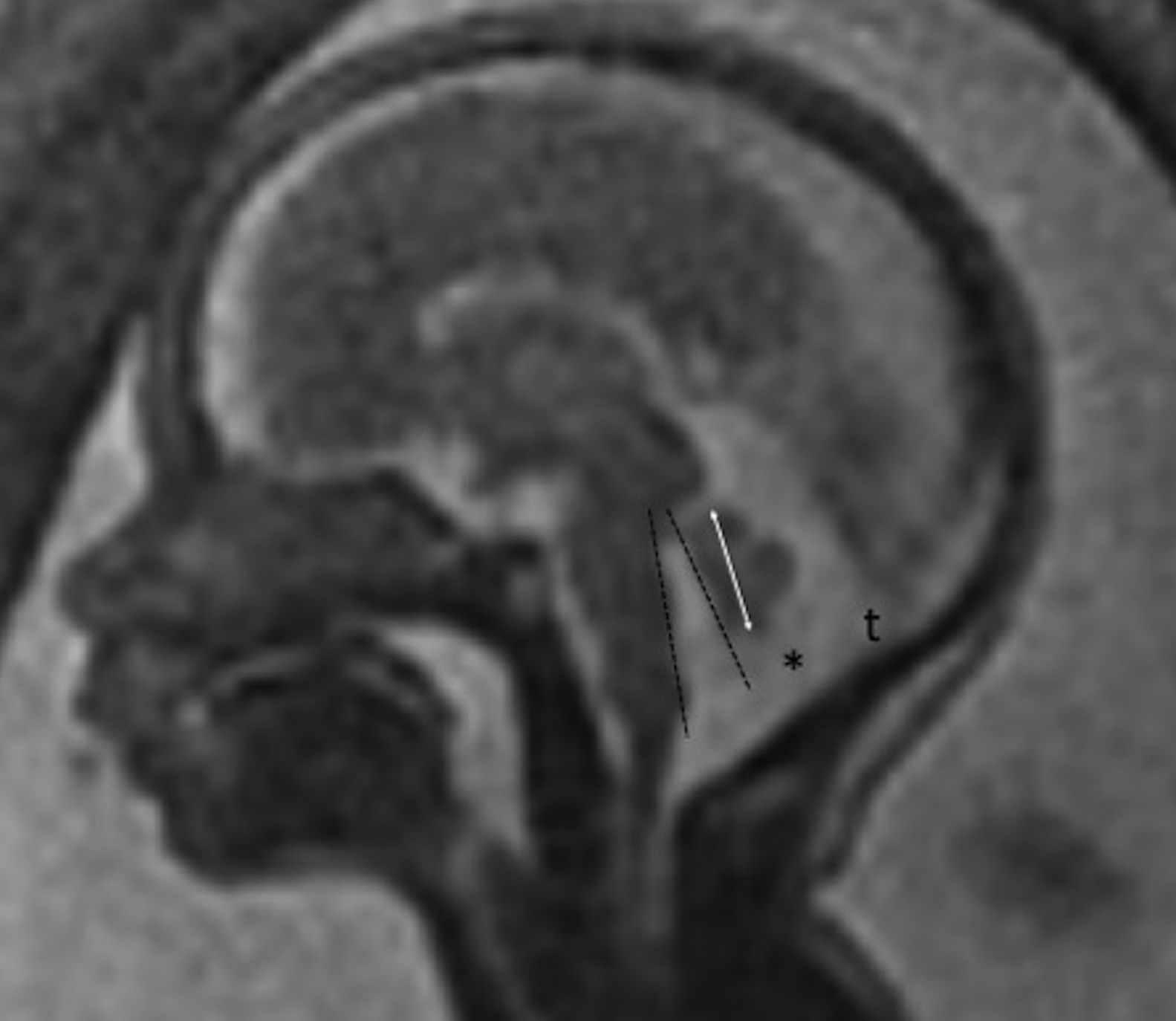
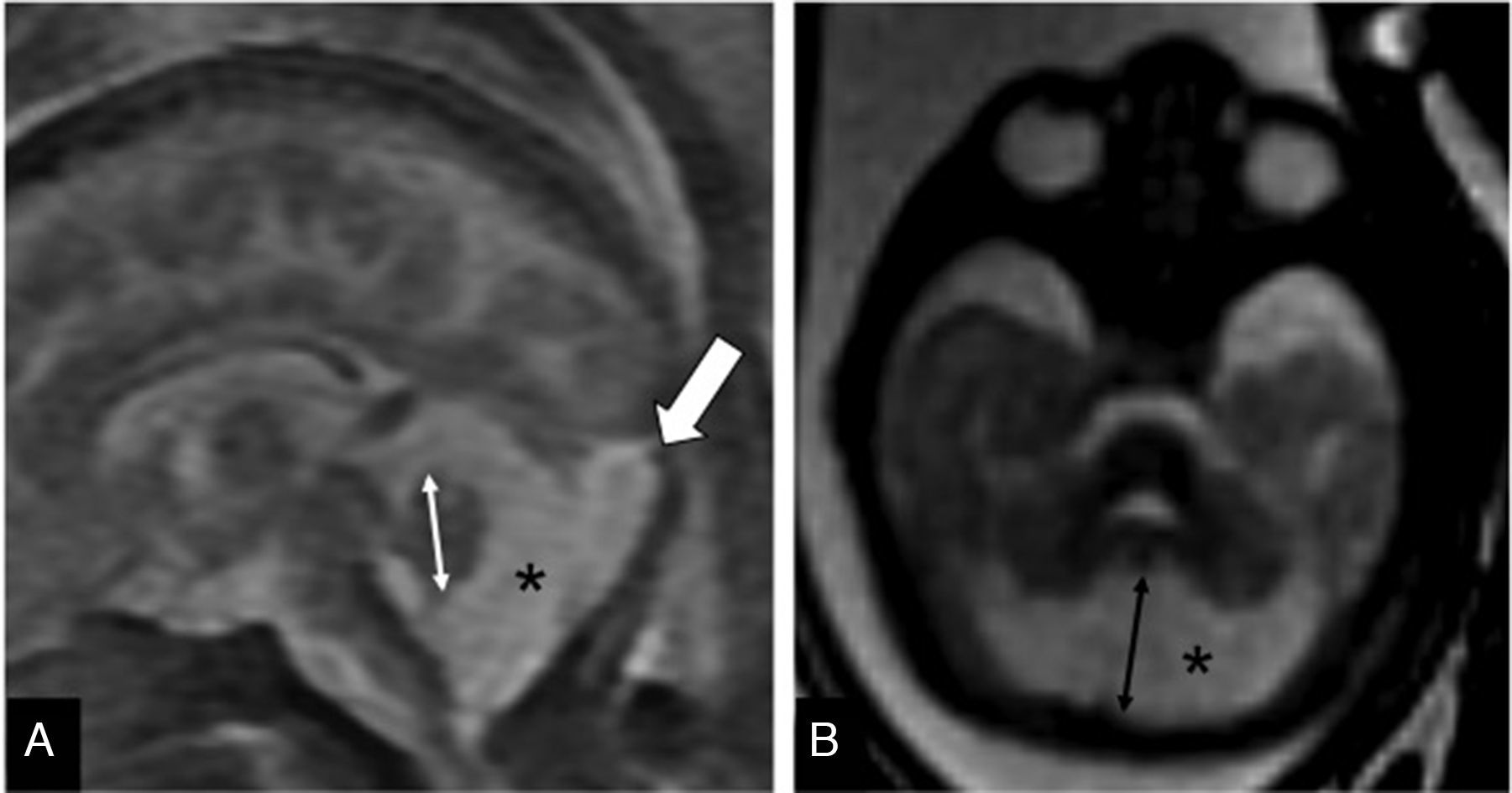
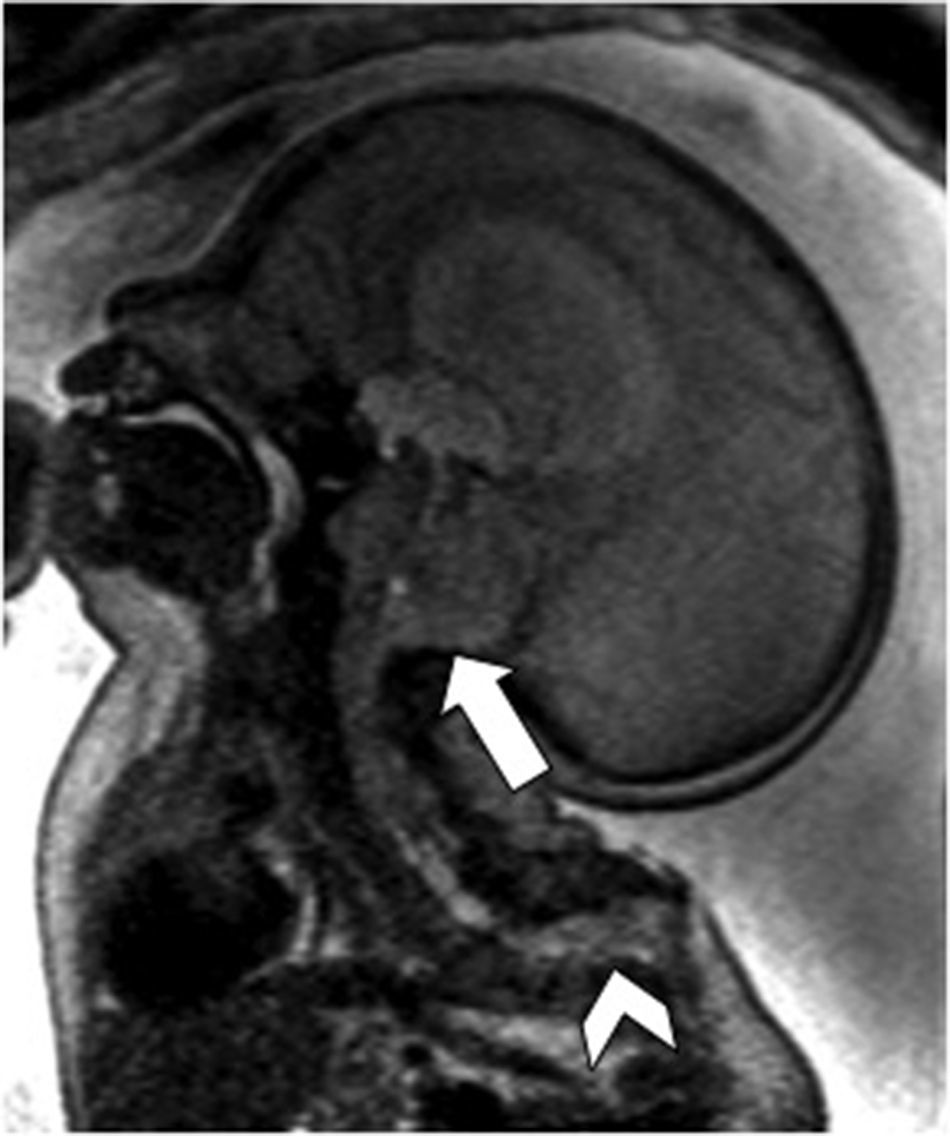
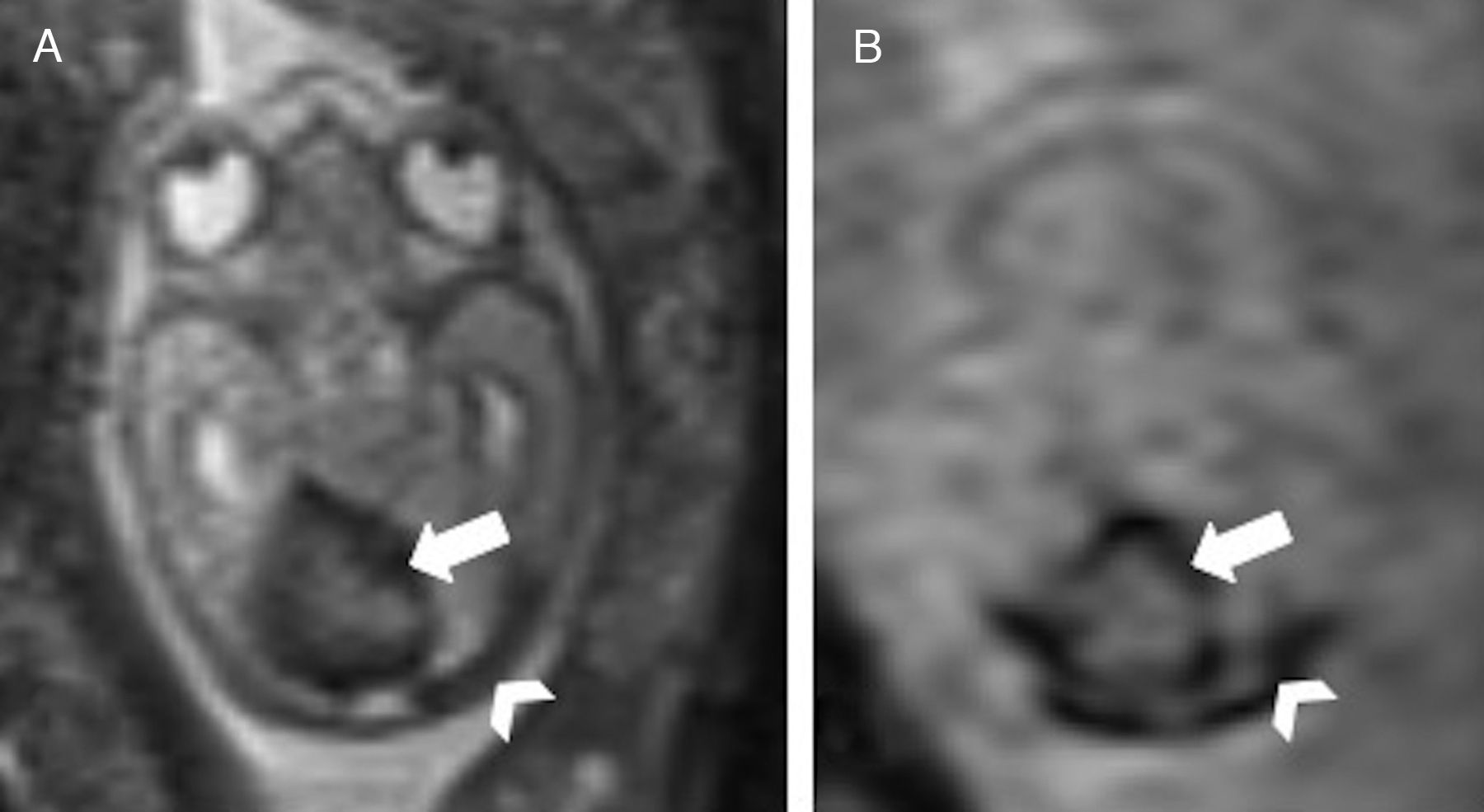
Tentorium of normal insertionLack of cerebellar compressionBlake's pouch cyst (BPC): Blake's pouch is a structure that stems from the posterior membranous region and that, when closing at the end of pregnancy week #10, originates Magendie's foramen. If its fenestration is delayed or never happens, it causes the formation of the BPC that elevates and compresses the inferior vermis that remains malrotated until the Magendie's foramen is perforated. It is diagnosed whenever the sagittal midline of the cerebellum shows normal vermis and cisterna magna, and a wide tegmento-vermian angle secondary to mild or moderate anti-clockwise rotation of the vermis (Fig. 3). The MRI conducted in between pregnancy weeks #20 and #23 is not enough, since the fenestration of Blake's pouch can occur in weeks #24 and #26.12 In approximately 50 per cent of the cases, the BPC is associated with heart defects and trisomy 21, which is why on suspicion of fetal BPC, determining the karyotype and conducting one fetal ultrasound are both recommended. For the lack of other associated defects, its postnatal prognosis is good.13
Blake's pouch cysts. Twenty-two week-old-fetus. T2-weighted sagittal image of one cerebellum of normal morphology and biometry according to its age (double arrow). Presence of excessive CSF in the posterior fossa (*) and slightly enlarged tegmento-vermian angle (dotted line) that translates the communication between the fourth ventricle and the cisterna magna, suggestive of the diagnosis. Normal position of the torcula (t) may be seen here.
Mega cisterna magna. It is defined as an increased CSF in the posterior fossa subarachnoid spaces, with a distance in the transverse plane >10mm in the vermis-occipital axis, a normal cerebellar anatomy, and in the absence of arachnoid cysts.11 Although some authors consider it a Dandy–Walker variant, it is a confusing term that should be avoided in the clinical practice.9 The mega cisterna magna as an isolated finding may be considered a benign entity with no repercussions on the psychomotor development in the postnatal age13,14 (Fig. 4).
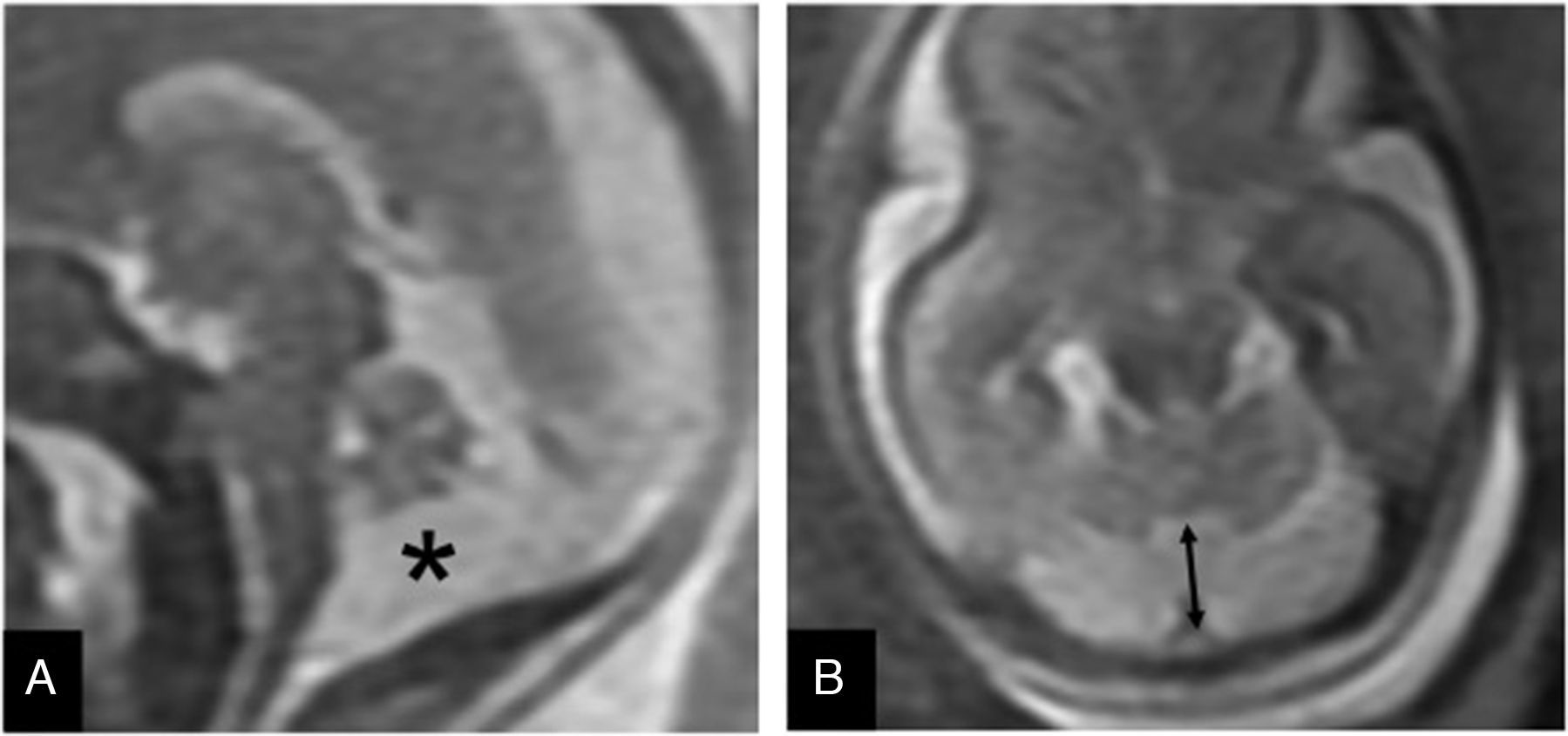
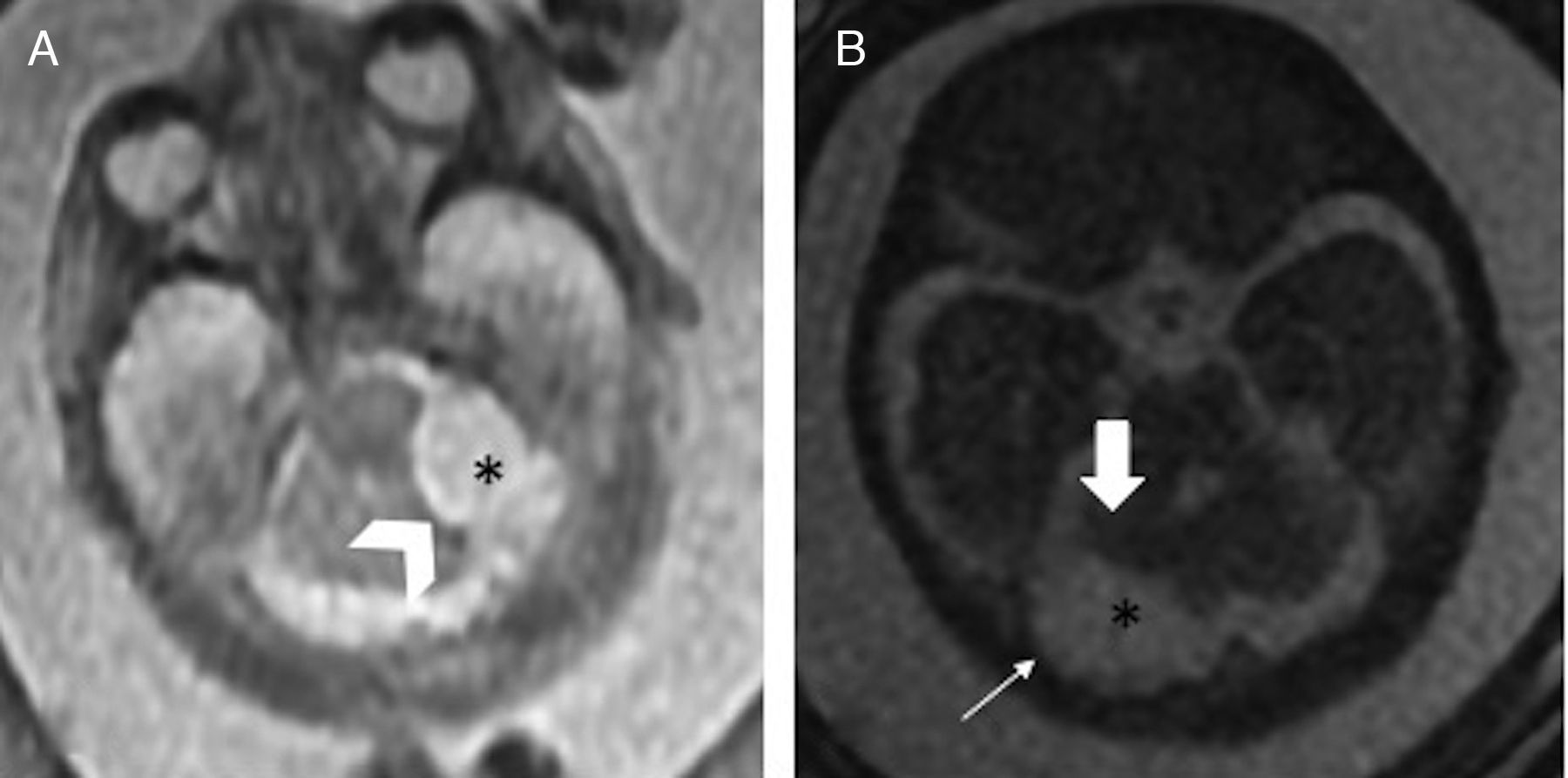
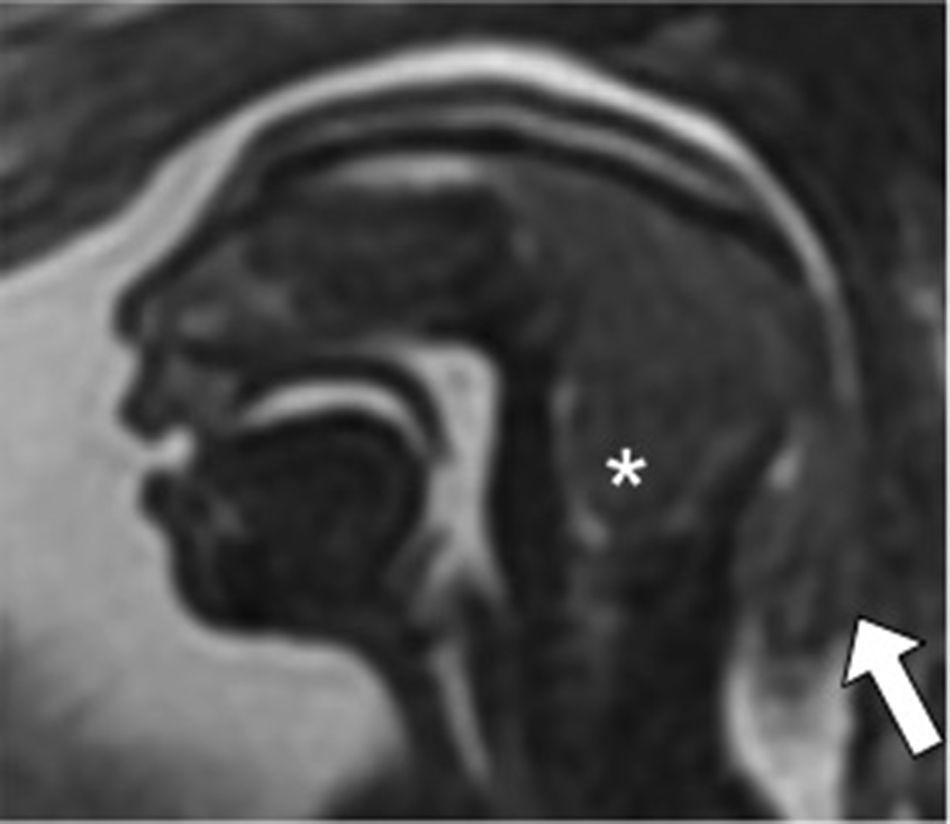
Inferior vermis hypoplasia (IVH). Traditionally defined as a Dandy–Walker variant, the IVH is diagnosed whenever the cerebellar biometry is lower than expected for its pregnancy age.15 Its prenatal diagnosis is difficult and there is