To review the pathophysiology of Fontan-associated liver disease, its histologic changes, and its radiologic manifestations.
ConclusionsFontan-associated liver disease is the result of a set of structural and functional changes in the liver that occur secondary to hemodynamic changes brought about by Fontan surgery. The radiologic manifestations of Fontan-associated liver disease consist of changes in the size and shape of the liver, alterations in the signal intensity or pattern of enhancement, abnormalities in the vascular structures, and focal lesions, which include benign nodules with intense uptake in the arterial phase and hepatocellular carcinoma. Radiologists need to be familiar with this disease and its complications, because the number of patients who undergo Fontan surgery continues to increase, and these patients undergo an increasing number of imaging tests.
Revisar la fisiopatología de la enfermedad hepática crónica asociada a la cirugía de Fontan, sus cambios histológicos y sus manifestaciones radiológicas.
ConclusionesLa enfermedad hepática crónica asociada a la cirugía de Fontan corresponde al conjunto de cambios estructurales y funcionales hepáticos secundarios a las modificaciones hemodinámicas propias de esta cirugía. Sus manifestaciones radiológicas comprenden: alteraciones en el tamaño y contorno hepático, alteraciones en el patrón de realce o intensidad de señal, anomalías en las estructuras vasculares y lesiones focales, que incluyen nódulos benignos hipercaptantes en fase arterial y carcinoma hepatocelular. El radiólogo debe de estar familiarizado con esta patología y sus complicaciones, puesto que los pacientes con cirugía de Fontan representan una población cada vez mayor, candidata a un número creciente de pruebas de imagen.
The Fontan procedure (FP) is a palliative procedure in which systemic venous return is redirected to the pulmonary circulation bypassing the right ventricle. This technique is used in complex congenital heart conditions whose common characteristic is having only one functioning ventricle.1 The congenital heart defects most commonly treated with the FP are: tricuspid atresia, pulmonary atresia with intact ventricular septum, hypoplastic left heart syndrome and double-inlet ventricle.2
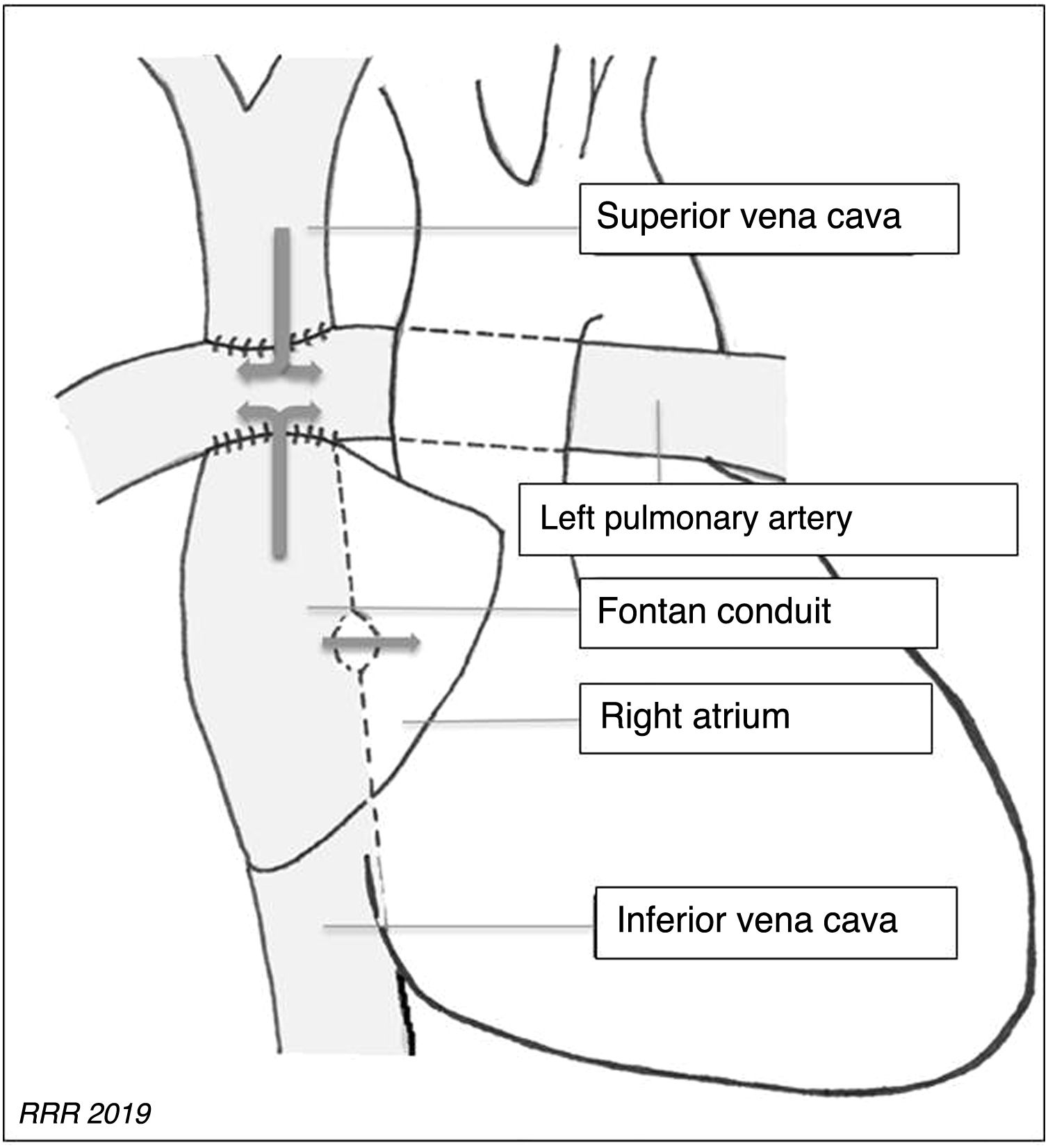
The original Fontan procedure involved directly connecting the right atrium to the pulmonary arteries (atriopulmonary variant). This technique was associated with a significant risk of arrhythmia and thrombosis and is now considered obsolete.1 The current FP (total cavopulmonary variant) is performed in two stages, which allows the patient to adapt to the haemodynamic changes and reduces surgical morbidity and mortality.2 These stages are:
- 1
Partial cavopulmonary connection or bidirectional Glenn procedure: connects the superior vena cava (SVC) to the pulmonary arterial circulation.
