Expiratory CT scan is a complementary technique of inspiratory CT that provide valuable physiological information and may be more sensitive to detect air trapping than pul-monary function tests. It is useful in many obstructive airway diseases, including obliterative bronchiolitis, asthma, Swyer-James syndrome, tracheomalacia, hypersensitivity pneumonitis and sarcoidosis. In obliterative bronchiolitis, expiratory CT scan may be the only imaging technique that shows abnormalities in the early phase of disease. In order to obtain a good quality study, we should explain the procedure to the patient, use precise instructions and do some practice before image acquisition. Here we describe strategies to optimize the techni-que and propose an algorithm that help in interpretation of imaging findings in patients with obstructive airway disease.
La TC torácica en espiración es una técnica complementaria de la inspiración que aporta valiosa información fisiológica y puede ser más sensible que las pruebas de función respiratoria para detectar atrapamiento aéreo. Tiene múltiples indicaciones, entre las más frecuentes están la enfermedad obstructiva de la vía aérea producida por bronquiolitis obli-terante, asma, síndrome de Swyer-James, traqueomalacia, neumonitis por hipersensibilidad o sarcoidosis. En alguna de ellas, como la bronquiolitis obliterante, la TC espiratoria puede ser la única técnica de imagen que detecta alteraciones en las fases iniciales. Si queremos que sea de utilidad diagnóstica, hay que asegurarse de que el estudio tenga calidad suficiente. Para ello se recomienda explicar al paciente en qué consiste la prueba, emplear instrucciones precisas y realizar un breve entrenamiento antes de iniciar la adquisición. En este trabajo sugerimos estrategias para optimizar la técnica y proponemos un algoritmo para interpretar los hallazgos radiológicos en el contexto de la patología obstructiva pulmonar.
In 1993, Richard Webb published an article in the journal Radiology describing the technique called "dynamic lung CT scan", in which the images were acquired continuously during the patient's inspiration and expiration.1 He demonstrated that the lung increases diffusely in density during expiration, especially in sloping areas, since its density is the combined result of the volume of air, blood, and lung tissue, and as the former decreases, the overall density increases proportionally. Since then, expiratory chest computed tomography (CT) scan has been increasingly used as an adjunct to inspiratory scanning to obtain physiologic information on regional lung function in patients with both large and small obstructive airway disease.
A small airway is defined as that of a non-cartilaginous nature that measures less than 2 mm and is made up of the terminal and respiratory bronchioles. Under normal conditions, it is not visible on CT scan due to its small size. However, when there is bronchiolar pathology, it can manifest as direct signs (tree-in-bud and centrilobular nodules) or indirect signs (density, attenuation or mosaic pattern on inspiration and air trapping on expiration). The Fleischner Society defines air trapping as the retention of air distal to a bronchiolar obstruction, generally partial.2 They are areas of the lung with a lower increase in density than the rest of the lung parenchyma during expiration and that do not decrease in volume during this phase. The presence of air trapping is a definitive sign of small airway disease and in some pathologies such as bronchiolitis obliterans it may appear as the only finding during expiration, with the inspiratory study being normal.3 However, it is often possible to suggest the presence of small airway disease on inspiratory CT scan as well, where it manifests as a mosaic density pattern with areas of lower attenuation than the rest of the lung parenchyma.
The small airway has traditionally been considered a "silent zone" of the lung, since it only represents 10–15% of the overall airway resistance.4 That is why in the initial phases its affectation is not detected in the pulmonary function tests (PFTs) and the patients are asymptomatic, being reflected only late in the spirometry when it is already very affected.5 Thus, these tests are considered insensitive for detecting mild degrees of small airway disease and some authors maintain that expiratory CT scan could be a more accurate technique, since it identifies air trapping early.6
The three main indications for performing a chest CT scan on expiration would be: 1) to evaluate patients with known or suspected airway pathology; 2) to identify air trapping in patients with normal inspiratory CT scan; and 3) to differentiate between the three possible causes of a mosaic pattern: small airway disease, obstructive vascular disease and infiltrative lung disease.
However, obtaining a good expiration study is not always easy. In order to obtain useful functional information that complements the inspiratory study – and avoid non-diagnostic studies that increase radiation to the patient without added benefit – it is necessary to achieve a sufficiently deep expiration without movement artifacts. This requires an adequate prior explanation of the technique, some training and the maximum collaboration of the patient, who can often present limitations such as dyspnoea, hearing problems or respiratory coordination.
In this article, we review the expiratory chest CT scan technique, suggest tips to improve its quality and propose a diagnostic algorithm to interpret radiological findings in the context of obstructive pulmonary disease.
Expiratory CT scan: how I do itDespite the importance of respiratory manoeuvres in expiratory chest CT scan, the radiological literature offers little information on how to obtain a quality study. In general, the expiratory part is usually done after the inspiratory CT scan, but not immediately, instead allowing the patient to rest for a few minutes between them. It is important to carry out a preliminary test with the patient in the same position as the acquisition, i.e., with the arms raised. After several normal breaths, we will instruct the patient to take a deep breath and then let it out "until there is no air left in your lungs", holding the end of the exhalation without moving and trying to avoid the Valsalva manoeuvre. It is important to give the patient enough time to complete the forced expiration, since in obstructive diseases the emptying of air from the lungs is slower than in the normal lung. For this reason we must extend the instruction "blow, blow, blow…" for at least six seconds before the "hold", and then acquire the study.7,8 The goal would be to achieve residual volume, or at least functional residual capacity.
When the patient has understood the instructions, we will proceed to carry out the acquisition, avoiding using the automatic orders of the equipment, since it has been shown that the quality improves a lot when the instructions are issued by the person who performs the test, i.e., the technician or the radiologist.9 Since we do not usually use respiratory volume monitoring systems (e.g., spirometer), the role of technicians is critical in obtaining a good study. The radiologist, for his or her part, will be responsible for ensuring that the technicians are aware of the instructions for the respiratory manoeuvres, as well as for regularly monitoring their application and evaluating the final quality of the studies (trying to minimise the cases of "exhalation equals inspiration"). In the event that sufficient expiration is not achieved despite the instructions described, the recourse of performing a CT scan in lateral decubitus remains, which restricts the movements of the declining hemithorax, forcing its expiration.10
In the past, expiratory CT scan acquisition used to be performed as an incremental scan with thin slices, typically 1 mm, every centimetre, or covering only the lower half of the lungs. Currently, however, with multi-detector row equipment, it is recommended that a low-dose, full-coverage helical acquisition (tube current 20–50 mAs) be conducted, in order to perform multiplanar reconstructions and compare lung parenchyma in inspiration and expiration.11 This also allows us to obtain minimum intensity projection (MinIP) images that make air trapping zones more evident.
