Irreversible Electroporation (IRE) is a non-thermal tumor ablation technique. High-voltage electrical pulses are applied between pairs of electrodes inserted around and/or inside a tumor. The generated electric current induces the creation of nanopores in the cell membrane, triggering apoptosis. As a result, IRE can be safely used in areas near delicate vascular structures where other thermal ablation methods are contraindicated.
Currently, IRE has demonstrated to be a successful ablation technique for pancreatic, renal, and liver tumors and is widely used as a focal therapeutic option for prostate cancer.
The need for specific anesthetic management and accurate parallel placement of multiple electrodes entails a high level of complexity and great expertise from the interventional team is required. Nevertheless, IRE is a very promising technique with a remarkable systemic immunological capability and may impact on distant metastases (abscopal effect).
La electroporación irreversible o IRE (Irreversible Electroporation) es una técnica de ablación tumoral no térmica basada en la aplicación de pulsos eléctricos de alto voltaje entre pares de agujas insertadas alrededor de un tumor. La corriente generada favorece la creación de nanoporos en la membrana plasmática, desencadenando la apoptosis. Por ello, la IRE puede utilizarse de manera segura en localizaciones cercanas a estructuras vasculares delicadas, contraindicadas para el resto de técnicas termoablativas.
Actualmente la IRE se emplea con éxito para ablación de tumores en páncreas, riñón e hígado y, de manera muy extendida, como opción terapéutica focal para el cáncer de próstata.
La necesidad de un manejo anestésico específico y la colocación precisa y en paralelo de múltiples agujas implican un alto nivel de complejidad, siendo necesaria una gran experiencia del equipo intervencionista. No obstante, se trata de una técnica muy prometedora con una gran capacidad inmunológica sistémica que puede provocar un efecto a distancia del tumor tratado (efecto abscopal).
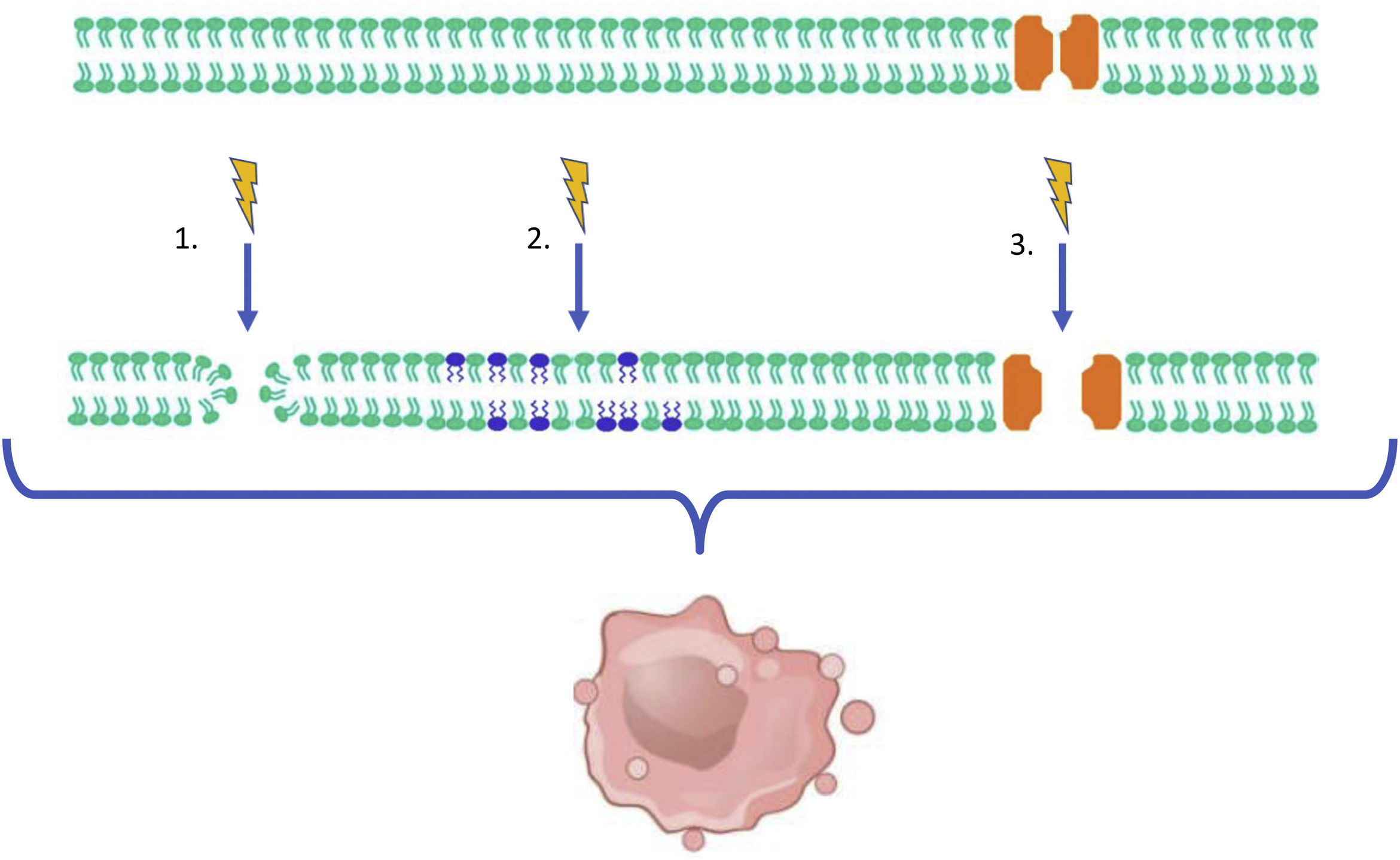
Irreversible Electroporation is a non-thermal ablation technique first described in 2003.1 Its ablation effect is not based on a thermal mechanism, but on the induction of an electric field through the application of multiple short, high-voltage electrical pulses (up to 3kV) between pairs of needles placed around a tumor.2 The resulting current favors the formation of nanopores in the tumor cell's membrane. Furthermore, it promotes an increase in the permeability of the membrane's phospholipids and triggers the activation of voltage-gated ion channels. The final outcome is an irreversible injury to the cellular structure resulting in cell death, which is primarily mediated by apoptosis3 (Fig. 1).