- 2
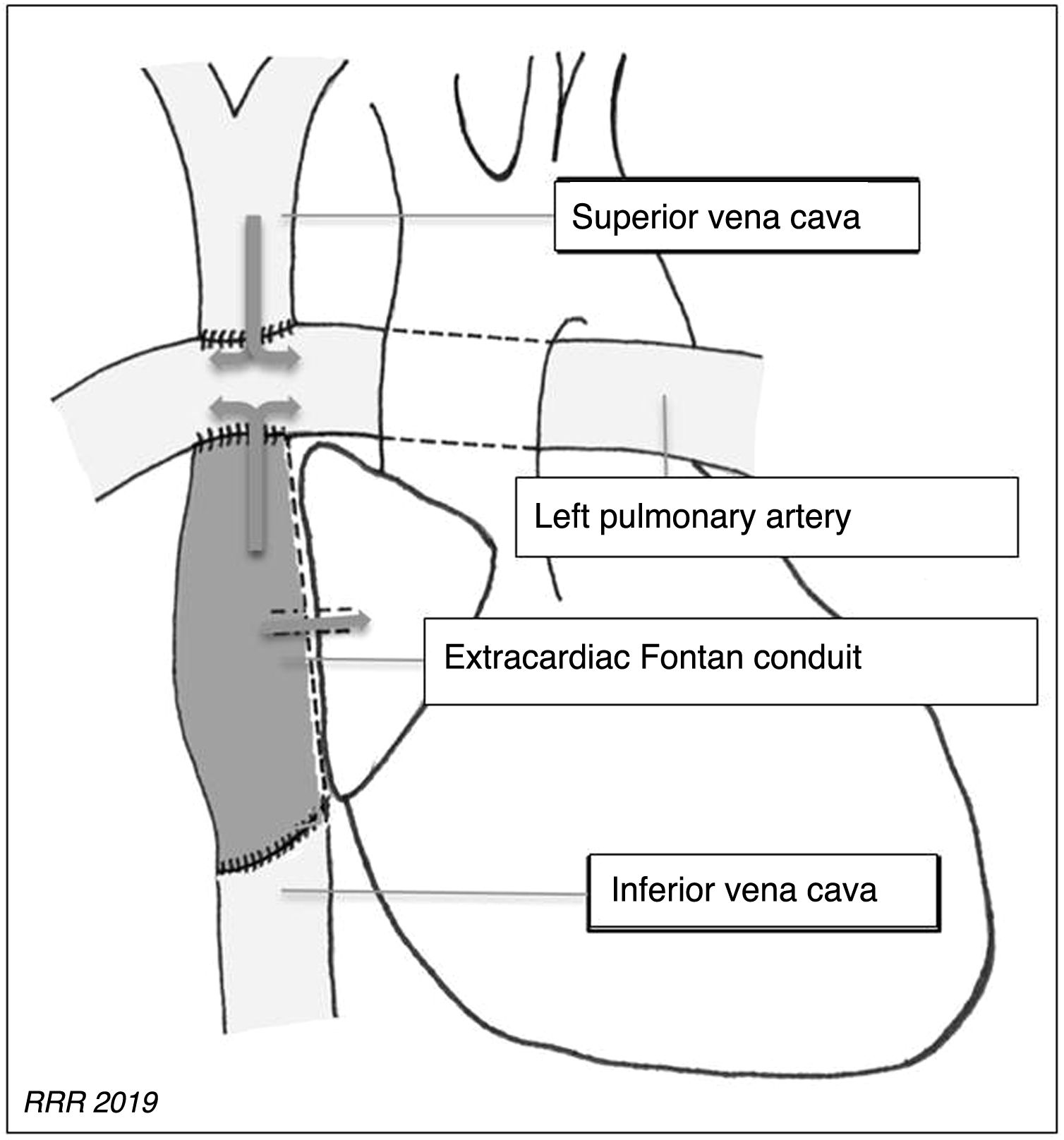
Total cavopulmonary connection or modified Fontan procedure: creates a direct connection between the inferior vena cava (IVC) and the pulmonary arterial circulation, preserving the previous Glenn shunt. The connection between the IVC and the pulmonary arteries is created using a conduit that can be intra-atrial or extracardiac (Figs. 1 and 2).
FP patients represent a growing population that mostly survives into adulthood. With the current technique, 15-year survival after surgery is 95%.3 However, the haemodynamic changes after the FP lead to significant long-term complications in multiple organs, such as peripheral venous insufficiency, protein-losing enteropathy and Fontan-associated liver disease (FALD).4
FALD is defined as the structural and functional liver abnormalities resulting from the haemodynamic changes caused by Fontan circulation, excluding other plausible causes of liver damage: viral, drug toxicity or alcohol.5
It must be remembered that FALD is a broad term that encompasses morphological and structural changes in the liver parenchyma, hypervascular liver nodules and liver cirrhosis.6
The pathophysiology of liver dysfunction after FP involves an elevation of central venous pressure (CVP) and a decrease in cardiac output.1,5–7
The FP invariably causes a sustained increase in CVP and chronic passive venous congestion.4,8 The elevation of the CVP is transmitted to the suprahepatic veins and the sinusoids, which reduces portal venous flow, partially compensated by increased arterial vascularisation. It also leads to sinusoidal hypertension and congestion leading to oedema in the perisinusoidal space, situated between the hepatocytes and the sinusoidal endothelium. The oedema in the perisinusoidal space and the reduced portal flow hinder the diffusion of oxygen and nutrients to the hepatocytes and, ultimately, lead to atrophy and hepatocellular necrosis with fibrogenic phenomena.1,4,9–11
The majority of patients with FP have a reduced cardiac output leading to hypoxaemia in different organs, including the liver. Hypoxaemia aggravates and contributes to hepatocellular necrosis and to the profibrogenic state generated by the elevation of CVP.1,4,7
In addition, deficient lymphatic drainage in patients with FP could increase the oedema in the liver interstitium and contribute to the pathophysiology of FALD.12 Nevertheless, the role of lymphatic dysfunction in FALD requires more study.
Histological changes in FALDThe pathophysiological phenomena described and the complex architecture of the liver, with its double vascular supply and a single venous drainage, determine the histological abnormalities of FALD.
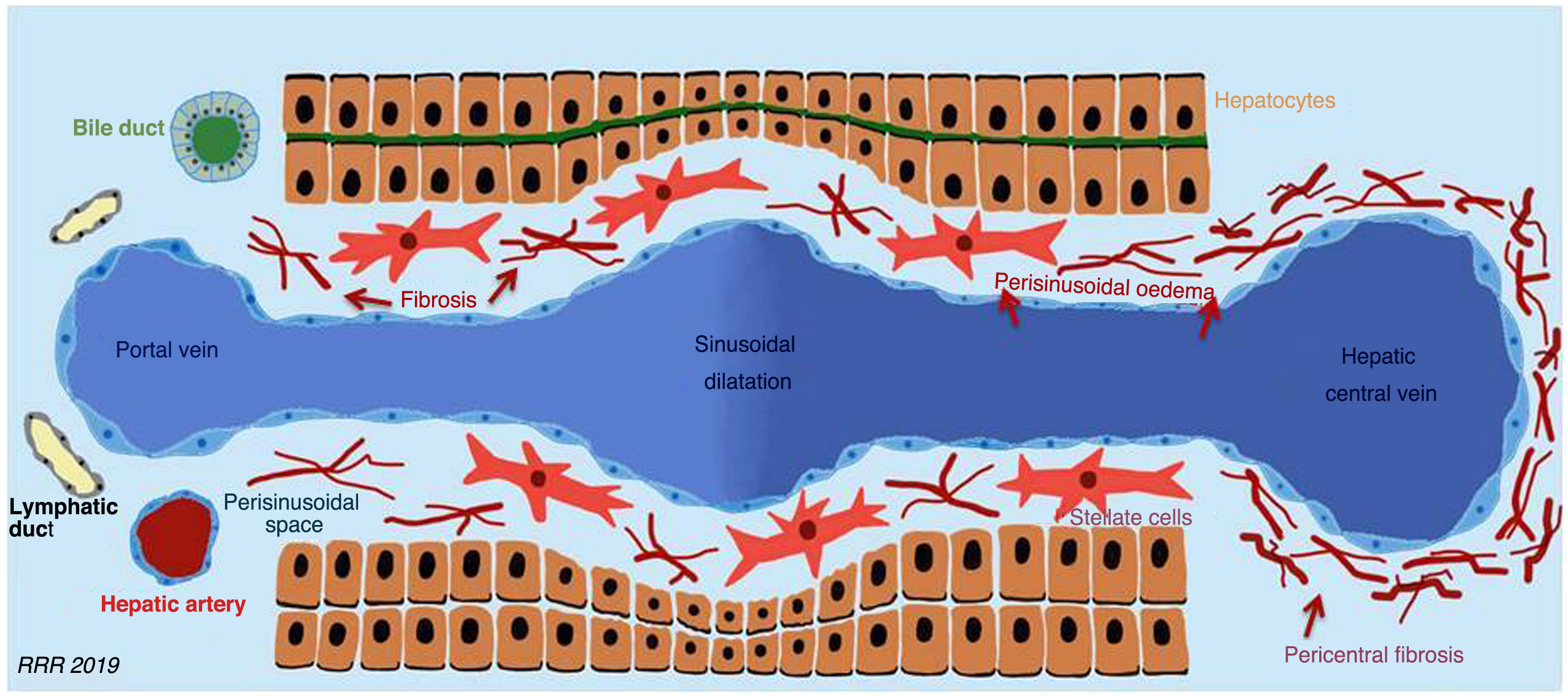
The histological changes start with the dilatation of the hepatic central vein, of the hepatic sinusoid and of the periportal lymph vessels. Hepatocyte atrophy then occurs due to the reduced supply of oxygen and nutrients to the hepatocytes, which induces the activation of stellate cells, with great fibrogenic capacity, that favour the progressive deposition of collagen. Fibrosis, atrophy and hepatocellular necrosis are more evident in the proximity of the hepatic central vein (zone 3), as it is harder for oxygen to reach these areas. In more advanced stages, fibrous bridges are established between the centrilobular zones, which end in global cirrhosis with areas of regeneration. This pattern, known as “inverse cirrhosis”, contrasts with fibrosis secondary to alcoholic cirrhosis or hepatitis C-induced fibrosis, where bridging fibrosis develops between the portal spaces1,4,5,9,10 (Fig. 3).