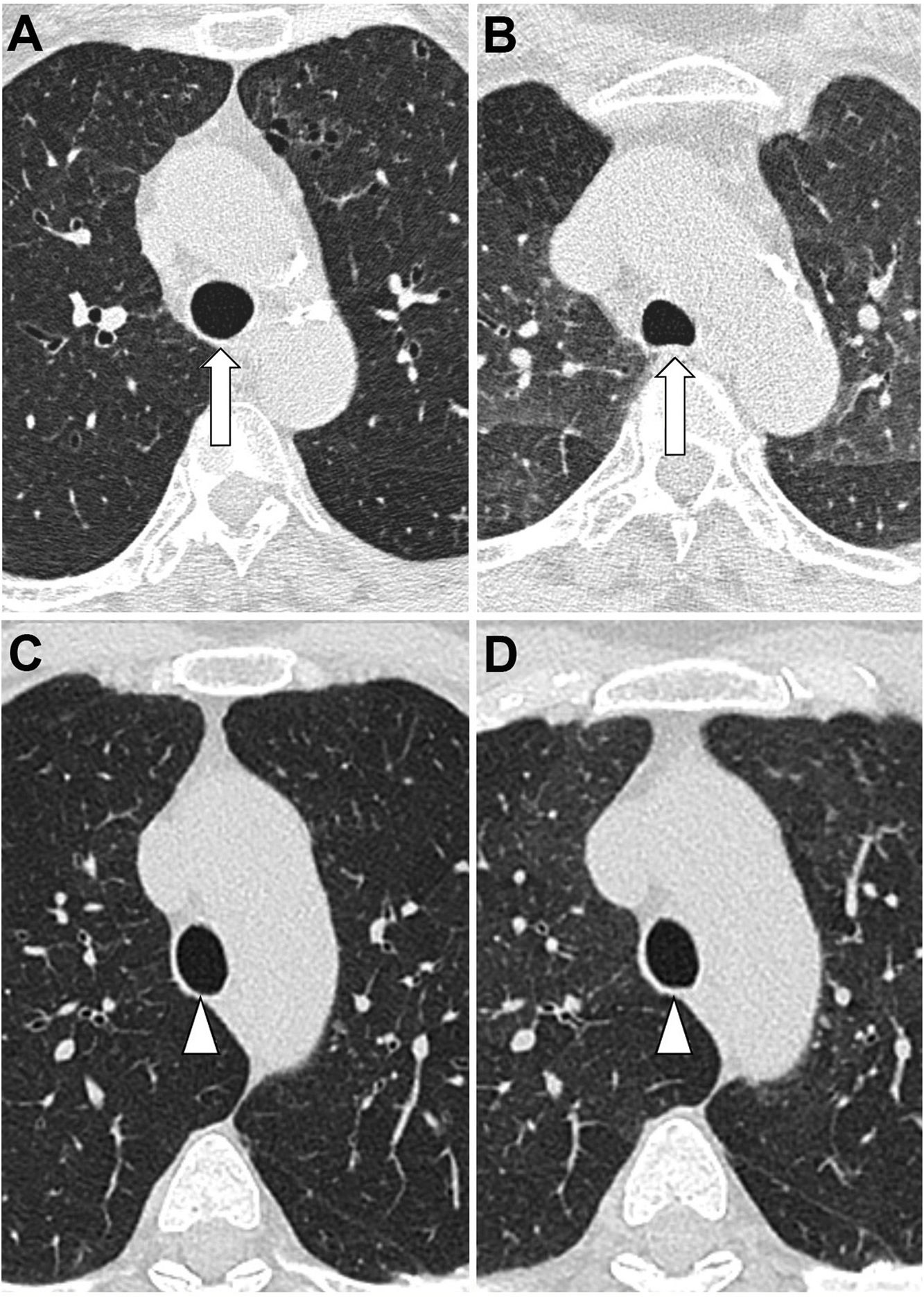
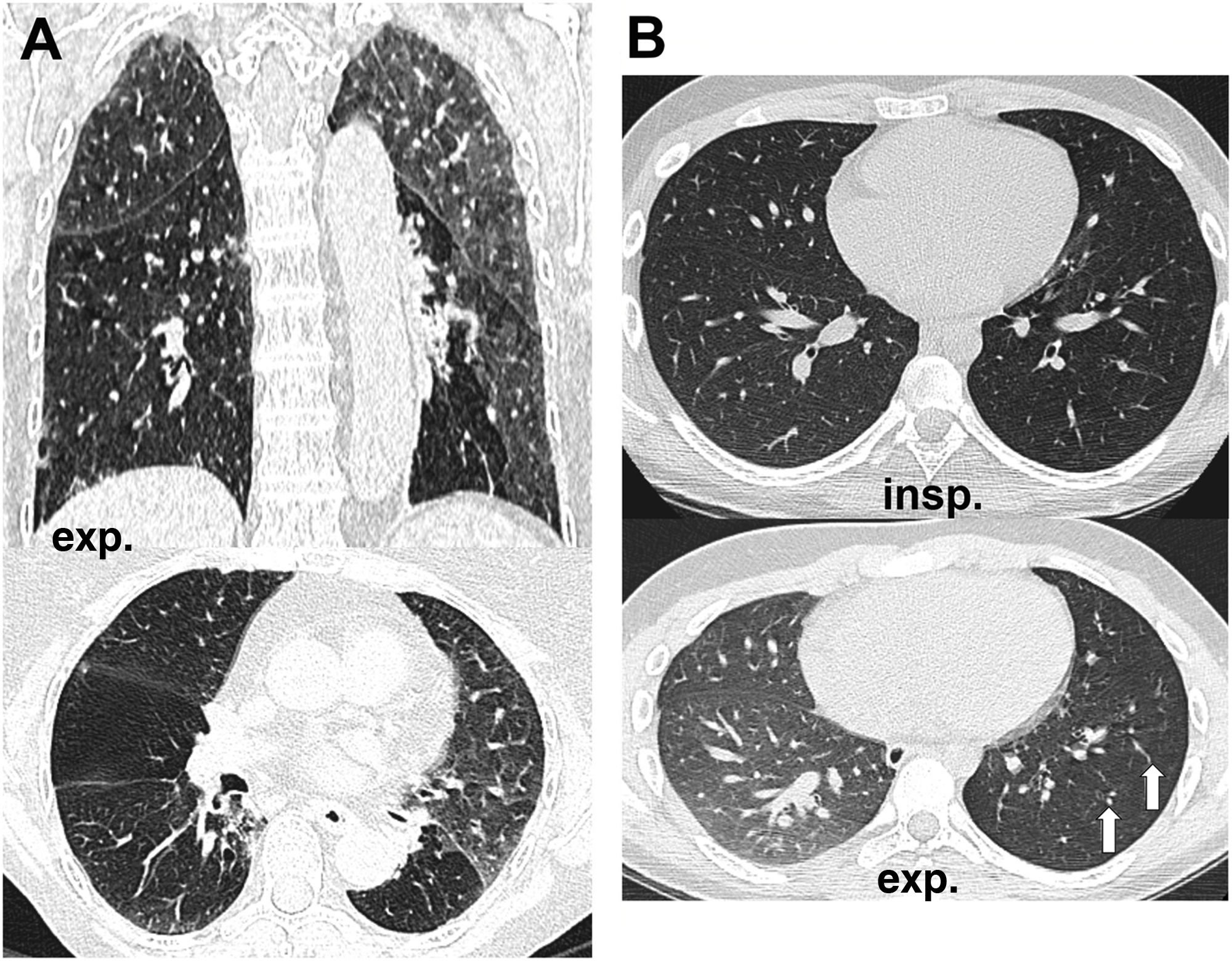
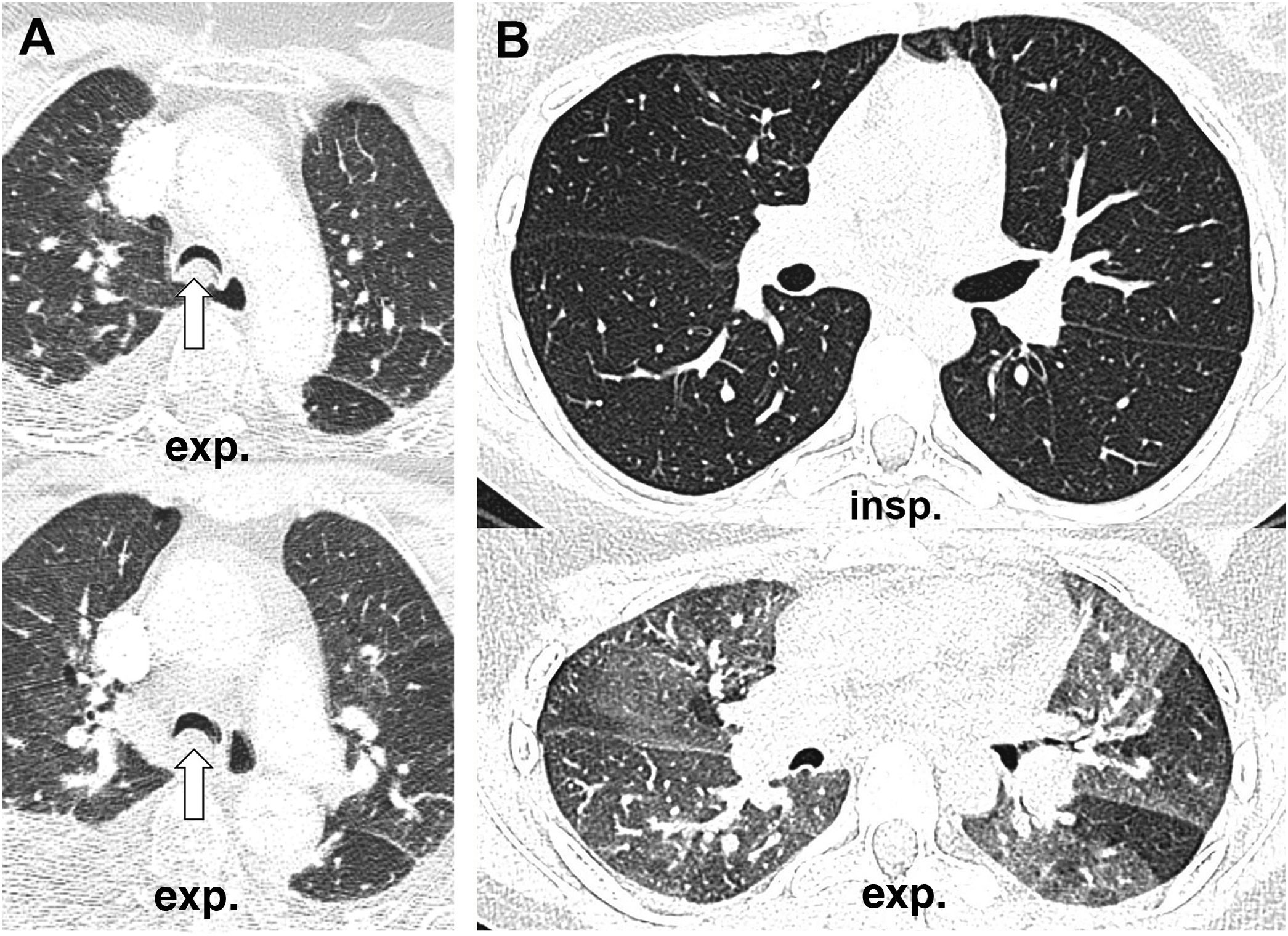
How to know if a good exhalation has been achievedThere is no accepted measure to determine whether a sufficient degree of expiration has been obtained on CT scan, with changes in tracheal morphology or increased lung attenuation being the most commonly used criteria. The posterior wall of the trachea, which is membranous in nature, moves backwards during inspiration, giving it the shape of the letter "O", while on expiration it moves forward, becoming straight (in the shape of the letter "D") or bulging slightly forward (Fig. 1A,B). However, it has been shown that in healthy volunteers the trachea can show a great variety of morphologies regardless of the degree of expiration reached, even remaining identical in both phases despite adequate expiration12 (Fig. 1C,D).
Variability of movements of the posterior tracheal membrane with respiration. In the patient on the upper images, posterior displacement is observed during inspiration (A) and anterior displacement during expiration (B), while in the patient on the lower images there are hardly any changes in the morphology of the trachea during inspiration (C) and expiration (D). The increase in lung density can be seen in the last picture.