Delicate tissues such as blood vessels, nerves and biliary and urinary tracts are spared during IRE ablation. The electrical current flows through the collagen structures present in these tissues permitting the safe utilization of IRE in locations situated in their proximity.4,5
Despite being a non-thermal ablation technique, there may still be some heat generation due to the transformation of electron kinetic energy (Joule effect). However, unlike thermal ablation procedures close to vascular structures, IRE is not influenced by the heat sink effect.6
These advantages allow IRE to overcome the limitations of thermal ablation techniques in certain tumor locations.
This article aims to review the technique, planning, and anesthetic management of the IRE procedure. Furthermore, this publication also seeks to facilitate a comprehensive assessment of the scenarios for IRE application and its oncological results as an ablation technique for abdominal tumor pathology. Finally, it is our goal to illustrate the great systemic immunological potential and promising future of the technique.
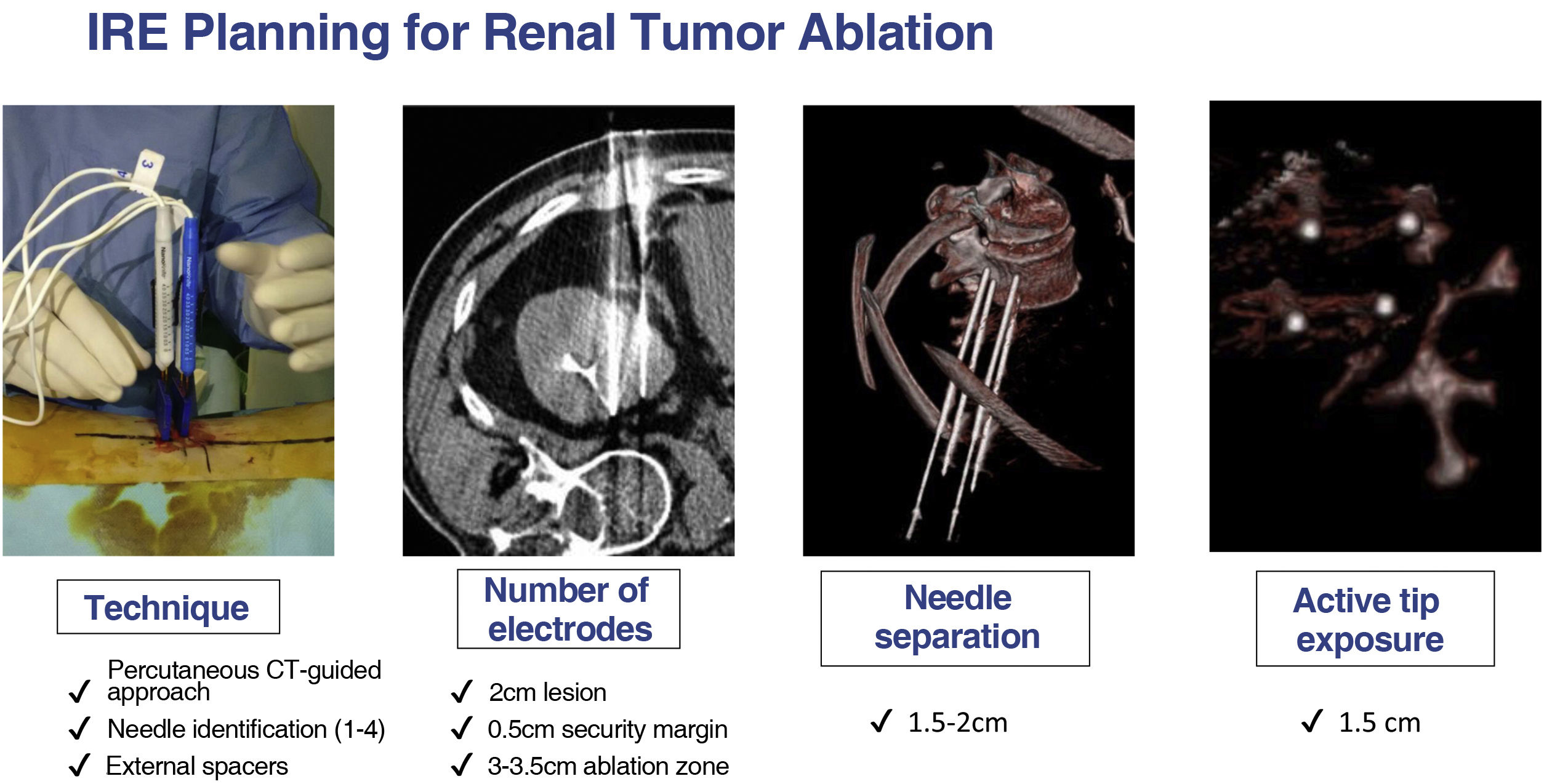
PlanningThe therapeutic goal of IRE is to accomplish the complete ablation of the tumor volume with an oncologic safety margin of 5−10mm.3,7,8
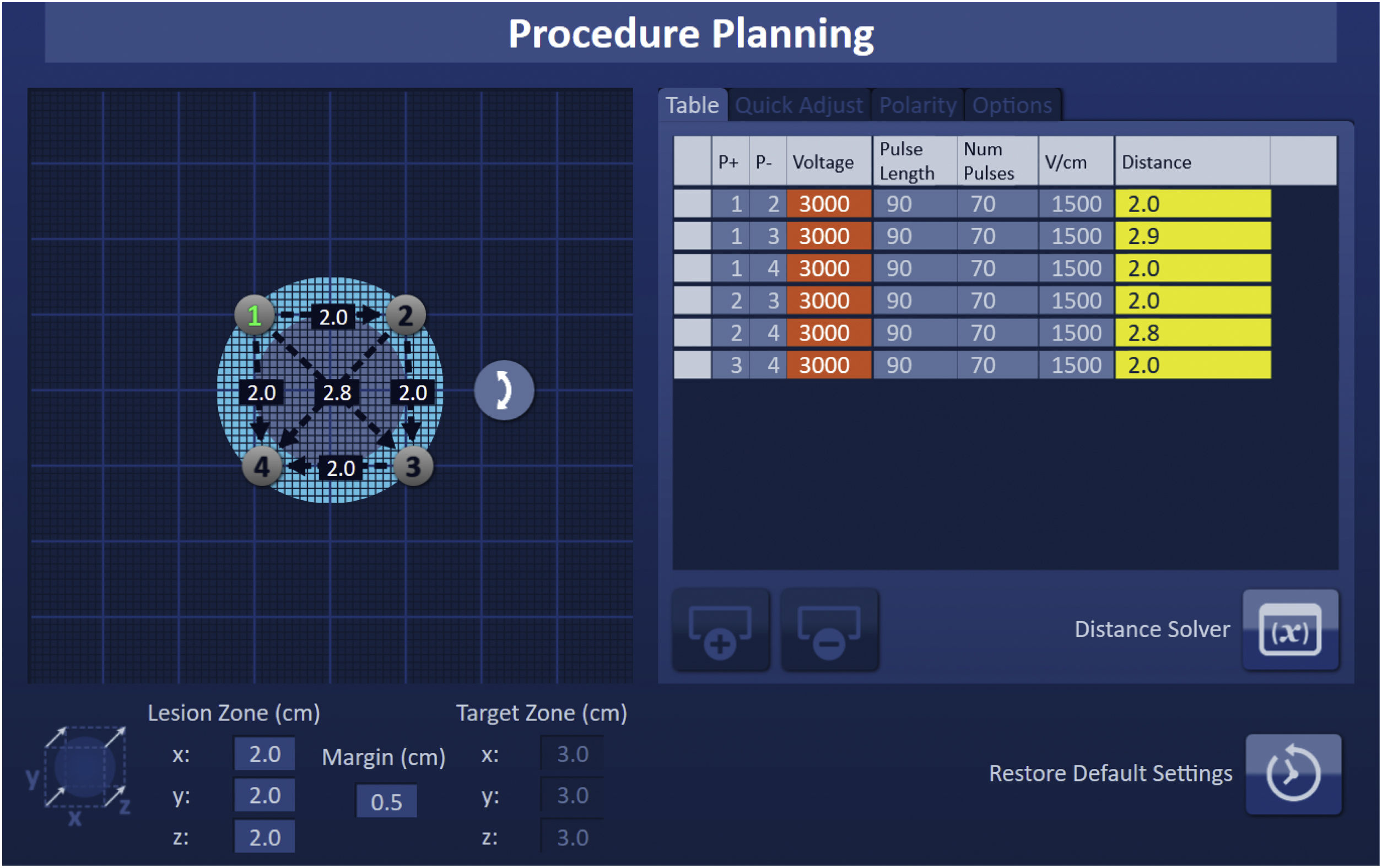
Detailed planning of the procedure is essential in order to correctly predict an electric field that is effective for ablation. There are numerous software solutions available for IRE procedure planning with tools for image segmentation and needle position simulation. Typically, the number of needles used for IRE ranges from 2 to 6 and they must be parallel to each other with a 1−2cm distance between them. The adjustable active tip of the electrode and the need for overlapping ablations is also determined during planning.