Histological changes in Fontan-associated liver disease that include the dilatation of the hepatic central vein and of the liver sinusoid, hepatocyte atrophy and the secondary activation of hepatic stellate cells. The fibrosis process is more intense in the region close to the hepatic central vein.
The natural history of FALD is hard to predict and its course essentially depends on the heart condition.1 Nevertheless, the time elapsed since the procedure is a determining factor for the degree of hepatic fibrosis, which is a universal histological finding in patients with FP.13
Role of imaging techniques in FALDUltrasound, computerised tomography (CT) and magnetic resonance imaging (MRI) are used to evaluate the presence of signs of FALD and its complications, and they are especially important in the detection and characterisation of liver nodules.
The usefulness of elastography is limited in patients with FALD, due to its inability to distinguish between the contribution of the venous congestion and the contribution of the fibrosis to the liver stiffness values (LSV).14 Monitoring the change in the LSVs in these patients could be more clinically beneficial than an isolated evaluation.15,16
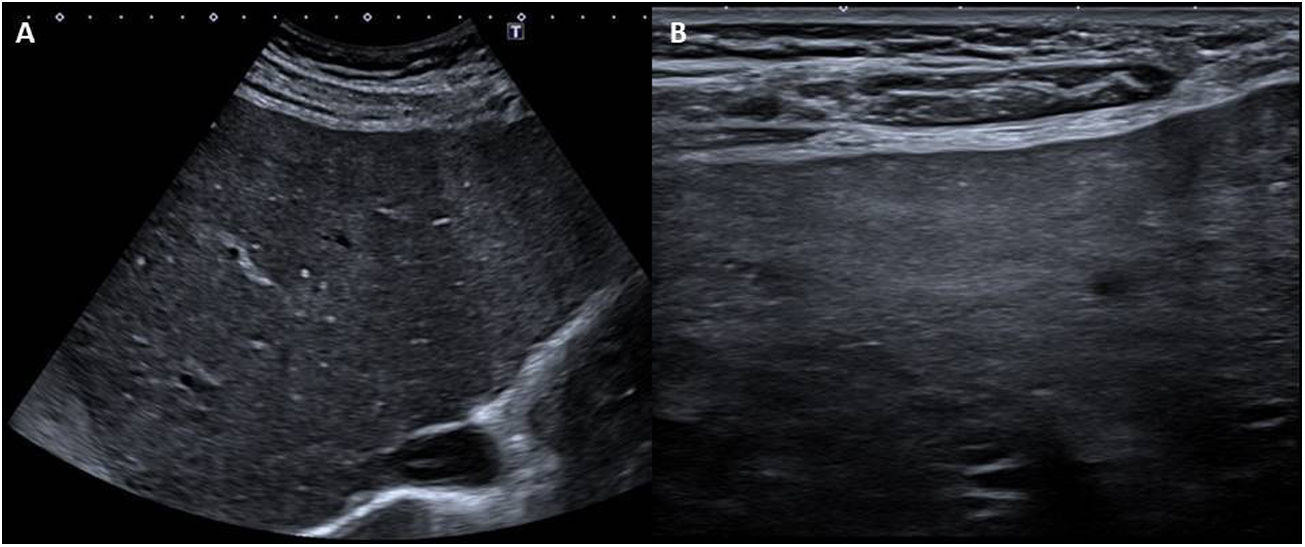
Radiological findings in FALDParenchymal and vascular abnormalities in FALDUltrasound and doppler ultrasoundAt the onset of the disease, the size of the liver is usually normal and its echogenicity is normal or slightly hypoechogenic. With the development of fibrosis and cirrhosis, nodularity and irregularity of the liver contour develop, and these are visible with high and low frequency probes, heterogeneous parenchymal echogenicity (favoured by the presence of nodules), areas of focal atrophy and hypertrophy of the caudate lobe3,5,6,17–20 (Fig. 4).
Woman aged 26 years with extracardiac Fontan procedure 20 years ago for double inlet single left ventricle heart with pulmonary atresia. Ultrasound images with low and high frequency probes (A and B, respectively), which show moderately heterogeneous parenchymal echogenicity, as well as nodularity and irregularity of the liver contour (more visible with the linear probe), signs of advance Fontan-associated liver disease.
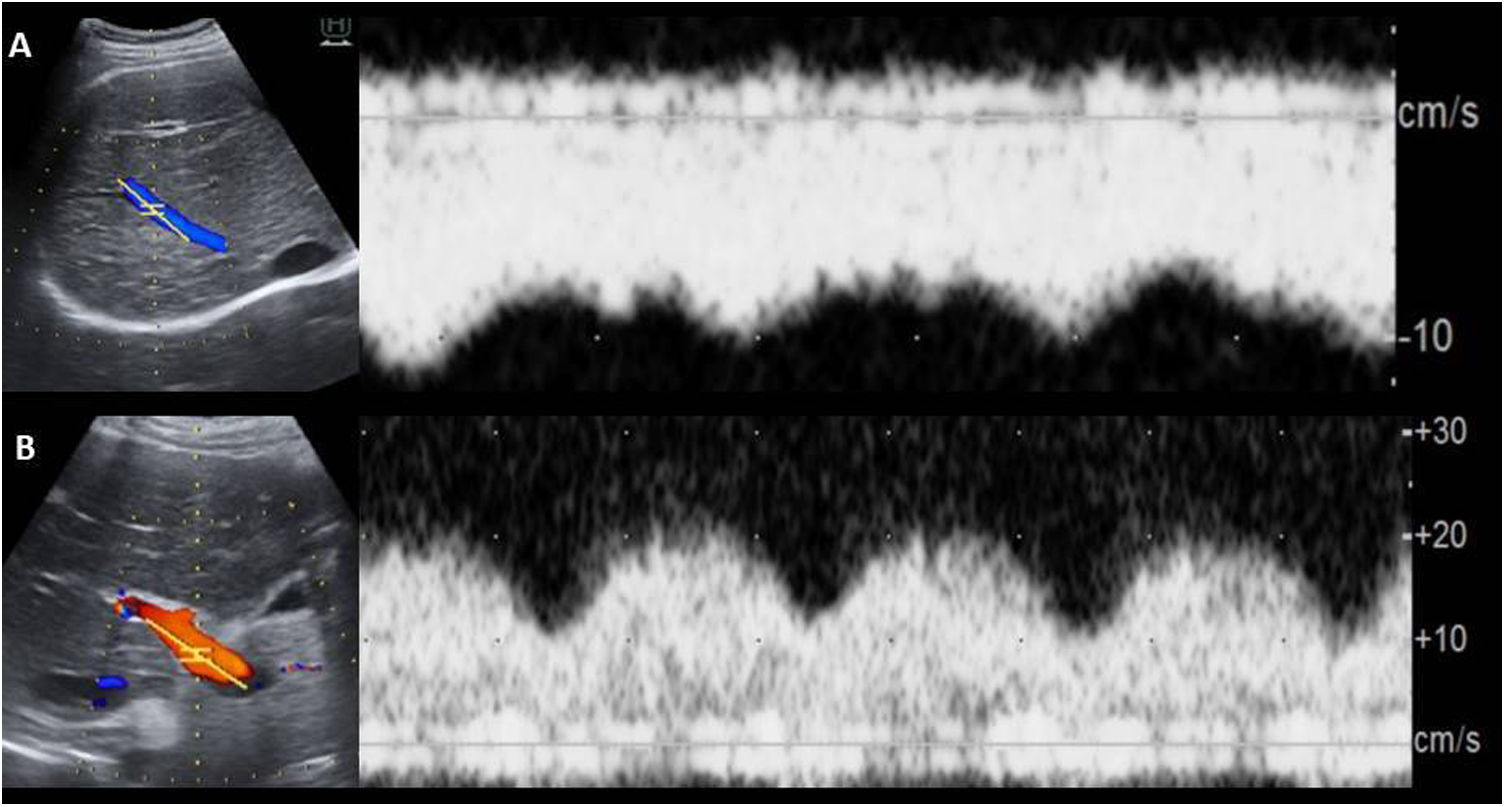
The dilatation of the IVC and suprahepatic veins is a common finding similar to other cases of hepatic congestion.6,9,10 In Doppler ultrasounds, the loss of the triphasic pattern in the hepatic veins is universal in patients with FP.1,6 The loss of hepatopetal flow may represent an increase in liver stiffness secondary to fibrosis or cirrhosis6 (Fig. 5).