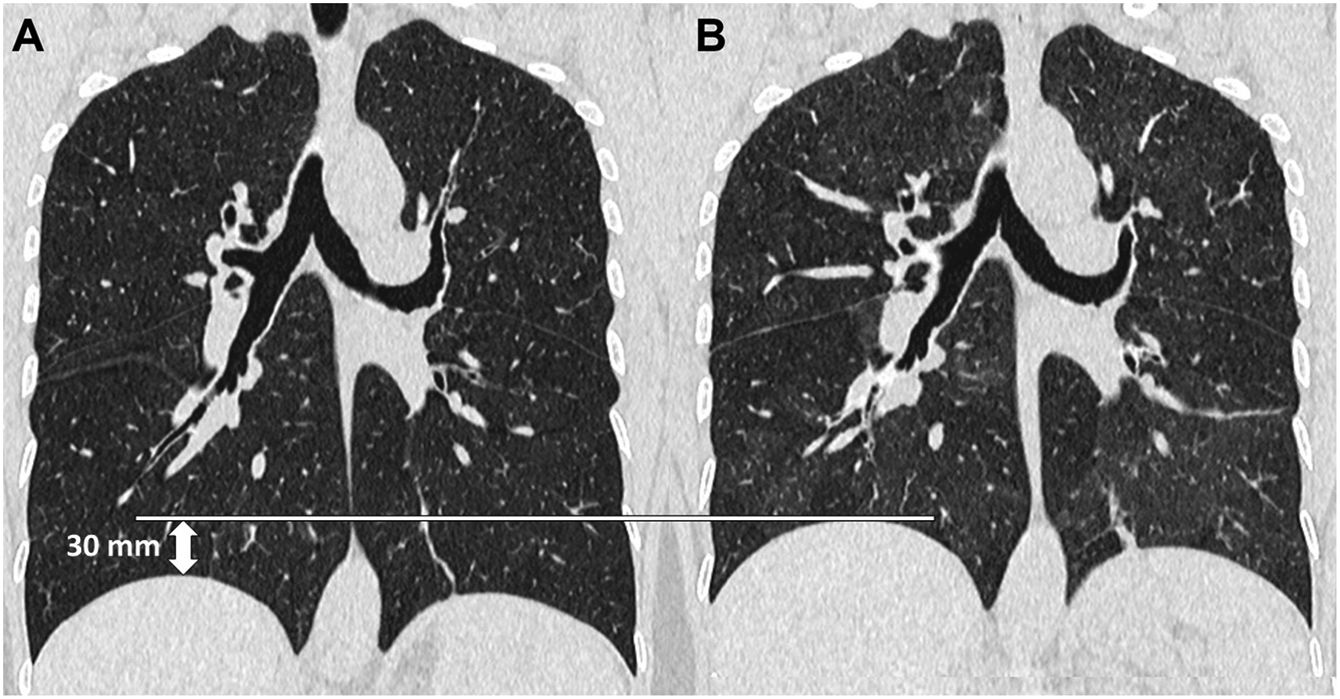
The ideal, therefore, would be to have an easily reproducible quantitative measure to assess the expiratory effort. The change in lung volume is the most reliable data, but this value is not routinely obtained in clinical practice. In its place, Azour et al.12,13 have proposed diaphragmatic excursion, a measure of the range of diaphragmatic movement during respiratory manoeuvres, as a marker of lung volume change. The diaphragmatic excursion can be quickly calculated by the radiologist using the first axial image in which the lung apex appears as the upper end and the one in which the diaphragm appears as the lower end, multiplying the number of images by the slice thickness and subtracting from the result in inspiration that obtained in the study in expiration. The mean value obtained by these authors in a group of 83 patients was 31 mm, considering a limited respiratory excursion if the value was less than 30 mm and acceptable if it was greater than or equal to 30 mm. This parameter can be useful when qualitative data such as tracheal morphology or increased density of the lung parenchyma do not clearly demonstrate that adequate expiration has been achieved (Fig. 2).