even a risk of false positives on the MRIs7 (Fig. 5). Its prognosis is hard to tell since it can show a dominant trait associated with a benign prognosis, or be part of a genetic fragile X syndrome.3
Inferior vermis hypoplasia. Thirty week-old-fetus. (A) T2-weighted sagittal image showing the midline of one undersized cerebellum (double arrow), excess of cerebrospinal fluid (CSF) in the posterior fossa (*) and a normally inserted torcula (thick arrow). The fourth ventricle is not communicated to the cisterna magna. (B) FIESTA transverse image showing an excess of CSF (*) in the enlarged cisterna magna (double arrow).
Posterior fossa arachnoid cyst. Arachnoid cysts are cystic dilations surrounded by arachnoids that may appear at anywhere on the posterior fossa without any communication with the fourth ventricle. They may be retrocerebellar or lateral and compress one hemisphere only, and cause fetal hydrocephaly. The MRI allows us to assess any underlying parenchymal anomalies, above all, in advanced gestation ages16 (Fig. 6).
Arachnoid cyst of the fetal posterior fossa. (A) T2-weighted transverse image in a 21 week-old-fetus showing one hyperintense lesion (*) located laterally to the cerebellum and compressing the left cerebellar hemisphere (arrowhead). (B) FIESTA transverse image of another 22 week-old-fetus with one retrocerebellar arachnoid cyst (*) compressing the right hemisphere (thick arrow), and causing the thinning of the adjacent occipital bone (thin arrow).
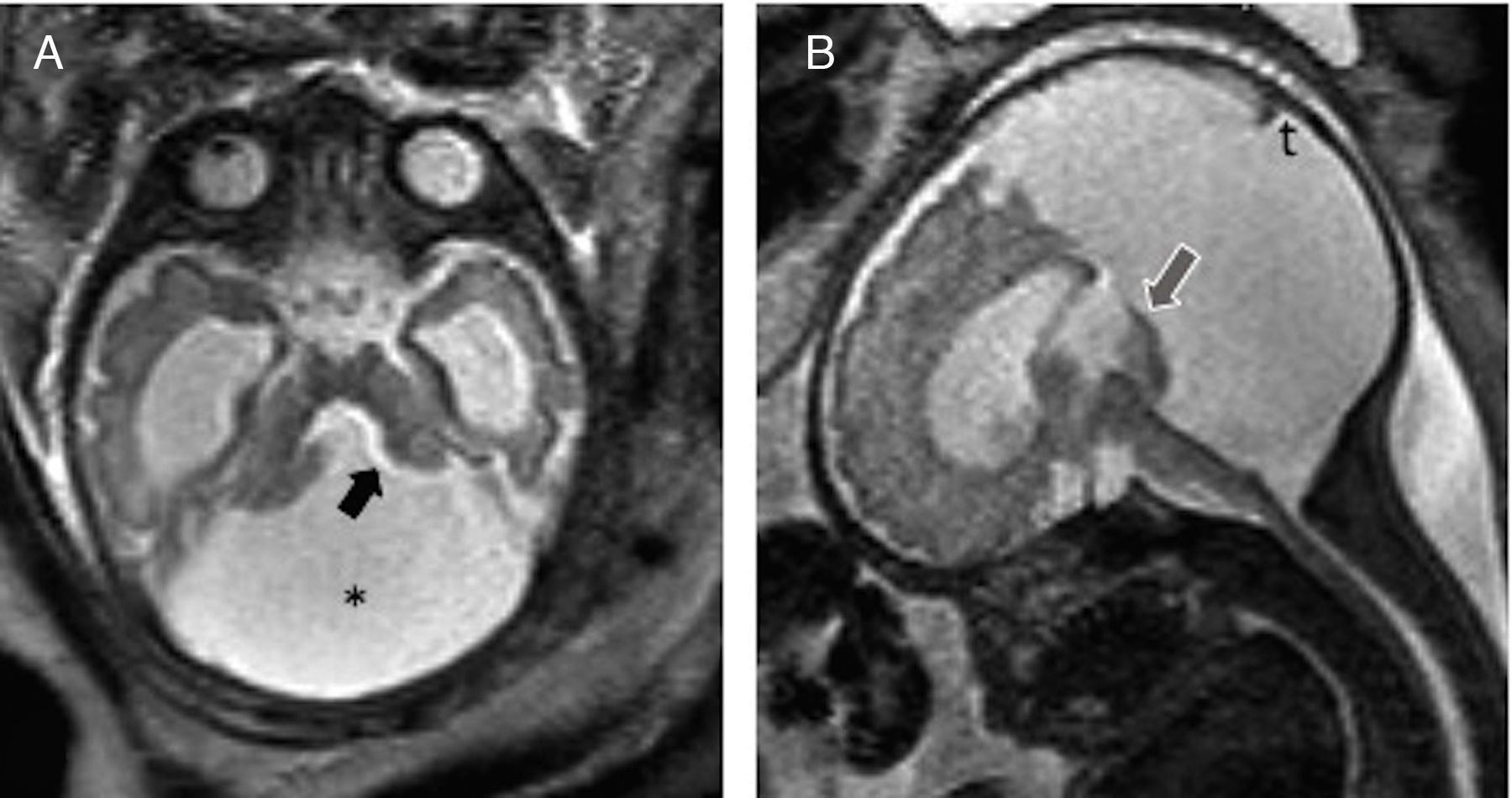
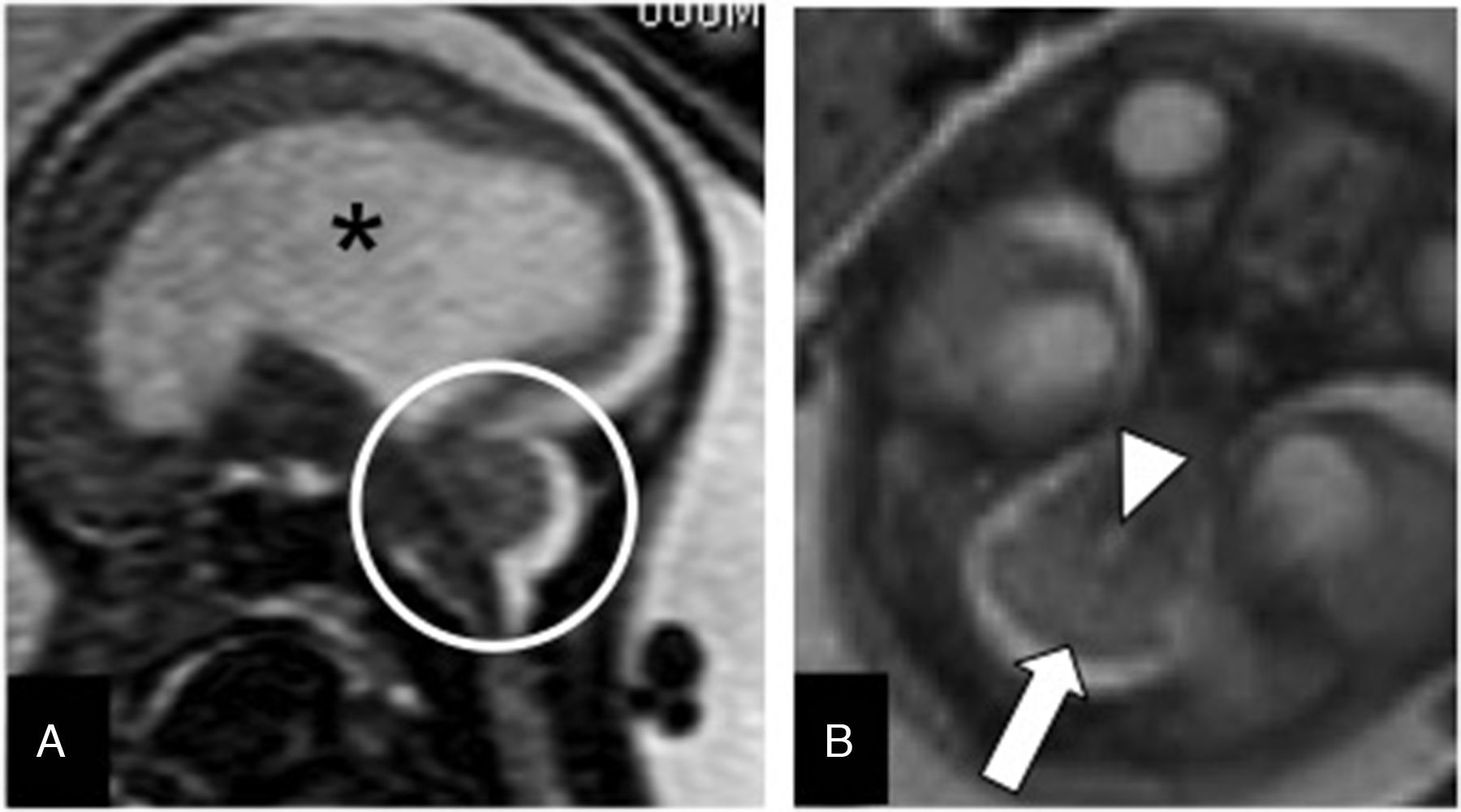
Dandy–Walker malformation (DWM). It is the most common cerebellar malformation of unclear etiology. It is defined through variable hypoplasia and anti-clockwise rotation of the vermis, cystic dilation of the fourth ventricle, and a wide cisterna magna with an abnormally high implantation of the torcula and the tentorium (Fig. 7). Fifty per cent of the cases associate supratentorial abnormalities such as agenesis of the corpus callosum or heterotopias. Since the neurological prognosis of the DWM is associated with the degree of vermian development and the associated supratentorial abnormalities17,18 assessment using MRIs is recommended. The “tail sign” on the MRI may be indicative for its diagnosis19 (Fig. 7).
Dandy–Walker malformation. (A) T2-weighted transverse image of a 34 week-old-fetus with an excess of CSF in the posterior fossa (*) and wide communication with the fourth ventricle (black arrow). (B) T2-weighted sagittal image showing partial agenesis of the vermis, the “tail sign” (arrow), and an elevated torcular implantation (t).