A numerical model of the electric field can be generated considering all the previous variables, thus ensuring the entire tumor volume and safety margins are within the ablation zone.3,7,8
As with other ablation techniques, the needles entry points and paths are planned based on computed tomography (CT) or magnetic resonance imaging (MRI) (Fig. 2).6,9,10
It is paramount not to perform IRE if there are stents, surgical staples or other metallic devices near the ablation field, as these could distort the electric field.
Both ultrasound or CT may be used for needle guidance. Although an exclusively ultrasound-guided approach is technically feasible, CT offers additional advantages. Firstly, it permits the evaluation of adjacent structures. Secondly, it enables the three-dimensional assessment of electrode parallelism, allowing for the measurement of actual distances between the needles, which must be entered into the IRE console before generating the electric pulses.7,11 Except in the surgical context, CT is usually the preferred imaging technique. The IRE procedure may also be performed in the angiography room with ConebeamCT and needle guidance software.
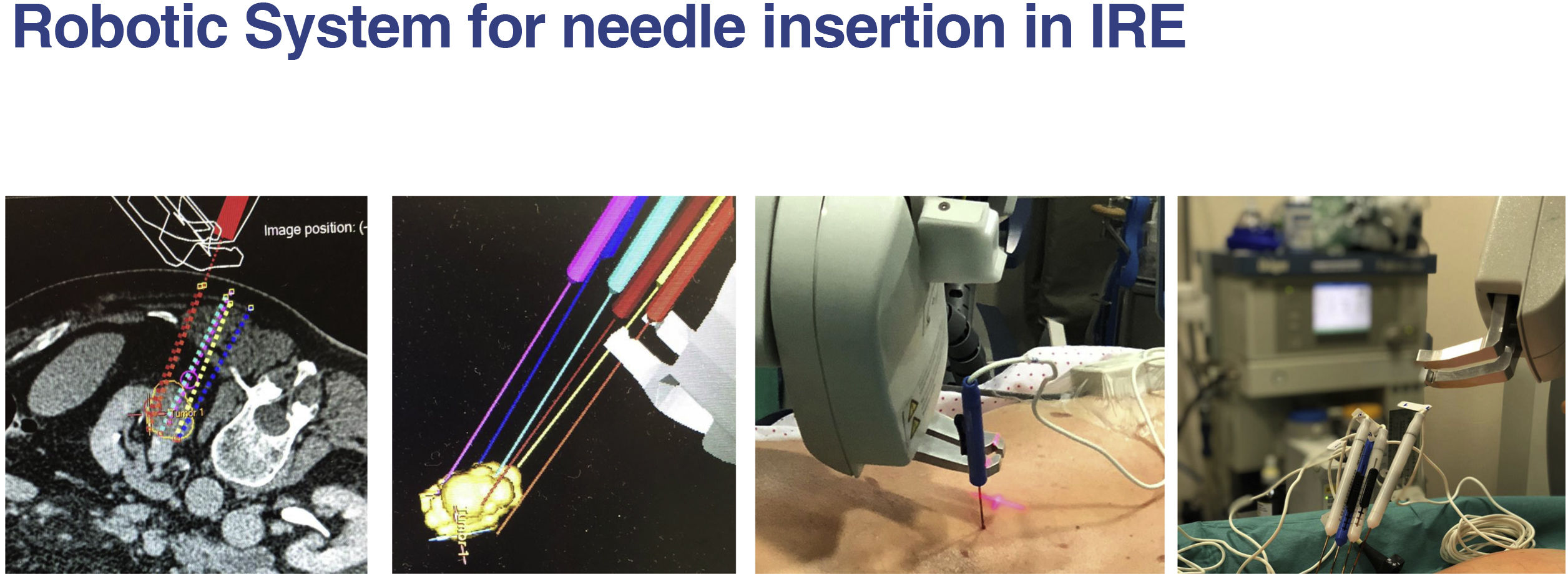
The increasingly available navigation systems can aid in the process of needle placement, reducing both the level of expertise required for technical success and the duration of the procedure12 (Fig. 3).
The surgical or percutaneous approach for IRE is a debated topic. Regardless of the approach, IRE requires broad experience in the field of image-guided tumor ablation.13
The percutaneous approach is less invasive. Its disadvantages include suboptimal tumor delimitation by CT, the frequent need for transgastric or transhepatic approaches, the necessity for general anesthesia (with multiple apneas) in an environment different from the operating theatre and the prolonged CT occupancy time (4h on average). On the other hand, associated pain, hospital stay and complication rate are lower.
The surgical or open approach requires a laparotomy in the operating room, an unfamiliar environment for the interventional radiologist, who must become familiar with the use of special “T-shaped” ultrasound probes. Intraoperative ultrasound allows for real-time differentiation between resectable and non-resectable tumors, as well as the detection of micro metastases that may change the therapeutic plan.14 In addition, the open technique favors control over adjacent structures (which can be manipulated for protection) and the real-time detection and treatment of complications.
Procedure and anesthetic managementFollowing the placement of the 19G needle electrodes in the desired location, they are connected to their designated cables in the Nanoknife® system (Angiodynamics, Latham, NY, USA). Distances between the pairs of needles are then introduced into the console (Fig. 4).