Male aged 20 years with Fontan procedure at 8 years of age for pulmonary atresia and double inlet ventricle with D malposition. Loss of triphasic pattern in suprahepatic veins (A) and markedly pulsatile portal flow (but with normal mean velocity) in the context of Fontan-associated liver disease.
It is common to observe a decrease in velocity (<16cm/s) and an increase in pulsatility in the portal vein.21 In patients with FP, portal flow is invariably hepatopetal and the diameter of the portal vein is normal or smaller than usual6 (Fig. 5).
The elevation of the resistance (>0.71) and pulsatility (>1.3) indices of the superior mesenteric artery, celiac trunk and hepatic artery is a non-specific finding that can occur in these patients.1,21
The radiological findings in portal hypertension (PHT) secondary to FALD – splenomegaly, ascites and collateral venous circulation – are identical to those present in PHT due to other causes. Nevertheless, severe PHT is rare in patients with FALD. The appearance of varicose veins from the systemic to the pulmonary circulation does not necessarily reflect a significant transhepatic pressure gradient. In addition, the presence of ascites may indicate lymphatic overflow and peritoneal cavity decompression.4
CT and MRIModifications in the size, contour, hepatic vessels and signs of PHT are similar to those described for ultrasound (Fig. 6).
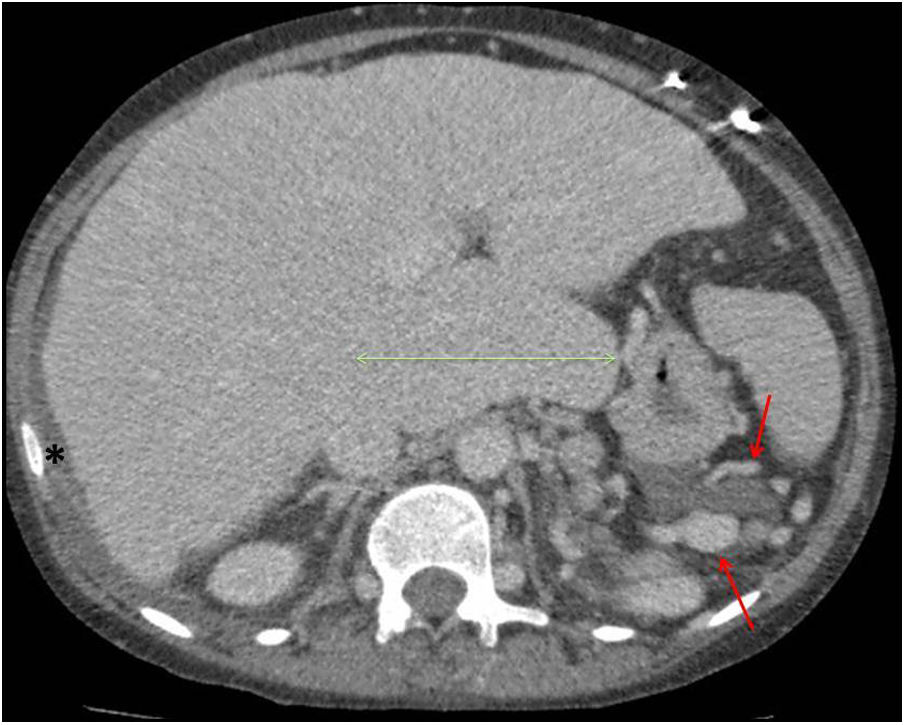
Woman aged 24 years, with truncus arteriosus type I, right ventricular hypoplasia and stenosis of both pulmonary branches, who underwent extracardiac Fontan surgery 11 years ago. Abdominal computerised tomography scan with intravenous contrast that shows signs of advanced Fontan-associated liver disease, in which the lobulated liver contour and the marked hypertrophy of the caudate lobe are particularly noteworthy. There is also prominent collateral circulation in the left subdiaphragmatic region (red arrows) and a small amount of ascitic fluid in the right subdiaphragmatic region (asterisk) suggestive of portal hypertension and hydropic decompensation.
In contrast CT and MRI scans, passive hepatic congestion manifests with a mosaic pattern of enhancement. It consists of mottled and reticular bands of hypoenhancement, predominantly peripheral, visible during the portal phase, which become isocaptant in the equilibrium phase5,6,18,20 (Fig. 7).
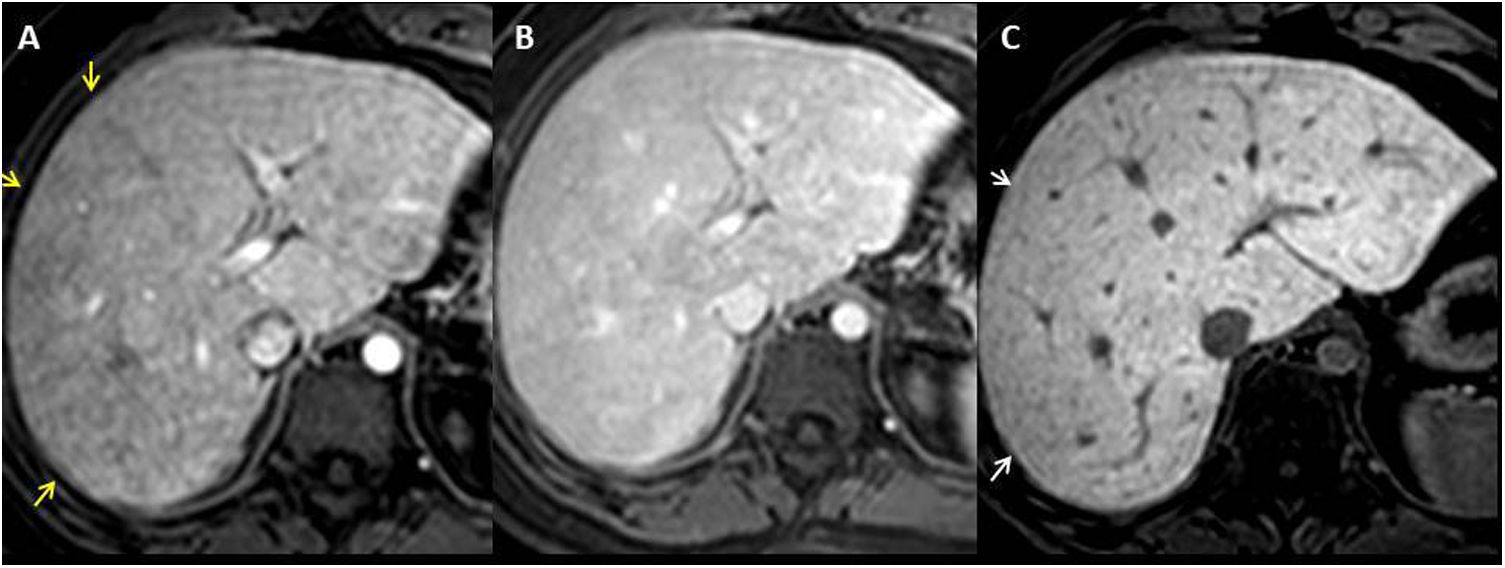
Man aged 22 years with Fontan procedure 17 years ago for single left ventricle heart with malposition of large vessels. T1-THRIVE sequence after extracellular gadolinium in portal phase (A), in which a mosaic pattern of enhancement (yellow arrows) can be observed and which becomes isointense in the equilibrium phase (B), secondary to passive congestion. C) T1-THRIVE sequence in hepatocellular phase after the administration of gadoxetic acid that shows peripheral hypointense reticular bands due to congestion and decreased liver function. Note the correlation with the mosaic pattern visible in the portal phase.