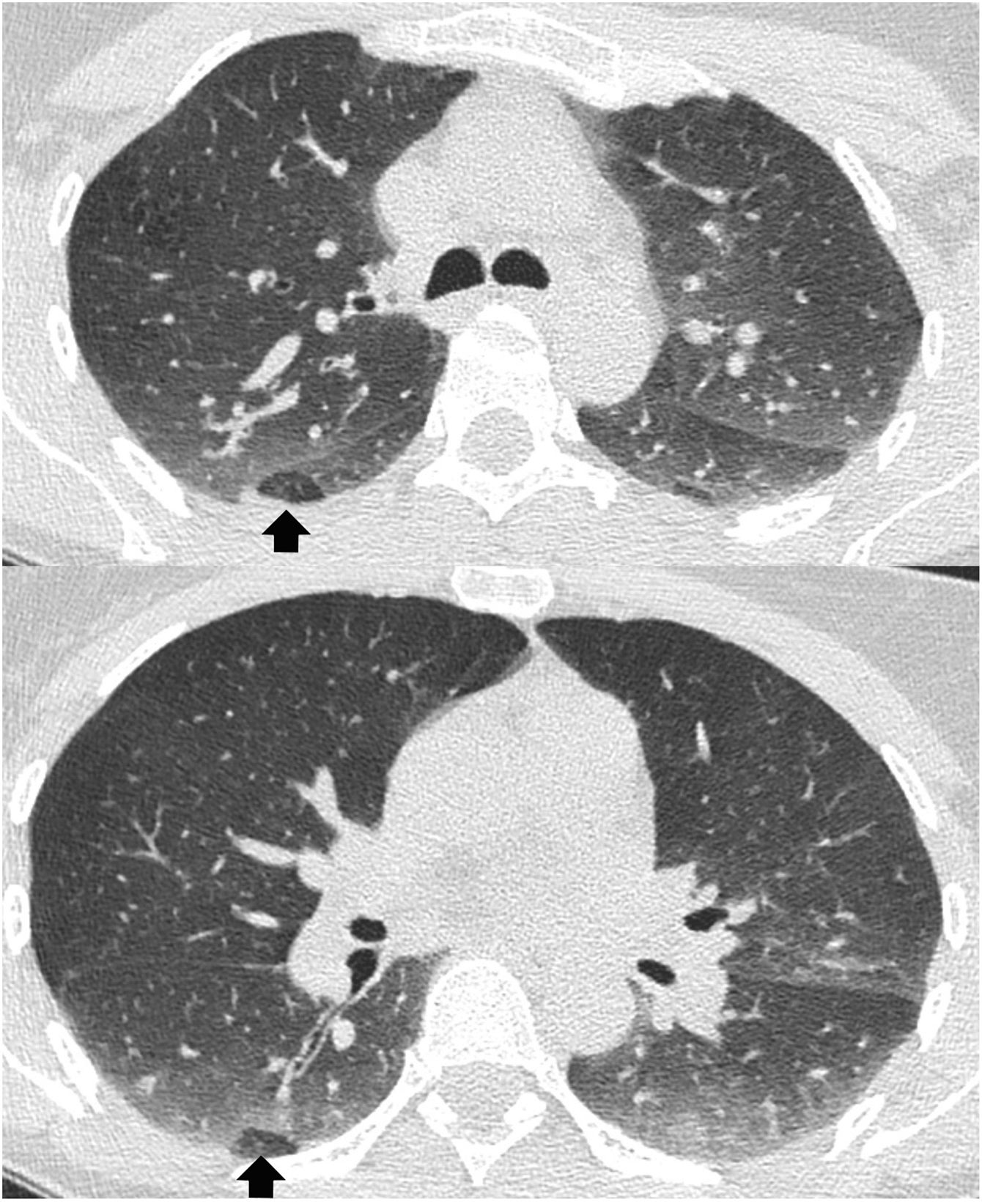
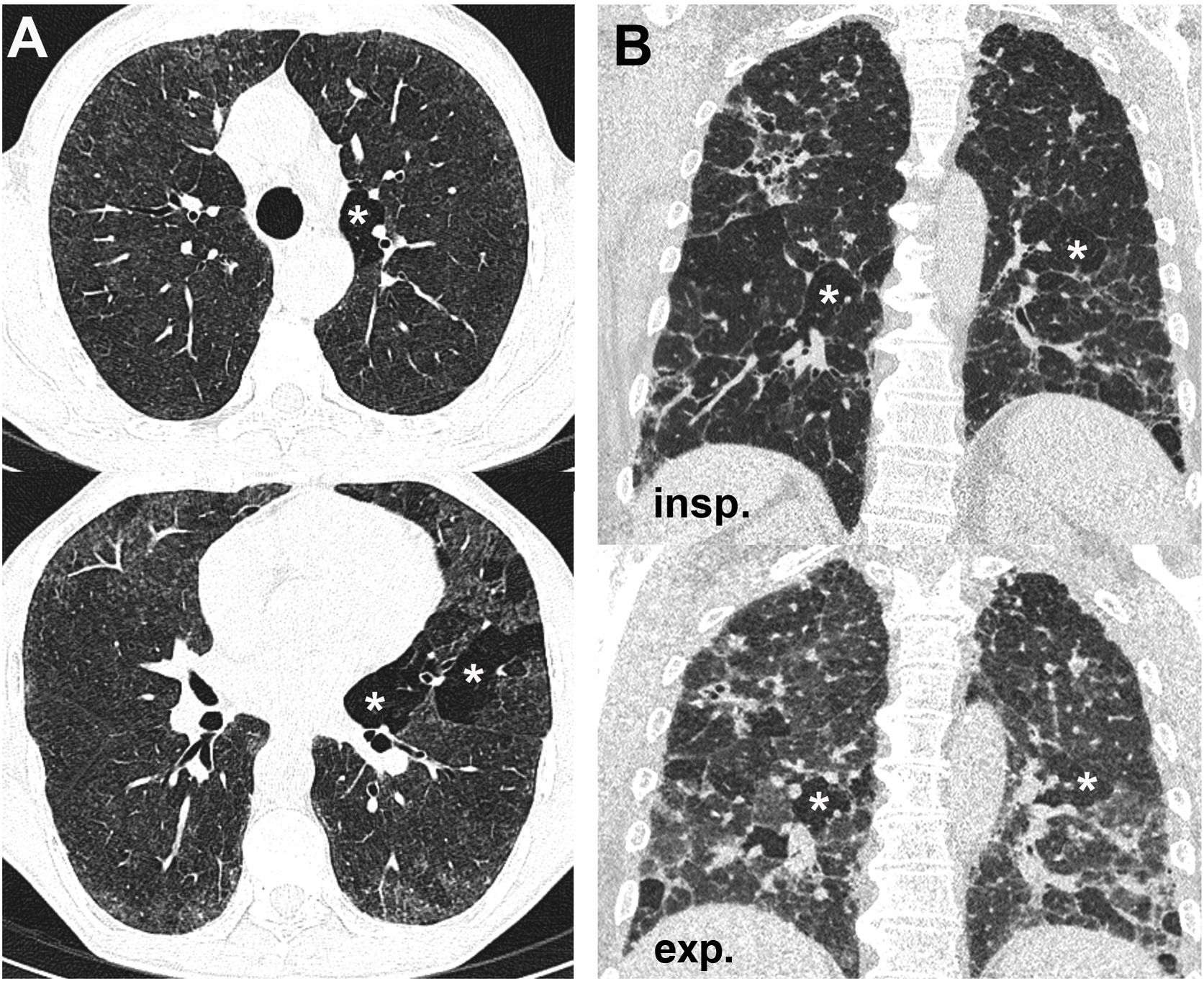
Air trapping in asymptomatic subjectsThe presence of some degree of air trapping may be an incidental finding unrelated to underlying pathology. In the original article by Webb et al.1 from 1993 the existence of small foci of air trapping during expiration was described in 40% of asymptomatic individuals with normal PFTs. Two possible causes of this high prevalence of lobular air trapping in patients with normal PFT have been suggested: on the one hand, differences in focal lung compliance and muscle tone of the small airway, but without alteration of the airway, and, on the other, small airway pathology too mild to be detected on PFTs. A later study with more volunteers showed that this type of trapping appeared in 62% of participants14, especially in the posterior and inferior areas of both lungs. Up to three adjacent secondary lobules or less than one lung segment were affected in 47% of them (lobular air trapping), and the authors interpreted this finding as not representing small airway disease (Fig. 3). However, in cases of major involvement of a lung segment, which occurs especially in smokers and heavy smokers, it is due to true bronchiolar obstructive pathology.