In order to verify that the current employed is appropriate, a series of 10 test pulses comprising 1500V/cm and lasting between 70–90μs are first applied to a specific pair of electrodes. The console detects whether the current emitted from the test pulses falls within the desired range of 20–50A. Consequently, voltage parameters can be manually adjusted in the event of excessive or insufficient current prior to completing the application of the remaining pulses in the protocol. The electrical objective is for the ablated tissue to receive 90 effective pulses between the different pairs of needles with a current intensity greater than 20 A.7,10,15
The application of high-voltage electrical pulses can induce undesirable effects, such as arrhythmias, elevated blood pressure, muscle contractions, and convulsions. Thus, the procedure should always be performed under general anesthesia and maximum muscle relaxation.16,17 The emission of pulses must be synchronized with the refractory period of the ECG to avoid the onset of arrhythmias. Therefore, IRE is contraindicated in patients with ventricular arrhythmias, pacemakers, or defibrillation devices,16 as well as in patients with a high anesthetic risk (American Society of Anesthesiologist Physical Status (ASA PS)>3) (Fig. 5).
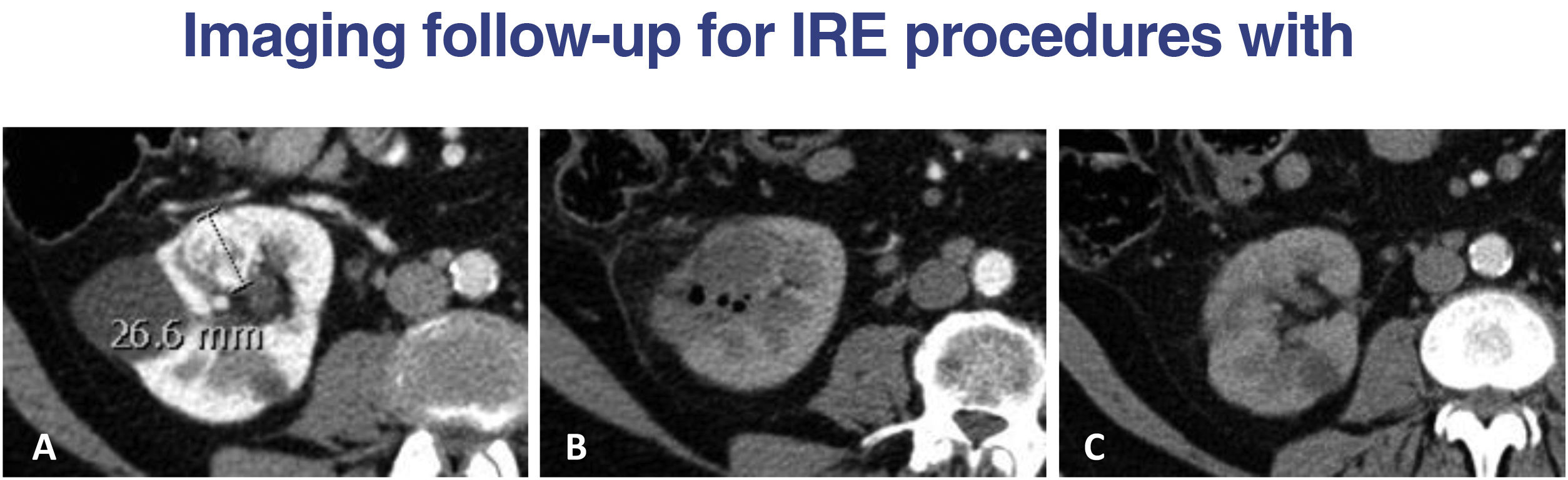
A) Cortical, hypervascular renal carcinoma protruding into the renal sinus. B) After ablation with IRE complications such as bleeding, vascular thrombosis or urinary fistula are ruled out zone (excretory phase not shown); typical gas bubbles and hypodense area in the ablated. C) CT control in 6 months, resulting in a “regeneration” of the renal parenchyma, making it difficult to identify the ablated area.
Uncontrolled arterial hypertension, congestive heart failure, and active coronary artery disease are relative contraindications, and the risk-benefit of IRE should be assessed in these patients. As it is the case with other solid organ ablation techniques, International Normalized Ratio (INR) values<1.5 and platelet counts>50×109/l are required.18
Postoperative management of IRE does not possess significant peculiarities. Following the procedure, a moderate elevation of enzymes from the ablated organs is typically observed without other notable analytical alterations.