It should be remembered that the mosaic pattern of uptake is a common finding in FALD and is secondary to a relatively slow enhancement around the congested hepatic veins. Its presence correlates with a higher degree of liver fibrosis.6
In the hepatobiliary phase after hepatospecific contrast, there is heterogeneous enhancement that reflects the congestion and reduced liver function (Fig. 7). The hypointense reticular bands may be caused by dilated veins or the existence of fibrous septa.6
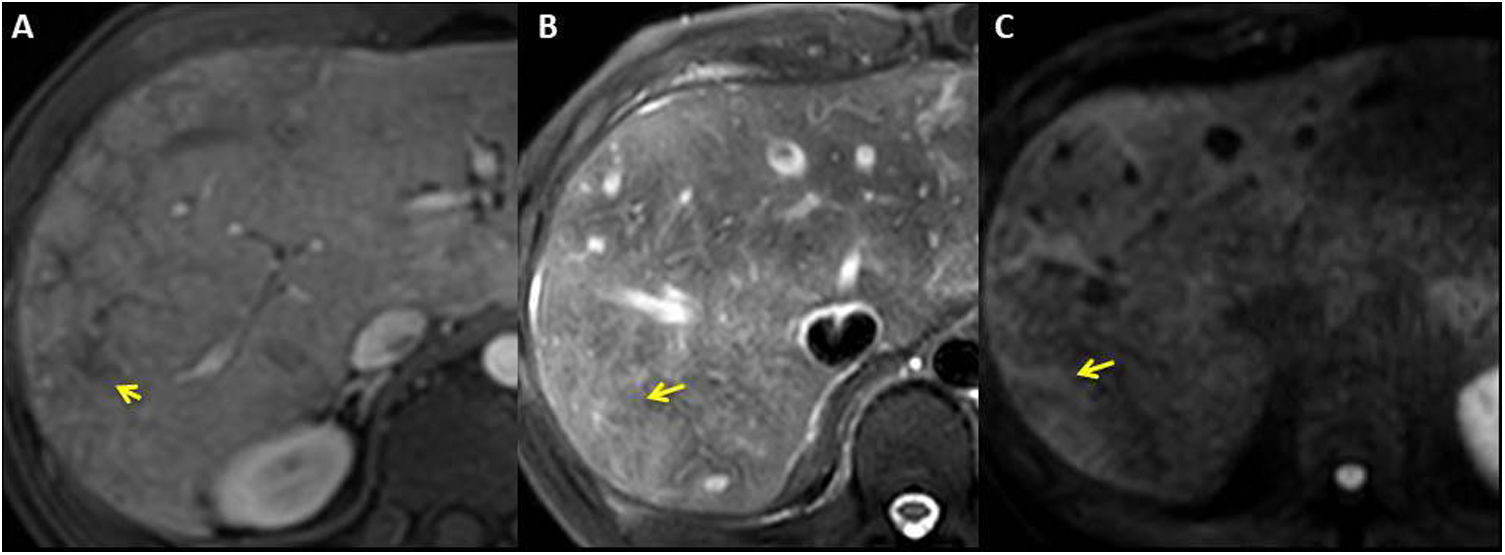
The areas of abnormal enhancement in the periphery of the liver may show high signal intensity in T2/STIR sequences and in diffusion, and hypointense in T1 sequences (Fig. 8). The presence of low apparent diffusion coefficients (ADCs) and high signal intensity in diffusion with high “b” value suggests progressive liver damage due to passive congestion.4,6,22,23
Woman aged 20 years with single left ventricle heart, with double inlet, aorta in L-malposition and pulmonary atresia with extracardiac Fontan procedure at 8 years of age. Post-gadolinium T1-THRIVE sequence in portal phase (A) shows peripheral reticular enhancement (yellow arrows) that corresponds to hyperintense peripheral area in T2-SPAIR (B) and diffusion (‘b’ value 1000).
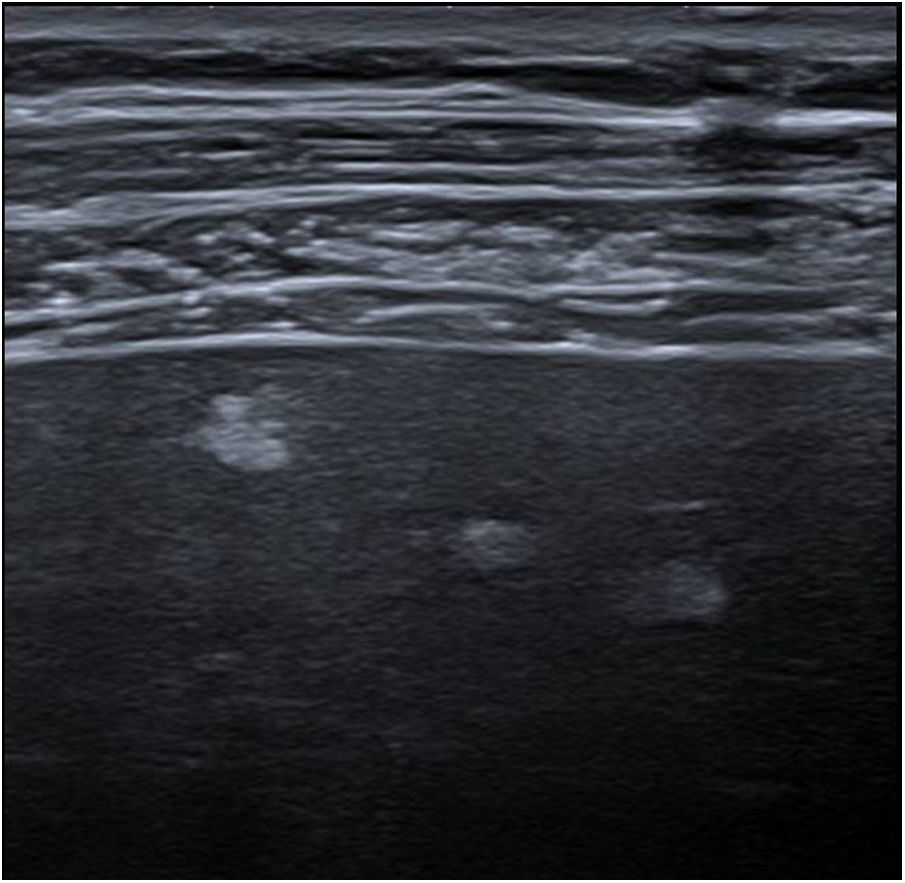
It is common for ultrasounds to detect multiple small peripheral hyperechogenic focal lesions (<5mm) with irregular borders, which are visible with high frequency transducers (Fig. 9). With a low frequency probe, they can generally only be observed as areas of heterogeneous echotexture; they are also not visible in CT or MRI scans. It is thought that they could represent early stages of liver fibrosis and do not show a clear correlation with the duration of the Fontan procedure.18
Male aged 31 years with extracardiac Fontan procedure 21 years ago for single left ventricle heart. Ultrasound image with high frequency linear probe showing several hyperechogenic nodules, smaller than 5mm, characteristic of Fontan-associated liver disease. These lesions were not visible with low frequency probe or dynamic CT study.