Quantification of air trappingAlthough in clinical practice we usually assess air trapping on expiratory CT scan subjectively (visually), its quantification as a marker of small airway disease has been a very active area of research in recent years, especially in patients with chronic obstructive pulmonary disease (COPD). COPD produces irreversible structural changes in the lung, including destruction of the parenchyma (emphysema), remodelling of the large airway, and reduction in the calibre of the small airway.15 In an initial phase of the investigation, thickening of the bronchial walls was used as a surrogate marker of small airway remodelling, but more recent work has focused on the direct quantification of air trapping.16 Traditionally, air trapping has been quantified using physiological measurements obtained from spirometry, of which the most sensitive is forced flow in the mid-expiratory phase (FEF 25–75%).17 The forced expiratory volume in the first second (FEV1), for its part, reflects the initial portion of the expiratory curve and is affected only in more advanced phases. Currently, some authors consider that expiratory CT scan is more sensitive than PFTs17,18, since it detects air trapping earlier than spirometric indices, and is a powerful tool to determine the severity of bronchiolar obstruction.
There are several ways to quantify air trapping using combined inspiration and expiration CT scan. The simplest would be to use the ratio between the mean lung density (or volume) in expiration and in inspiration. This density (or volume) E/I ratio correlates well with spirometric indices of small airway disease and respiratory morbidity indices (dyspnoea, quality of life and 6-minute walk test), but does not discriminate between emphysematous and non-emphysematous air trapping.19 Both tend to coexist in COPD, since in addition to producing bronchiolar narrowing, lung destruction in areas of emphysema causes the elastic recovery force necessary to expel air to be lost. To specifically measure non-emphysematous air trapping, which represents true small airway disease, an application called "Parametric Response Map" (PRM) has been proposed, in which the images in inspiration and expiration are distorted to be able to compare them voxel to voxel.19 The parameter obtained would be the percentage of voxels with a density greater than −950 HU on inspiration (normal lung value, which would exclude emphysema) but less than −856 HU on expiration (below the normal value: non-emphysematous trapping). The PRM therefore, would provide spatial information on the distribution of emphysema and small airway disease, allowing the measurement and monitoring of both components separately.19
When to perform an expiration CT scanIn the latest version of the ATS/ERS/JRS/ALAT guidelines for the diagnosis of idiopathic pulmonary fibrosis20 it is recommended to systematically perform an expiratory CT scan in the study of interstitial disease, since if there is extensive air trapping, the diagnosis will be "alternative to the usual interstitial pneumonia." It would also be indicated in the evaluation of disease of the large or small airways, including tracheomalacia (Fig. 11A), bronchiolitis obliterans, Swyer-James syndrome, hypersensitivity pneumonitis, cystic fibrosis, sarcoidosis, and tobacco-related interstitial diseases. Although CT scan is not considered essential in the diagnosis of COPD, detection of air trapping can help in early identification, since small airway involvement precedes lung destruction (emphysema).21 In the same way, asthma affects not only the bronchi, but also the small airways, and the detection of air trapping can be an early sign of the disease.22
Sometimes, however, a mosaic pattern may appear incidentally on inspiratory CT scan without a clear cause, forcing the prescription of a complementary expiratory CT scan to be performed immediately (if the radiologist is available for review) or at a new appointment.
Gaeta et al.6 suggest performing an expiratory CT scan, even with a normal inspiratory CT scan, in the following scenarios:
- •
Patients with PFTs that show an obstructive pattern, especially of the small airway.
- •
Patients with chronic cough and/or wheezing.
- •
Patients with exertional dyspnoea.
- •
Patients with diseases that have a component of involvement of the small airway (suspected or proven), mainly hypersensitivity pneumonitis, sarcoidosis and diseases associated with bronchiolitis obliterans.
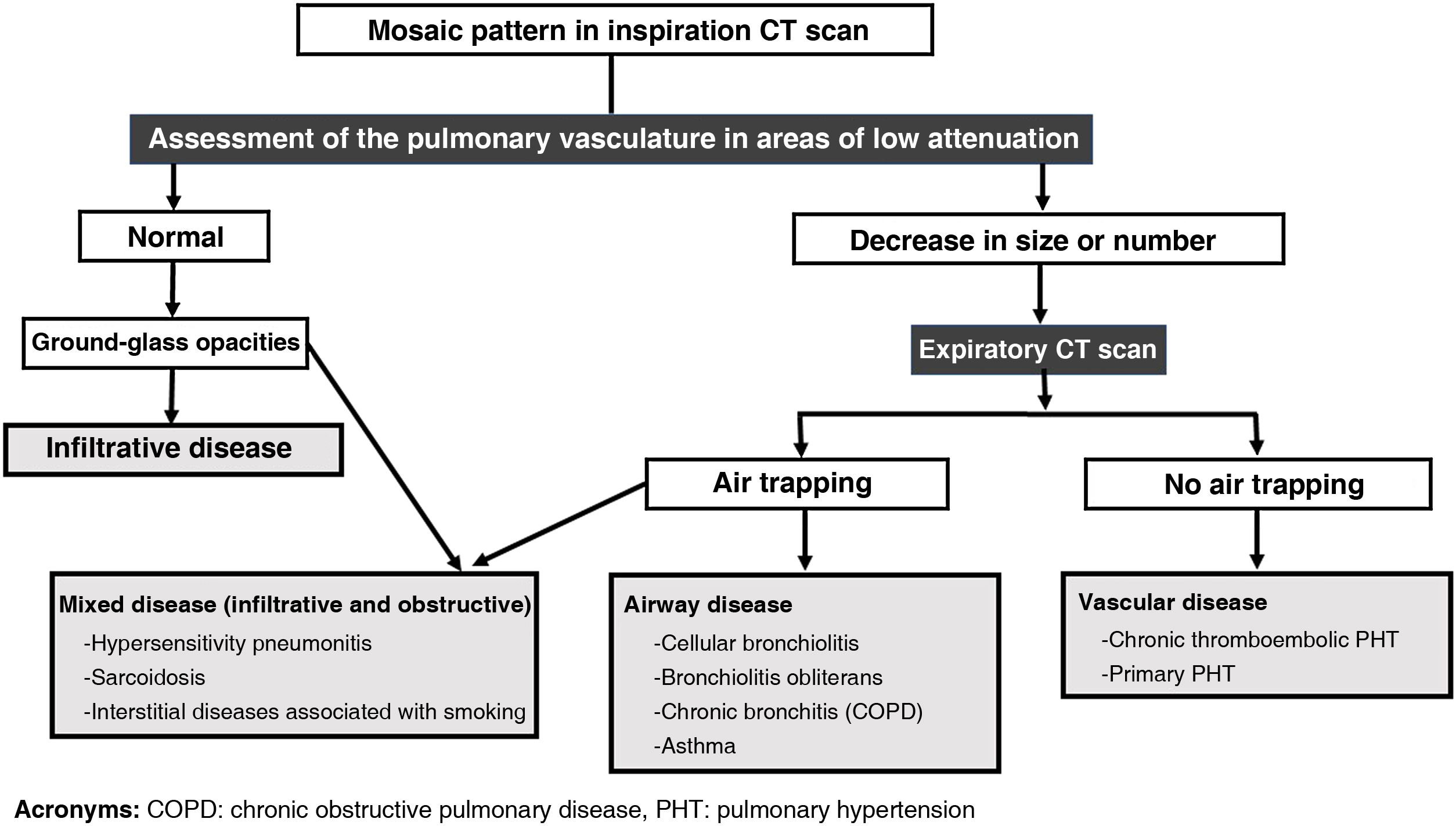
The main objective of the expiratory CT scan is to identify air trapping, a definitive sign of small airway disease. Air trapping may not be evident on inspiratory CT scan or may manifest as "mosaic density," a radiological pattern defined by the Fleischner Society as heterogeneous attenuation of the lung parenchyma on inspiratory CT scan, with patchy areas of major and minor density.2 This finding is not a diagnosis in itself, but rather a sign that may be due to multiple entities that can be grouped into three categories: obstructive pathology of the small airway, pulmonary occlusive disease, and interstitial disease with a ground-glass pattern of patchy distribution. Interstitial disease accounts for approximately half of the cases, small airway disease accounts for a third of them, and the rest would correspond to pathology of the pulmonary vasculature.23,24
The first step in identifying the cause of the mosaic pattern on inspiratory CT scan is to determine which areas of the parenchyma have normal attenuation and which are abnormal. In some cases it may be obvious, but in others it is difficult to know if the pathological areas are the least dense, the most dense, or both.