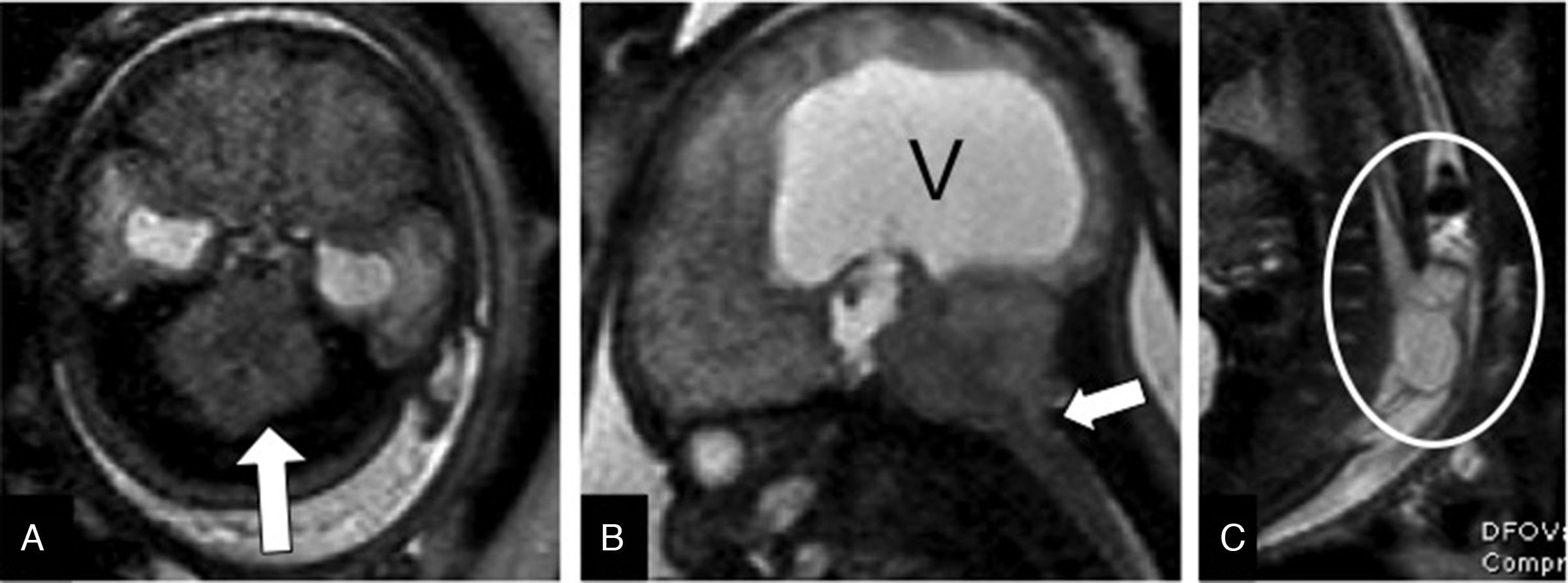
Chiari malformation type II. The open spinal dysraphisms like the myelomeningocele are associated with the posterior fossa malformation known as Chiari type II. It is believed that this association is due to a loss of CSF through the spinal defect that collapses the primitive ventricular system, avoiding the expansion of the rhomboencephalic vesicle. This translates into a small posterior fossa with a vermian herniation and a cerebellar tonsil herniation. These changes may be mild in the early stages of pregnancy and progress toward the end of the pregnancy.10
It usually associates other cranial anomalies such as a reduced subarachnoid space, dysgenesis of the corpus callosum, or subependymal heterotopias, most of them better seen through an MRI20 (Fig. 8).
Arnold–Chiari malformation type II. Thirty five-week old fetus. (A) T2-weighted transverse showing the “banana sign cerebellum” due to the absence of CSF in the cisterna magna (arrow). (B) T2-weighted sagittal image showing serious ventriculomegaly (V), cerebellar tonsil herniation, and inferior vermis herniation through the foramen magnum toward the cervical spine (arrow). (C) T2-weighted sagittal image of the rachis showing wide-open spinal dysraphism with lumbar myelomeningocele (circle).
When one cephalocele in the craniocervical junction accompanies the changes of a Chiari type II, then this malformation is known as a Chiari type III malformation.21 The differential diagnosis is established with the iniencephaly, a rare form of spinal dysraphism that associates one cervico-occipital junction malformation (Fig. 9), and also with the occipital encephalocele, a protrusion of encephalic material through an occipital bone defect that may associate syndromes with characteristic findings such as the Meckel-Gruber syndrome, or the Klippel-Feil syndrome (Fig. 10).22,23
Occipital encephalocele. Thirty week-old-fetus. FIESTA sagittal image showing microcephaly and herniation of occipital encephalic material (arrow). Absence of cerebrospinal fluid in the posterior fossa (*) whose elements are unrecognizable in this fetus with serious cerebral malformations.
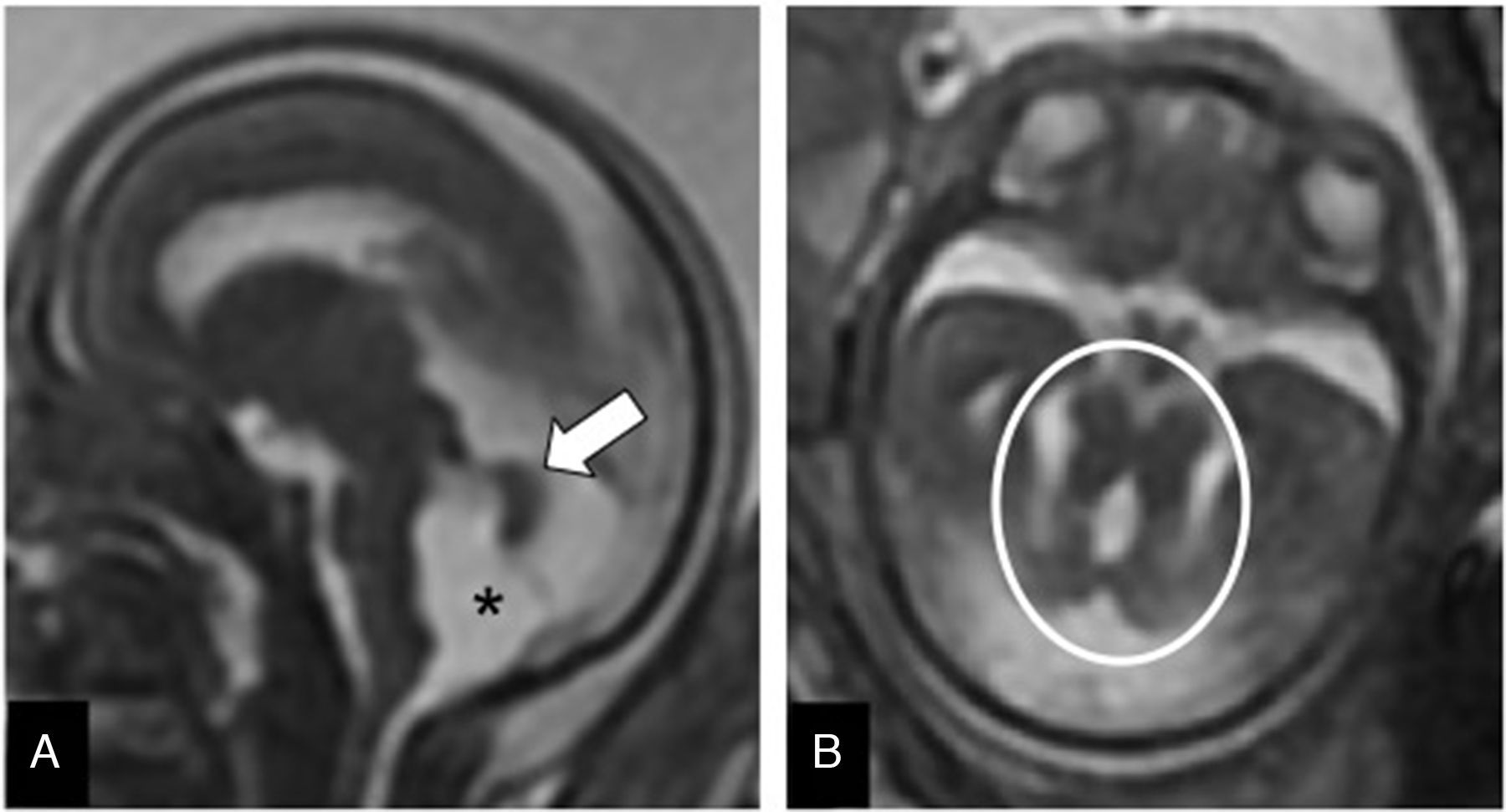
Rhombencephalosynapsis. It is the complete or partial absence of vermis with fusion of the cerebellar hemispheres (Fig. 11). It may associate other extracranial malformations (cardiac, vertebral, anal) or cerebral malformations. The MRI facilitates its diagnosis and determines whether it is isolated or associated with other encephalic malformations such as dysgenesis of the corpus callosum, or aqueductal stenosis.24 Its prognosis is unfavorable in the complete forms with other malformations.