More than a third of patients with FALD have focal lesions including benign nodules with high contrast uptake in the arterial phase and hepatocellular carcinoma (HCC).24
The benign nodules are called regenerative nodules or focal nodular hyperplasia-like (FNH-like) nodules in the scientific literature and they are similar to the nodules visible in patients with hepatic venous flow obstruction, as in Budd-Chiari syndrome or right heart failure.6,25
FNH-like nodules have identical macroscopic and microscopic characteristics to traditional FNH, but located in a pathological liver.18,20,25 Its prevalence in adults with FP is 20-30%.6,18 The imbalance in hepatic microcirculation caused by passive congestion, the subsequent reduction in portal flow and the compensatory increase in arterial flow are considered to be the pathophysiological mechanisms for the development of these nodules.6,18,20,26 The malignant potential of FNH-like nodules is not clear.27
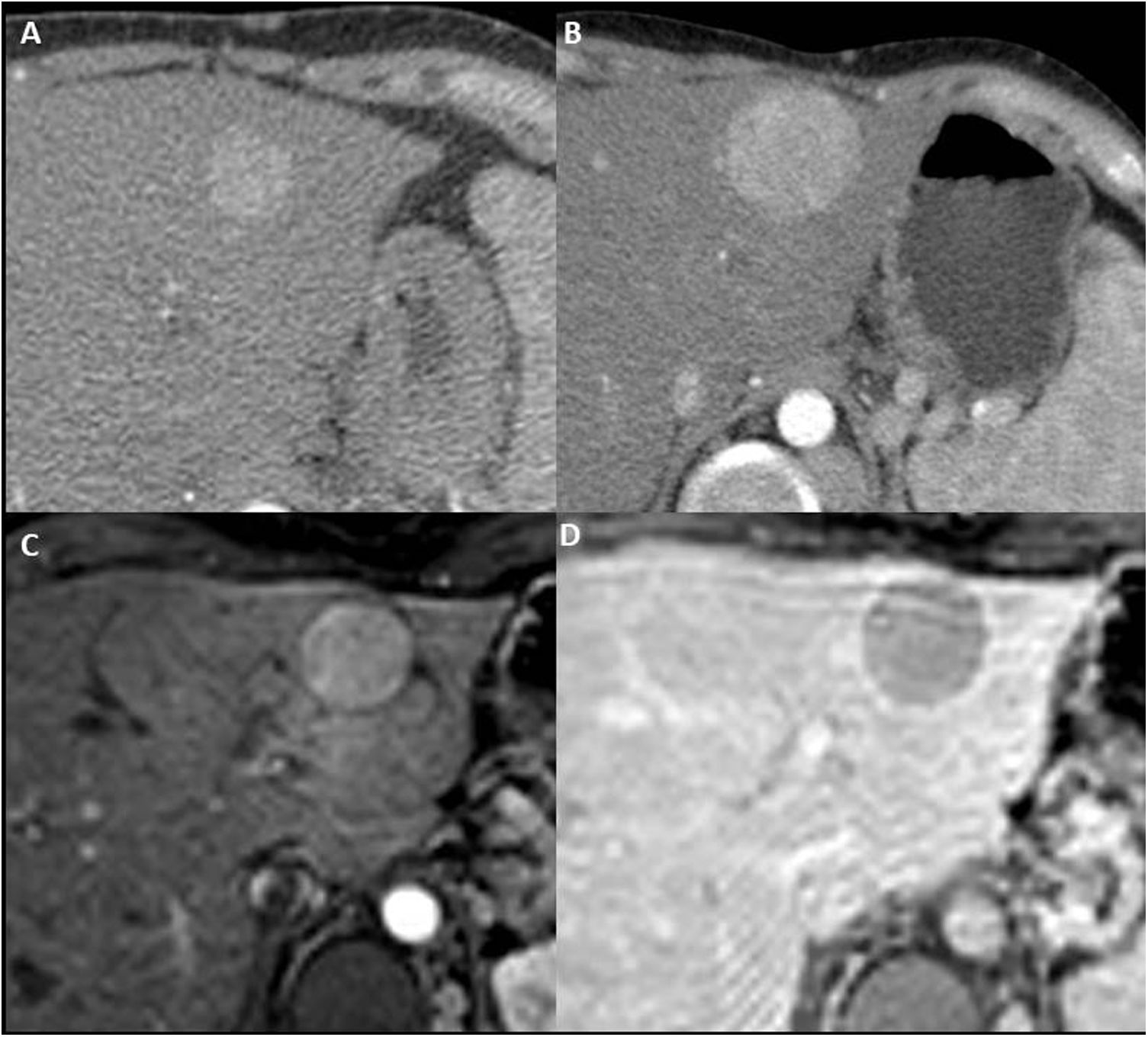
FNH-like nodules are usually small (<3cm) and multiple6,25, with a predilection for the right lobe and frequently less than 2cm from the surface6,18 (Fig. 10). They are usually homogeneous in all sequences, slightly hypointense/isointense in T1, slightly hyperintense/isointense in T2, with absence or slight restriction of diffusion (Fig. 11). After contrast in MRI, CT or ultrasound, they are homogeneously hypervascular in the arterial phase and take up as much contrast as the parenchyma in the equilibrium phase6,25,27 (Fig. 10). Larger lesions may also have a central scar, similar to that of traditional FNH lesions6,25 (Fig. 11).
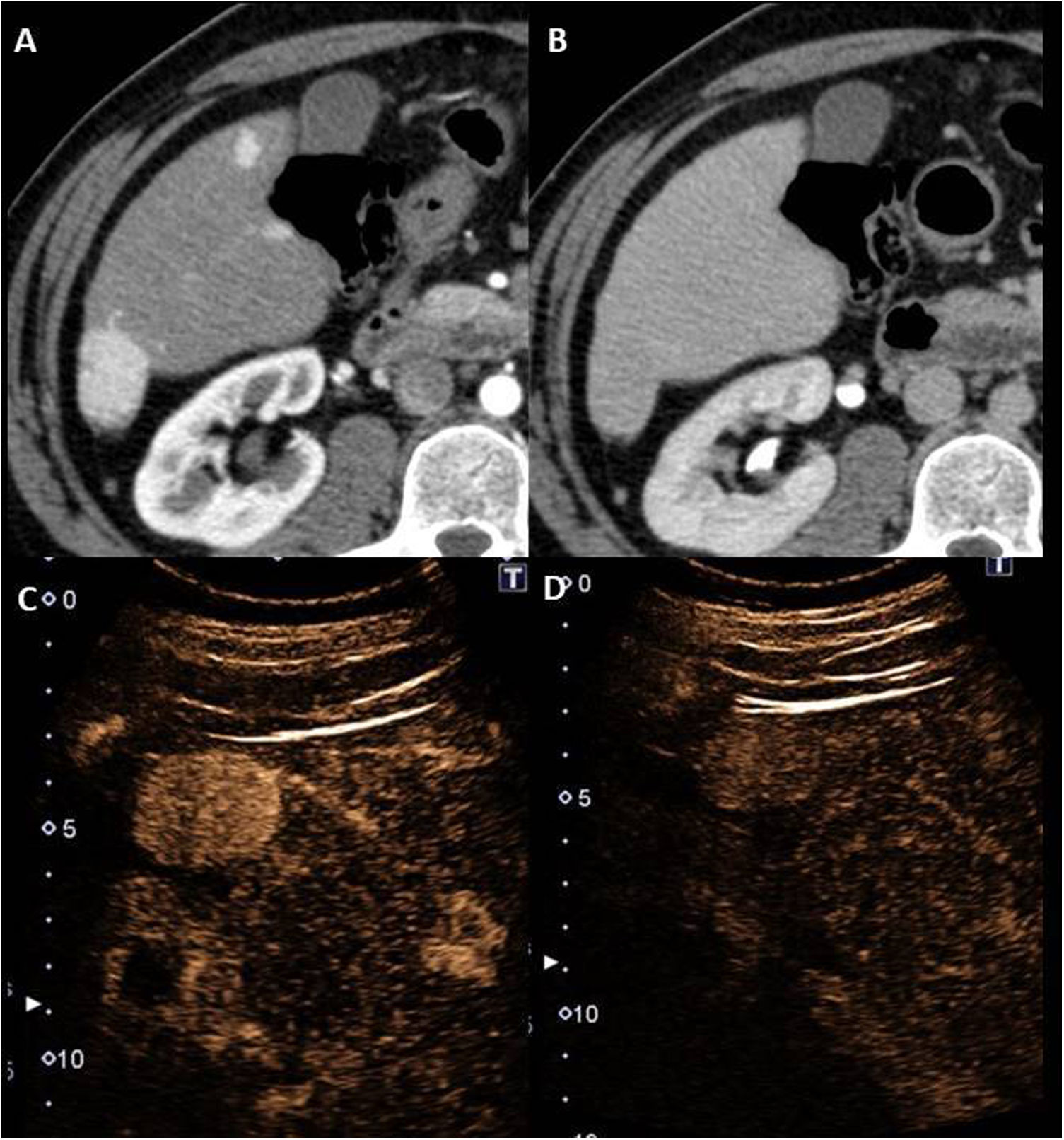
Male aged 35 years with single left ventricle heart with two atrioventricular valves, restrictive bulboventricular foramen and subpulmonary stenosis, who underwent a Fontan procedure 20 years ago. Images from dynamic computed tomography in late arterial (A) and equilibrium (B) phases that show FNH-like nodules with typical characteristics. In the ultrasound with Sonovue® (C and D) focused on the lesion in segment VI of the larger entity, its behaviour is superimposable (hypervascular in the arterial phase without subsequent washout).