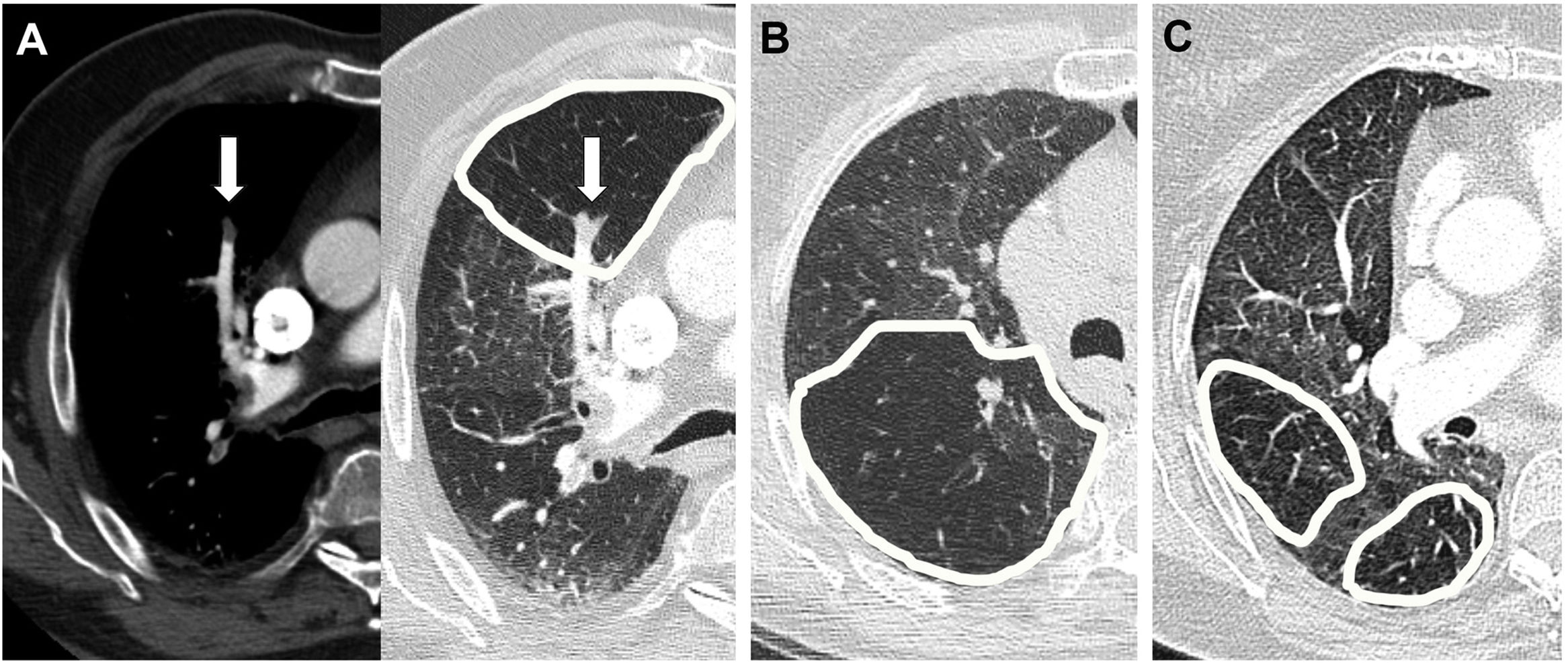
An important clue is provided by the size of the peripheral pulmonary arteries in the areas of lower density (Figs. 4 and 5): when the cause is vascular, for example in chronic thromboembolic pulmonary hypertension (CTEPH), segmental pulmonary arteries and subsegmental cells are of smaller calibre in low-density areas, as they are stenotic or occluded. This finding can also be found, although less pronounced, in small airway disease, since the obstruction or fibrosis of the bronchioles leads to hypoxic vasoconstriction in the affected lung, with a reduction in the calibre of the vessels and diversion of blood towards adjacent areas that have better gas exchange. In the case of patchy ground-glass interstitial disease, however, the size and number of peripheral pulmonary arteries are uniform throughout the lung parenchyma, in both the denser and less dense portions. Thus, when we find a decrease in the size of the peripheral pulmonary vasculature in the less dense areas, we must think that the cause of the mosaic pattern may be vascular in nature or be related to a disease of the small airway, ruling out an infiltrative disease.
Mosaic pattern: assessment of the size of the peripheral pulmonary arteries in less dense areas. A) In obstructive vascular disease (chronic thromboembolic pulmonary hypertension, CTEPH) there is a regional decrease in blood flow due to thrombosis; B) in small airway disease (bronchiolitis) hypoxia and reflex vasoconstriction occur with decreased vessels, while in (C) patchy interstitial disease the vessels are normal in areas of lower density.
A second step would be to perform an expiratory CT scan to detect air trapping (Fig. 5), which would differentiate small airway disease from the rest of the mosaic pattern causes. In patients with a mosaic pattern unrelated to the airway, the lung parenchyma shows a diffuse increase in density throughout the lung on expiration. For example, in CTEPH we will observe an increase in density both in the abnormal areas of lower attenuation (hypoperfused) and in the higher-density normal lung, and the same occurs in patients with patchy ground-glass interstitial disease, where the affected areas increase in density on expiration, like normal lung with lower attenuation. In contrast, in small airway disease, partial obstruction of the bronchioles produces a valvular phenomenon, allowing air to enter the alveoli during inspiration but not out on expiration. These areas of air trapping, therefore, do not increase in density or decrease in volume during expiration, unlike the rest of the normal lung.25 Occasionally, some degree of air trapping may also occur in patients with CTEPH due to reflex bronchoconstriction that occurs in the hypoperfused lung.