Post-ablation pain can be compared to that experienced following radiofrequency ablation and is generally manageable with conventional analgesia. It is common for patients to be discharged after six hours of monitoring in a resuscitation unit and 24−48h in a hospital ward.19
Ire in abdominal tumorsPancreasIn the field of abdominal malignancies, IRE is employed in the treatment of non-metastatic pancreatic adenocarcinoma. This disease is classified according to preoperative radiological findings as resectable, borderline resectable, and locally advanced or unresectable. IRE is indicated in the latter two cases, with the shared aim of increasing overall survival (OS), albeit with distinct therapeutic intents.20
IRE in irresectable tumors (Stage III)Locally advanced pancreatic cancer (LAPC) represents approximately one-third of all cases.21,22 Criteria for non-resectability include vascular invasion or involvement greater than 180° of the mesenteric artery, celiac trunk, hepatic artery, portal vein, or superior mesenteric vein.23–25 Patients in this category have a median survival of 11 months and have limited therapeutic alternatives beyond chemotherapy.26
As surgical removal is not technically feasible in these instances, the intent of IRE is palliative, aiming to prolong the patient's OS, control local disease progression, and reduce the prolonged use of systemic therapy.20 In situ IRE has been reported to achieve effective downstaging in isolated cases, enabling subsequent surgical resection.27
IRE in borderline tumorsBorderline tumors are generally defined as those with short vascular involvements amenable to surgical reconstruction. Generally, these patients have a median survival slightly superior to twelve months, which significantly increases when surgical resection is feasible. There exist few therapeutic alternatives to preoperative neoadjuvant chemotherapy with the intent of downstaging.28
As the microscopic involvement of the resection margin (R1) is widely underestimated, the goal of IRE in these patients is to accentuate safety margins that would not be feasible with surgery alone, thereby increasing the percentage of patients with complete tumor resection (R0).20,29 Thus, IRE for margin enhancement can achieve complete tumor resection with R0 margins in over 70% of patients, thereby increasing overall survival and reducing locoregional recurrence.30
Regardless of the therapeutic intent, IRE should be preceded by neoadjuvant chemotherapy, which serves several purposes. Firstly, it excludes aggressive tumor subtypes that do not remain stable and are unlikely to benefit from IRE. Secondly, it achieves downstaging in a large percentage of patients, allowing them to be considered for surgical candidacy. Lastly, reducing tumor volume makes IRE safer and more successful.13
Technical aspectsThe percutaneous approach guided by Computed Tomography (CT) does not provide adequate control of peritumoral vascular structures, nor does it optimally delineate the tumor. To remedy this, the procedure can be performed with endovascular support (a pigtail catheter is placed in the celiac trunk allowing for repeated contrast media injections)11 and image navigation and fusion systems.
On the other hand, an open approach allows the surgeon to assess the tumor's resectability first-hand. Once experience with intraoperative ultrasound is acquired, needle placement with ultrasound support, particularly in the cauda-cranial axis and around the mesenteric vessels, is highly effective.
Oncological outcomesRegardless of the approach type, reviewed cohort observational studies in the literature demonstrate a median OS ranging between 7–27 months post-procedure,31 with progression-free survival (PFS) between 5–23 months.32,33
A recent meta-analysis (Shuiqing et al., 2022), with a combined sample size of nearly 1000 patients, concludes that IRE may benefit survival in patients with pancreatic adenocarcinoma (LAPC), but attention must be paid to the risk of complications.34 One of the most relevant studies included in this meta-analysis, conducted by Martin et al., compares patients who received IRE treatment along with chemotherapy or chemoradiotherapy (CRT) to those who received only chemotherapy or CRT. Improvement was observed in OS (20 vs. 13 months), local progression-free survival (14 vs. 6 months), and distant progression-free survival (20 vs. 13 months).35
The heterogeneity of different series in terms of patient selection and various chemotherapy protocols used explains the wide difference in outcomes. Therefore, to adequately understand the promising results of pancreatic IRE, more prospective, randomized research with standardized protocols is needed.3,36
Pancreatic IRE is a high-risk procedure with a complication ratio of 10%–20%.34 The application of high-voltage pulses, the need to place multiple needles, and the delicate anatomical location justify this. In addition, complications are often major (grades 3–5 of the Clavien-Dindo classification), such as pancreatitis, bleeding, duodenal perforation, or biliary fistulas and leaks. Abdominal pain, nausea, vomiting, diarrhea, and loss of appetite are minor, self-limiting, and common complications.
In patients with biliary obstruction, drainage is necessary prior to chemotherapy and IRE. Caution should be exercised when using metallic biliary stents if the patient is a potential candidate for IRE.
KidneyTumor ablation is currently accepted as an alternative to partial nephrectomy for the treatment of stage T1a renal cell carcinoma (RCC). It plays an especially important role in the treatment of cortical tumors, smaller than 3cm in diameter, and in patients with a high surgical risk, such as those of advanced age, with multiple tumors or with a single kidney.37 However, the thermal ablative techniques commonly used present significant limitations when lesions are centrally located, primarily related to the risk of thermal damage to the collecting system and the rest of the vascular structures of the renal hilum.
For these central locations, other ablation methods exist, such as cryoablation, which are also frequently used but require urothelial protection techniques such as the instillation of warmed saline through a catheter placed in the renal pelvis.38 For IRE ablation of central renal lesions, it is not necessary to perform techniques to protect the collecting system, as it remains unaffected, with even some urothelial regeneration over time.39 Moreover, due to its mechanism of action, IRE is not affected by the heat dissipation that occurs in the thermal ablation of central lesions near the hilar vessels.
On the other hand, IRE also allows the safe approach of renal tumors located in the anterior margin of the kidney, adjacent to colonic and small bowel loops. These structures are also preserved with IRE ablation.
Technical aspectsPatients undergo surgery in a prone position, which can make anesthetic management more complex.19 The anatomical location of the kidney often requires placing the needles at angles that do not coincide with the gantry, entering through intercostal spaces, which makes the technique more complex. Care should be taken to ensure that the needles do not collide with the CT gantry, and efforts should be made to achieve maximum perpendicularity to the table to prevent its mobilization.