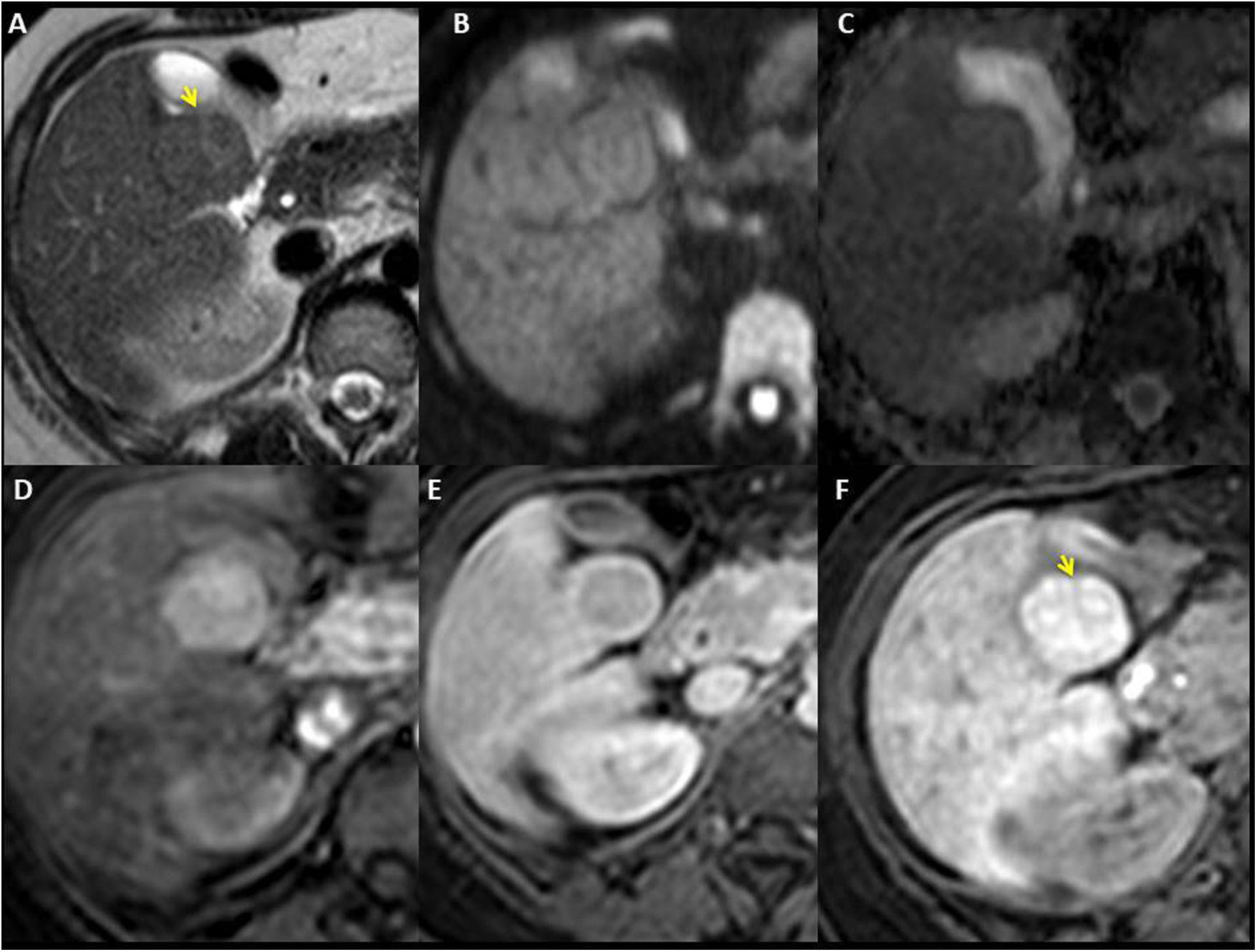
Woman aged 23 years who underwent Fontan procedure 13 years ago for pulmonary atresia with intact septum and right ventricle with sinusoids. FNH-like nodules in segment V, isointense in T2-TSE (A), diffusion (‘b’ value 1000) (B) and ADC map (C). The T2 sequence shows a hyperintense central scar (yellow arrow). T1-THRIVE after extracellular gadolinium in which the lesion shows a markedly hypervascular character in the arterial phase (D) with washout in the equilibrium phase (E) and a peripheral capsule of enhancement (atypical characteristics). F) T1-THRIVE sequence in hepatocellular phase after administration of gadoxetic acid, which shows intense homogeneous incorporation of the contrast medium (except for the central scar).
Contrast washout in the portal or equilibrium phase is uncommon, but can occur in FNH-like nodules, imitating the behaviour of HCCs6,24,27 (Fig. 11). Therefore, the LI-RADS criteria should not be applied to the nodules in these patients. The cause of the washout is not known, although it could be related to the congestion, fibrosis or hepatic arterial supply predominant in the surrounding parenchyma.27
It must be remembered that, in the hepatobiliary phase, after hepatospecific contrast media, the homogeneous retention of the contrast significantly increases the probability of a hepatic nodule in a patient with FALD being benign.6,20,27 The central scar present in some cases may remain hypointense during this phase (Fig. 11).
HCC in FALDHCC is now recognized as a rare complication in patients with FALD. The annual risk of HCC in cirrhotic patients with FALD is 1-5%.28 Some studies have even suggested that the incidence might be higher.29 HCC on FALD-associated cirrhosis has been described in very young patients, with one case in a patient of just 16 years of age.30
A progressive increase in the size of the nodule, changes in its radiological characteristics, restriction of diffusion, contrast washout, mosaic architecture, tumour thrombus or necrosis should raise suspicion of HCC in patients with FALD (Fig. 12). Serum alpha-fetoprotein elevation may be useful in the presence of a suspicious nodule.6,8,9,27
Woman aged 23 years with single left ventricle heart and Fontan procedure 19 years ago. Nodule in segment II hypervascular in late arterial phase (A) with washout in equilibrium phase (not shown) that increased significantly in size in the subsequent follow-up (B). In the post-gadolinium T1-THRIVE sequence in arterial (C) and equilibrium (D) phases, the radiological behaviour is similar, and an enhancement capsule is also identified. It was confirmed that the lesion corresponded to a hepatocellular carcinoma.