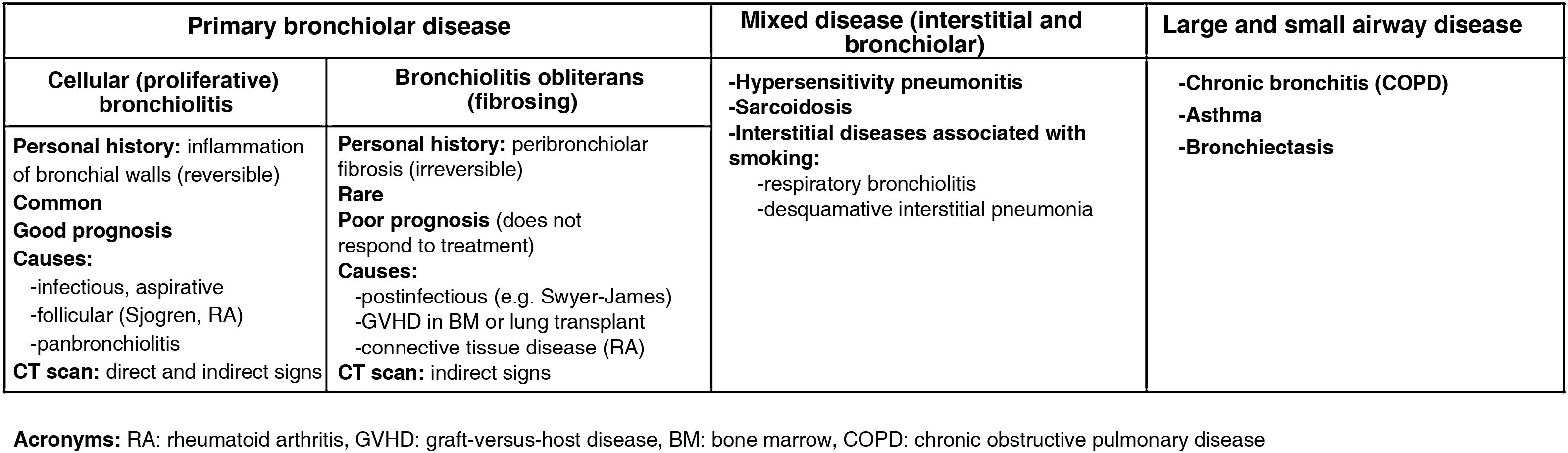
From a practical point of view, the pathology of the small airway can be classified into three groups: primary bronchiolar diseases (bronchiolitis obliterans, infectious bronchiolitis, aspiration bronchiolitis, follicular bronchiolitis, panbronchiolitis), mixed diseases with an associated bronchiolar and interstitial component (hypersensitivity pneumonitis, sarcoidosis, tobacco-associated interstitial pneumonia) and diseases of the large and small airways (chronic bronchitis, asthma, bronchiectasis) (Fig. 6).25
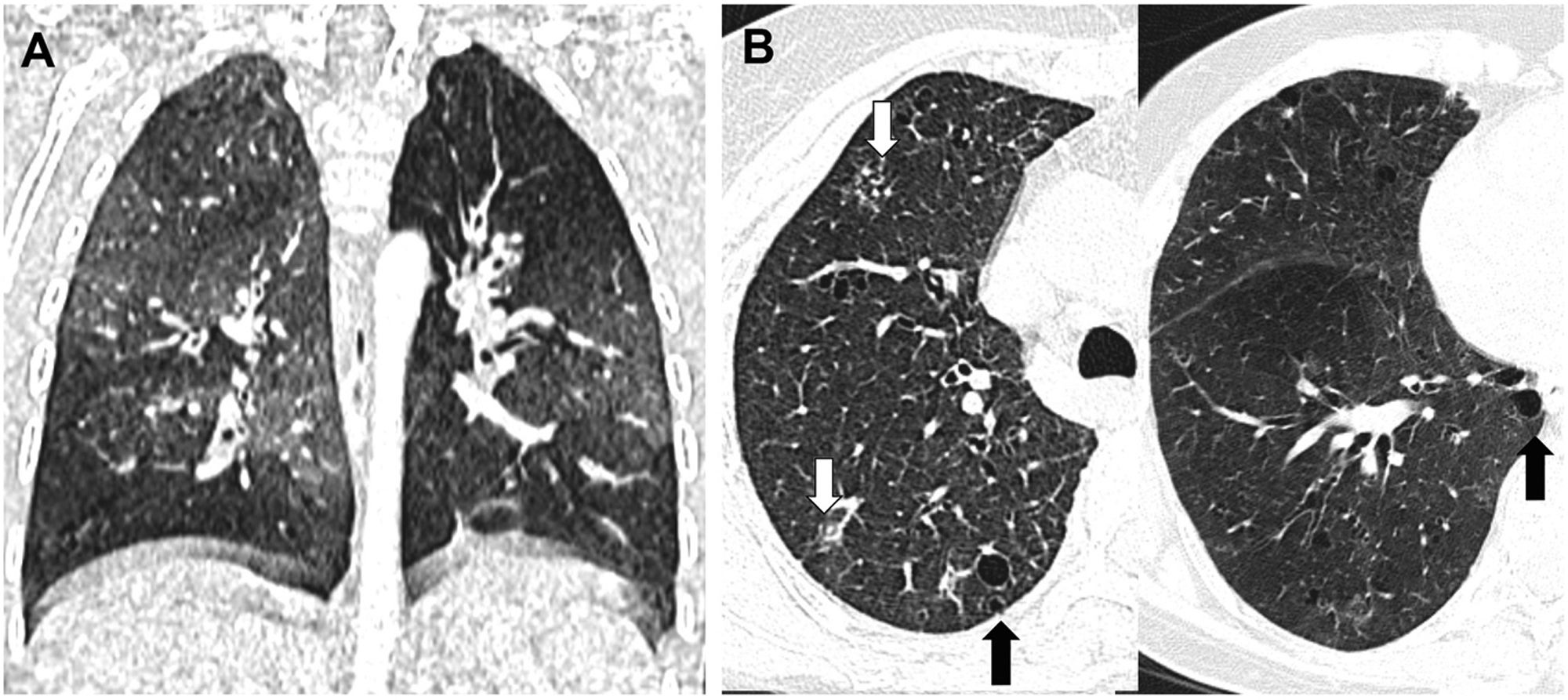
Bronchiolitis, in turn, can be divided into cellular or proliferative (reversible) bronchiolitis such as infectious bronchiolitis (the most frequent, caused by viruses, bacteria or mycobacteria), respiratory bronchiolitis (caused by tobacco smoke) and follicular bronchiolitis (lymphoid hyperplasia associated with autoimmune diseases such as Sjögren's syndrome), and on the other hand, fibrosing (irreversible) bronchiolitis such as bronchiolitis obliterans.26 In cellular bronchiolitis (Fig. 7) there are usually direct signs of involvement of the small airway on CT scan, such as tree-in-bud images produced by centrilobular micronodules that represent small bronchiolar mucosal impactions. However, in obliterative bronchiolitis, also called constrictive, there is irreversible peribronchiolar stenosis caused by submucosal fibrosis and, as there is no impaction or inflammatory phenomena, there will be no direct signs of bronchiolar involvement, but only indirect signs such as a mosaic pattern in inspiration or expiratory air trapping. Bronchiolitis obliterans can be an autoimmune complication of rheumatic diseases such as rheumatoid arthritis (Fig. 8A), a late sequela of viral infections in childhood (Swyer-James syndrome) (Fig. 8B) or a form of chronic rejection in patients with lung or bone marrow transplantation (Fig. 9). In the latter case, it manifests as a decrease in FEV1, dry cough and dyspnoea, and has a mortality of 12–27%.27 It usually appears as a mosaic pattern on inspiratory CT scan, and in late stages with associated bronchiectasis, although it often only manifests as air trapping on expiration.