Rhombencephalosynapsis. Twenty-two week-old-fetus. (A) FIESTA sagittal image showing ventriculomegaly (*), and one cerebellum with absence of primary fissure without identification of the lumen of the fourth ventricle (circle). (B) T2-weighted transverse image showing absence of vermis with fusion of both hemispheres (arrow), and a punctiform fourth ventricle (arrowhead).
Partial or complete absence of the vermis, and molar tooth sign. Both the Joubert syndrome and a series of associated genetic anomalies make up a group of diseases whose common and most representative characteristic is the presence of one mesencephalic malformation with the appearance of a “molar tooth sign” on the MRI (Fig. 12). These are disorders that many authors consider as ciliopathies, of autosomal recessive inheritance, or linked to chromosome X. Patients with these disorders show progressive hypotony and psychomotor retardation since birth, and may associate malformations in other organs like the retina, kidneys, liver, or the skeleton.25,26
Joubert syndrome. Twenty-two week-old-fetus. (A) FIESTA sagittal image that confirms the dilation of the fourth ventricle with wide tegmento-vermian angle (*), and undersized vermis (arrow). (B) FIESTA transverse image showing the molar tooth sign (circle). The fetus showed polydactyly too.
The hypoplasia of one cerebellar hemisphere may be due to the PHACE syndrome (Posterior fossa anomalies, Hemangioma, Cardiac or aortic coarctation, Eye abnormalities), also known as Pascual Castroviejo type 2 syndrome,27 in which the posterior fossa anomalies are usually the first finding and precede the appearance of hemangiomas. Nevertheless, the unilateral cerebellar hypoplasia is usually due to a congenital infection or vascular lesion that causes the volume loss of the cerebellar hemisphere.28 Thanks to their diffusion-weighted or gradient-echo sequences, the MRIs are superior to the ultrasounds when it comes to determining whether there are underlying ischemic or hemorrhagic causes to unilateral cerebellar lesions. It is essential to assess the cerebellar vermis because if infected, the postnatal prognosis is worse29,30 (Fig. 13).
Cerebellar hemorrhage, and venous thrombosis in congenital infection due to cytomegalovirus. Twenty-one week-old-fetus with signs of hydropsy. (A) Transverse image showing both the blood collection (arrow), and the enlarged and markedly hypointense transverse sinuses (arrowhead). (B) Gradient-echo transverse image showing the cerebellar hematoma (arrow) and thrombosis of the transverse sinuses (arrowhead).
Cerebellar hypoplasia consists of a morphologically normal cerebellum with reduced biometry. It may associate chromosomopathies, infections due to cytomegalovirus or metabolic disorders like glycosilation.9,10 When the protuberance does not show a normal curve, it is described as a pontocerebellar hypoplasia that may be seen in multiple entities such as the Walker-Warburg syndrome, or the muscle–eye–brain that usually associates serious disorders like lissencephaly.
ConclusionCerebellar malformations are common and most of them may be detected through ultrasounds. During the second half of pregnancy, the MRI plays a complementary role when the ultrasound conditions are deficient, and on suspicion of vascular lesions or complex pathology of the posterior fossa. The radiologist should be able to interpret the imaging findings of the different fetal posterior fossa malformations that exist, as well as the adequate terminology to describe them, since their prognosis is highly variable, and the consequences that the radiological report may have on perinatal counseling are relevant.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments with human beings or animals have been performed while conducting this investigation.
Confidentiality of dataThe authors confirm that in this article there are no data from patients.
Right to privacy and informed consentThe authors confirm that in this article there are no data from patients.
Authors- 1.
Manager of the integrity of the study: RLS.
- 2.
Study idea: RLS, AVS, GMF, FMS.
- 3.
Study design: RLS, AVS, AMF.
- 4.
Data mining: RLS, AVS, GMF.
- 5.
Data analysis and interpretation: RLS, AVS, GMF, FMS.
- 6.
Statistical analysis: N/A.
- 7.
Reference: RLS, AVS.
- 8.
Writing: RLS, AVS, AMF, FMS.
- 9.
Critical review of the manuscript with intellectually relevant remarks: RLS, AVS, AMF, GMF, FMS.
- 10.
Approval of final version: RLS, AVS, AMF, GMF, FMS.
The authors declare no conflict of interests associated with this article whatsoever.
Please cite this article as: Llorens Salvador R, Viegas Sainz A, Montoya Filardi A, Montoliu Fornas G, Menor Serrano F. Valoración del cerebelo fetal mediante resonancia magnética. Radiología. 2017;59:380–390.