Oncological outcomesIn a systematic literature review, Hilton et al. 2022 reported that IRE for kidney tumors is a safe technique with no intervention-related mortality at 30 days. Moreover, the procedure is well-tolerated, with patient discharge within 24h in 62/63 patients.40
Regarding oncological outcomes, the series by Dai et al. 2021, with a median follow-up of 50.4 months in 47 patients, demonstrated a local recurrence-free rate and metastasis-free rate of 81% and 93%, respectively, with an OS of 92%.41
Our experience is that renal IRE is as safe and effective as other ablative methods but should be performed by an experienced team. It has a clear indication in central tumors, as an alternative to nephrectomy, with a lower rate of complications and local recurrence compared to other ablative techniques.
Among the most frequent complications observed after renal IRE are perirenal hematoma and transient hematuria, and less frequently, pyelonephritis and urinary retention.40 Major complications, such as bleeding requiring transfusion and/or embolization or post-ablation abscesses requiring drainage, are infrequent.
LiverHepatic IRE is primarily indicated in those patients who are candidates for ablation, but for whom thermal ablation techniques are contraindicated due to tumor location near vital structures, such as blood vessels (inferior vena cava, suprahepatic or portal veins and hepatic artery), gallbladder, and bile duct.
Furthermore, the use of IRE in conjunction with other thermal ablation techniques allows the perivascular tumor component to be treated through electroporation, thus avoiding the risk of vascular thrombosis or incomplete tumor treatment that would exist with thermal techniques alone.
Technical aspectsHepatic IRE is usually performed with a percutaneous approach and the patient in the supine position. Due to their adjacency to vital structures,42–44 lesions often have a central location that allows needle placement by ultrasound, although it is advisable to ultimately verify their position with CT.45
An open approach is preferred when the patient is to undergo metastasectomy or hepatectomy and also presents with a non-surgical central lesion.
Oncological outcomesIn the two most recent meta-analyses conducted (Yu and Li 2022 and Gupta et al. 2021), an 86% efficacy of the technique is described, with an OS of 81% and 61% at 12 and 24 months, respectively.43,46 Although the series do not differentiate between the type of hepatic lesion (cellular hepatocarcinoma [CHC], cholangiocarcinoma, or metastasis), greater OS for CHC than for the rest of the etiologies is described.
The OS described for patients treated with IRE seems to be lower than for other thermal ablation techniques. However, it should be noted that these are patients not suited for radiofrequency or microwave due to the central location of their lesions. Central locations and confluence with large vessels limit the ablative or surgical treatment for CHC in Barcelona Clinic Liver Cancer (BCLC) A stage. In such cases, IRE ablation may be performed, thus avoiding treatment stage migration to non-curative methods (BCLC≥B) such as transarterial chemoembolization.45
The incidence of complications in the treatment with IRE in hepatic lesions is around 20%, consisting mainly of minor complications such as episodes of cholangitis, small hematomas, or portal branch thrombosis.46 Major complications such as biliary or intestinal fistulas and massive hemorrhages are infrequent.
IRE in extra-abdominal oncological pathologyProstatic IRE is one of the available options for the focal treatment of non-metastatic prostate cancer. Its oncological and functional outcomes surpass those of other therapeutic alternatives, with a lower incidence of erectile dysfunction and urinary incontinence.47 Therefore, the prostate is probably the most widespread extra-abdominal territory for IRE ablation.
Other locations in which IRE has been employed include the lung, bone, brain, breast, and cervix.
Clinical-radiological evaluation of IREGiven the high complexity and distinctive features of the technique, a comprehensive evaluation by the interventional radiologist in the consultation room is essential once the patient has been referred by the multidisciplinary committee. The first purpose of the consultation is to conduct a thorough anamnesis and review of the medical history, paying special attention to cardiac arrhythmias and pacemakers, as well as significant comorbidities that contraindicate general anesthesia. The existence of clips or metallic stents in the area to be treated will be ruled out. Based on the gathered information, relevant laboratory tests will be requested, and the need for CT or MRI prior to the procedure will be evaluated. Moreover, the consultation may also be the time to perform an ultrasound to help plan the type of approach and appropriate access.
It is crucial to clarify expectations and discuss therapeutic alternatives, leaving room to resolve patients’ questions and, ultimately, obtain informed consent. Patients referred directly for IRE from other physicians and bedside explanations result in lower-quality patient care.48
Imaging findings and radiological follow-up in IREImmediately following the IRE procedure, the ablated area appears as a hypoechoic or hypoattenuating area that reliably correlates with the zone of cell death. This finding allows for real-time monitoring, thus ensuring the entire tumor volume has been included.49 Common findings include gas bubbles, liquid collections and a hyperattenuating ring on the periphery of the ablation zone.50–54 This ring corresponds to a ridge of reactive hyperemia, which disappears approximately after a month and should not be confused with residual tumor.
Given that gas, the central hypoattenuating zone and a peripheric enhancing halo are also imaging findings present in hepatic abscesses, the patient's clinical and analytical context is essential to differentiate between the two situations.55 CT scanning further assists in ruling out other immediate complications such as bleeding or ectopic air caused by hollow viscus perforation.