Cellular or proliferative bronchiolitis. A) Infectious bronchiolitis due to adenovirus in a 2-year-old boy, with extensive air trapping; and (B) follicular bronchiolitis in a patient diagnosed with Sjögren's syndrome, showing direct signs of small airway involvement with centrilobular micronodules in the upper lobe (white arrows) and indirect signs in the form of air trapping in the lower lobe. The presence is observed in addition to air cysts suggestive of lymphoid interstitial pneumonia (black arrows).
Fibrosing bronchiolitis. A) Bronchiolitis obliterans in rheumatoid arthritis, with the presence of large bilateral areas of air trapping and (B) bronchiolitis obliterans as a late sequela to viral infection in childhood (Swyer-James syndrome). It usually affects more than one lung, which is why it was called "unilateral hyperlucent lung syndrome." The decrease in the calibre of the vessels associated with the extensive air trapping of the left lung is observed (arrows).
In addition to primary bronchiolar diseases, the second group would be that of mixed diseases., which combine involvement of the small airway with interstitial disease. In them, air trapping due to bronchiolar disease can coexist with interstitial disease, either in the form of ground-glass density centrilobular micronodules (for example, in hypersensitivity pneumonitis (Fig. 10A) or tobacco-related interstitial diseases) or in the form of solid micronodules with a perilymphatic distribution as in sarcoidosis. All of them can progress to fibrosis in the chronic phase and in hypersensitivity pneumonitis the “head cheese or three-density pattern” can also appear, resulting from the combination of areas of hyperdense lung (infiltrative component), normal lung and hypodense lung (air trapping) (Fig. 10B)28.
Hypersensitivity pneumonia in non-fibrosing (A) and fibrosing (B) phases. The first shows diffuse ground-glass centrilobular nodules with areas of air trapping (asterisks), while the second shows signs of fibrosis and areas of ground glass. The combination of the latter with normal lung and air trapping zones (asterisks) produces the typical "three-density pattern.".
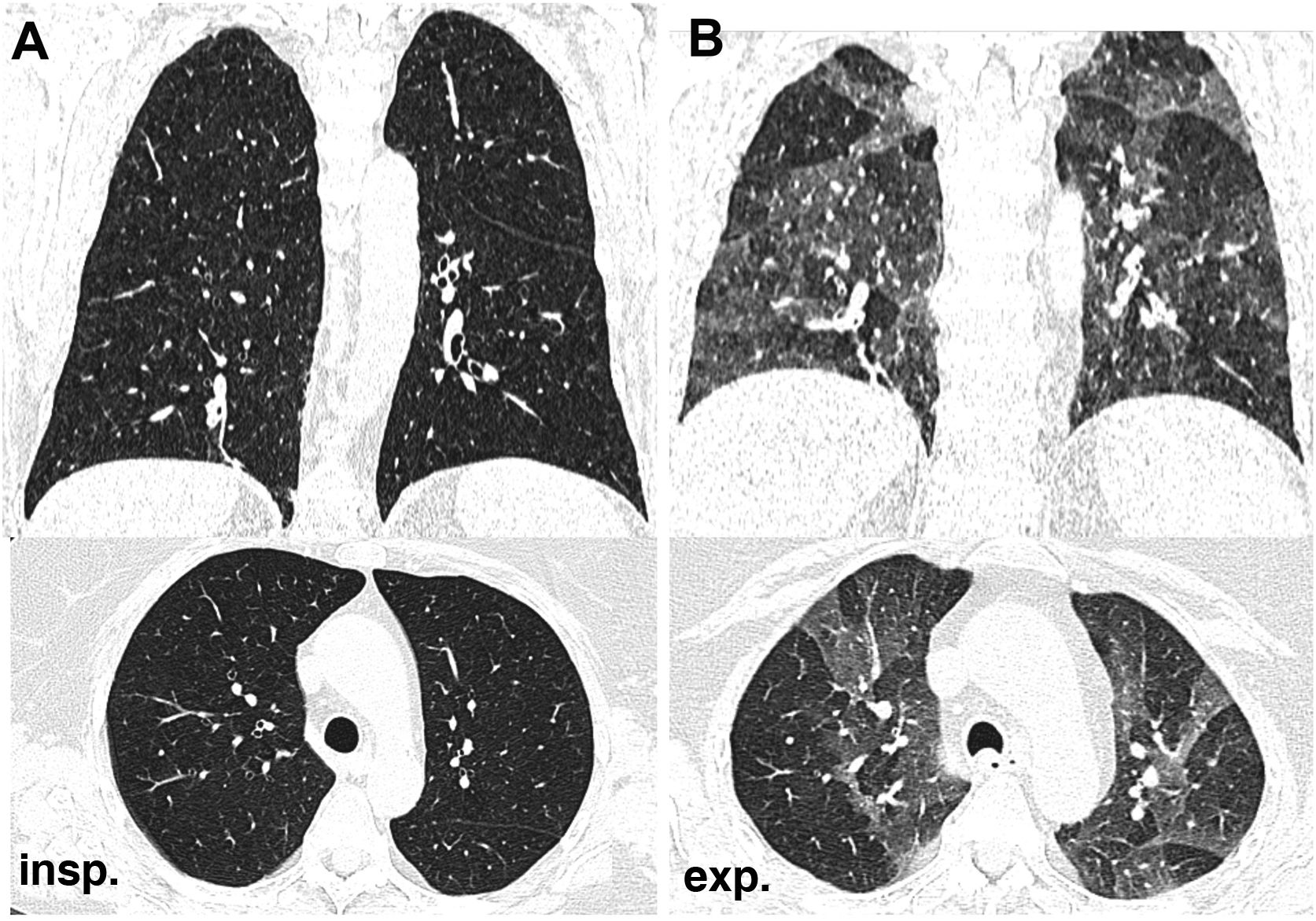
Large airway disease. A) Tracheomalacia, which can affect the trachea and main bronchi, observing excessive tracheal collapse during expiration that produces a crescent-shaped image of the tracheal lumen and (B) asthma, which combines involvement of the large and small airways. In this case, extensive areas of expiratory air trapping are observed with normal inspiratory CT scan. It can be associated with bronchiectasis.
Lastly, the third large group of diseases of the small airway is that associated with pathology of the large airway (chronic bronchitis, asthma and bronchiectasis). COPD is associated with irreversible structural changes in the lung, which include destruction of the parenchyma (emphysema), remodelling of the large airway (chronic bronchitis) and small airway obstruction. Similarly, air trapping together with thickening of the bronchial walls and bronchiectasis are the typical characteristics of asthma. In the initial phases, moreover, air trapping may be the only radiological sign of this disease (Fig. 11B).
ConclusionsExpiratory chest CT scan is a complementary technique to inspiration, especially useful in patients with suspected obstructive pathology of the small airway. To have diagnostic utility, the study must be of good quality. Demonstration of air trapping on expiration is an important finding that should always be assessed in conjunction with the rest of the clinical and imaging data.
Authorship- 1
Responsible for the integrity of the study: AMdAA.
- 2
Study concept: AMdAA, ABN.
- 3
Obtaining data: AMdAA, ABN, DUN, MDR.
- 4
Data analysis and interpretation: AMdAA, ABN.
- 5
Literature search: DUN, MDR.
- 6
Drafting of the article: AMdAA, ABN.
- 7
Critical review of the manuscript with intellectually significant contributions: AMdAA, ABN, DUN, MDR.
- 8
Approval of the final version: AMdAA, ABN, DUN, MDR.
The authors declare that they have no conflicts of interest.