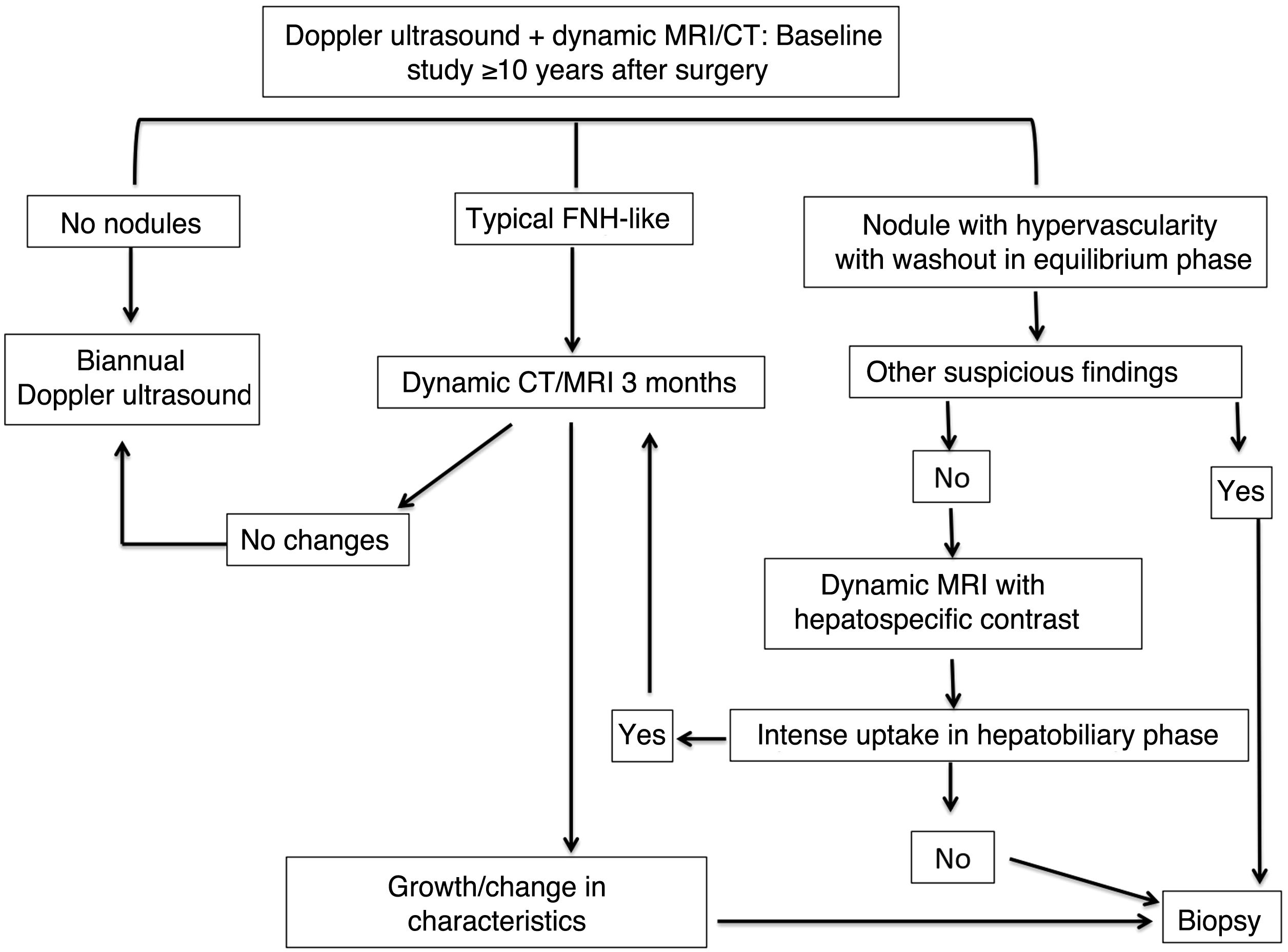
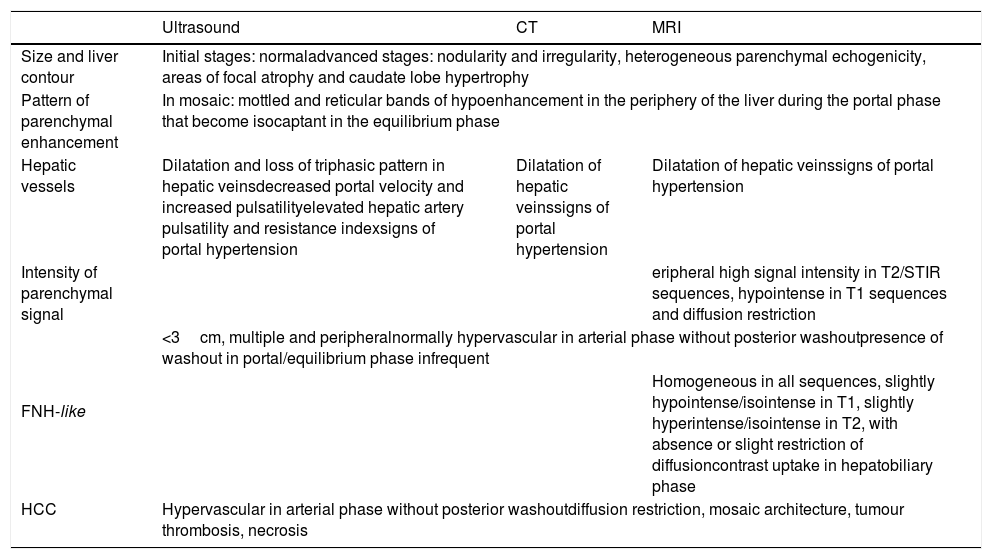
Given that FALD and HCC are significant complications in patients with FP, regular imaging studies are essential for adequate monitoring. There is no definitive consensus for the management of focal lesions in patients with FALD (the algorithm shown in Fig. 13 was proposed at our centre). Table 1 gives a summary of the principal radiological manifestations of this condition.
Summary of radiological characteristics of the parenchyma and the nodules in Fontan-associated chronic liver disease.
| Ultrasound | CT | MRI | |
|---|---|---|---|
| Size and liver contour | Initial stages: normaladvanced stages: nodularity and irregularity, heterogeneous parenchymal echogenicity, areas of focal atrophy and caudate lobe hypertrophy | ||
| Pattern of parenchymal enhancement | In mosaic: mottled and reticular bands of hypoenhancement in the periphery of the liver during the portal phase that become isocaptant in the equilibrium phase | ||
| Hepatic vessels | Dilatation and loss of triphasic pattern in hepatic veinsdecreased portal velocity and increased pulsatilityelevated hepatic artery pulsatility and resistance indexsigns of portal hypertension | Dilatation of hepatic veinssigns of portal hypertension | Dilatation of hepatic veinssigns of portal hypertension |
| Intensity of parenchymal signal | eripheral high signal intensity in T2/STIR sequences, hypointense in T1 sequences and diffusion restriction | ||
| FNH-like | <3cm, multiple and peripheralnormally hypervascular in arterial phase without posterior washoutpresence of washout in portal/equilibrium phase infrequent | ||
| Homogeneous in all sequences, slightly hypointense/isointense in T1, slightly hyperintense/isointense in T2, with absence or slight restriction of diffusioncontrast uptake in hepatobiliary phase | |||
| HCC | Hypervascular in arterial phase without posterior washoutdiffusion restriction, mosaic architecture, tumour thrombosis, necrosis | ||
HCC: hepatocellular carcinoma; FNH: focal nodular hyperplasia; CT: computerised tomography; MRI: magnetic resonance imaging.
The pathophysiology of FALD differs from the usual fibrotic and cirrhotic mechanisms. In the early stages of the disease, there are some radiological changes that differ from other chronic liver diseases (presence of hyperechogenic nodules smaller than 5mm, loss of triphasic pattern in suprahepatic veins or mosaic pattern of hepatic enhancement). Nevertheless, in advanced stages of the disease, it may be indistinguishable from other causes of liver fibrosis/cirrhosis.
FALD involves changes in morphology, contour, size, vascular structures and pattern of parenchymal enhancement. There is an increased incidence of FNH-like nodules and HCC, whose radiological behaviour may sometimes overlap. Radiologists should be aware that patients with FP are a growing population and FALD is an increasingly common condition. Monitoring using imaging techniques is justified in these patients because of the increased risk of HCC.
AuthorshipResponsible for the integrity of the study: BVS, MJP, DRV, JJB, JMB.
Study concept: BVS, MJP, DRV, JJB, JMB.
Study design: BVS, MJP, DRV, JJB, JMB.
Data collection: BVS, MJP, DRV, JJB, JMB.
Data analysis and interpretation: BVS, MJP, DRV, JJB, JMB.
Statistical processing: N/A.
Literature search: BVS, MJP, DRV, JJB, JMB.
Drafting of the article: MJP, BVS, DRV, JMB, JJB.
Critical review of the manuscript with intellectually significant contributions: BVS, DRV, JJB, JMB, MJP.
Approval of the final version: MJP, BVS, DRV, JJB, JMB.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Thanks to Dr Rafael Rodríguez Romero for his help with some of the illustrations in this article.
Please cite this article as: Parada Blázquez MJ, Rodríguez Vargas D, Mohigefer Barrera J, Borrero Martín JJ, Vargas Serrano B. Enfermedad hepática crónica asociada a la cirugía de Fontan. Radiología. 2021;63:159–169.