Due to their availability, CT and MRI are currently the most frequently used imaging techniques for monitoring tumor ablation with IRE. It is advisable to repeat imaging controls every three months during the first year and every six months afterwards.15
Generally, the ablation zone will appear with varying degrees of hypodensity in subsequent imaging controls, depending on the organ and the time elapsed.50–52,56 However, after IRE procedures it is not expected to encounter the markedly hypodense areas (caused by coagulative necrosis) commonly seen in other thermal ablation techniques.
Immediately after the procedure, it is normal for the ablation zone to extend beyond the original tumor due to expected inflammatory-edematous changes.50,56 Over time, a significant reduction in this initial ablation volume is characteristic of the IRE technique; the inflammatory-edematous changes diminish, and there is a clearing of cell debris, facilitated by preserved vascularization.51 Thus, a reduction of up to a third of the initial volume can be seen in just 6–12 months. In some cases, the original tumor zone becomes difficult to identify in late imaging controls, and there may even be some tissue regeneration.
On the other hand, late apoptosis up to 6–8 weeks after the procedure has been reported, so some increase in the initial volume size may be expected in relation to this phenomenon.50,54
It is crucial to highlight that, unlike with the World Health Organization criteria or the solid tumor response evaluation criteria (RECIST), size should not be considered the main or the only criterion. It is preferable to combine size with more functional criteria such as alterations in contrast media enhancement, restriction in MRI DWI/ADC sequences, and the use of PET-CT.50–52
Despite this, any increase in the size of the ablation zone after a period in which it has remained stable should be suggestive of recurrence and confirmed in subsequent image controls, along with a clinical evaluation and tumor markers assessment.50
Immunogenic response in IREIRE not only eradicates the tumor locally but also elicits a systemic immune response against the tumor cells themselves.57 This immunogenic cell death is triggered by the large release of DAMP (Damage-Associated Molecular Patterns) molecules and intracellular remnants that occurs after IRE-mediated tumoral cell death.3,57 Moreover, IRE preserves the regional large caliber vasculature from ablation damage and exerts a modulatory effect on the tumor stroma, reducing its rigidity and facilitating the recruitment and activation of antigen-presenting dendritic cells. Therefore, in IRE the systemic immunological effect elicited is significantly more robust than in other ablative techniques. Furthermore, IRE has been shown to induce the regression of metastases at a distance from the ablated tumor. This phenomenon is called the “abscopal” effect (meaning “off-target”) and it requires an effective activation of tumor-specific CD8+ T lymphocytes.57 To enhance this activation and increase the frequency of occurrence of the abscopal effect, there is currently a great interest in combining IRE with immunological checkpoint inhibitors. Recent studies suggest that IRE can counteract the immunosuppressive effect of the stromata of some tumors, such as pancreatic adenocarcinoma. Thus, the combination of IRE with immunological checkpoint inhibitors (such as anti-PD1) significantly improves its efficacy.58
Future advances in IRETechnical advancements have made IRE an increasingly reliable, reproducible, and user-friendly therapeutic modality. Additionally, the development of new planning software and needle navigation and guidance systems has improved the reproducibility of the procedure and reduced its technical complexity.7
The application of high-frequency electric pulses, known as H-FIRE (High Frequency IRE), can reduce muscle contractions and potentially even the need for cardiac synchronization.59
The concurrent use of IRE with chemotherapy has significant clinical potential. The penetration of chemotherapeutic drugs into the tumor stroma is limited, so their administration alongside IRE can facilitate better cellular penetration in the ablated area.60 This also reduces systemic toxicity and creates a synergistic effect between cell death pathways (apoptosis in IRE and mitosis inhibition in chemotherapy). The result is an increase in OS and a reduction in local and distant recurrence.
Numerous studies comparing IRE with the standard therapeutic modality for each organ will publish their results in the coming years, helping to determine what role IRE will play both as a standalone technique and in combination with other therapies.
In conclusion, technical progress and growing clinical experience with IRE make the future of this technique very promising.
ConclusionIn recent years, IRE has established itself as an alternative tumor ablation technique, with a growing level of clinical experience. The incorporation of this non-thermal ablation technique into the therapeutic arsenal of radiologists performing tumor ablation procedures can be highly beneficial and convenient. IRE surpasses the usual boundaries of tumor ablation, enabling ablative therapy in patients for whom it was previously not an option.
Despite the fact that IRE requires significant operating team for therapeutic success, advancements in the technique and navigation and planning systems make its use simpler and more reproducible.
The noteworthy systemic immunological potential of IRE and its use in combination with other therapies continues to be studied with great interest, so it is reasonable to anticipate promising advancements in the coming years.
Authorship- 1.
Responsible for study integrity: JMA and RA.
- 2.
Study conception: JMA.
- 3.
Study design: RA and JMA.
- 4.
Data acquisition: JMA, RA, EG, and MJÁ.
- 5.
Data analysis and interpretation: RA, JMA, and EG.
- 6.
Statistical processing: RA and JMA.
- 7.
Literature search: RA and JMA.
- 8.
Drafting of the manuscript: RA and JMA.
- 9.
Critical review of the manuscript with intellectually significant contributions: JMA, SM-A, and MÁdG-A.
- 10.
Approval of the final version: JMA, SM-A, and MÁdG-A.
There are no conflicts of interest on the part of any of the authors.