Liver transplantation is one of the treatments for patients with advanced stage chronic liver disease and for selected patients with hepatic tumors. Ultrasonography is the first-choice imaging technique to evaluate liver transplants. This article reviews the surgical technique, anatomy, and normal findings on ultrasonography in the immediate postoperative period in patients who have undergone liver transplantation, which will be used as a reference in later studies.
ConclusionEarly vascular (arterial and portal) complications can represent a threat for the graft or the patient. During the period after liver transplantation, the patient is recovering from surgery and the transplanted organ is adapting to its new environment. In this period, ultrasonography can show alterations in the parenchyma or Doppler findings that would be considered abnormal in other situations; these findings are usually transitory. Knowing how to interpret them is key to detecting or ruling out complications.
El trasplante hepático es uno de los tratamientos de la hepatopatía crónica en estadios avanzados y pacientes seleccionados con tumores hepáticos. La ecografía es la técnica de imagen de elección para su evaluación. En este trabajo se revisa la técnica quirúrgica, la anatomía del trasplante hepático y los hallazgos ecográficos normales en el postoperatorio inmediato, que servirán de referencia para evaluaciones posteriores.
ConclusiónLas complicaciones vasculares tempranas (arteriales y portales) pueden suponer una amenaza para el injerto o el paciente. Tras el trasplante hepático existe un periodo de adaptación del injerto al nuevo medio y de recuperación posquirúrgica en el que podemos observar alteraciones parenquimatosas o hallazgos en el estudio Doppler que difieren de los habituales y se pueden considerar como normales en esta situación; generalmente son transitorios. Su conocimiento e interpretación es clave para detectar o excluir complicaciones.
Liver transplantation is one of the treatments for chronic liver disease in advanced stages and in select patients with liver tumours.1 The number of transplants in Europe continues to grow, with an estimated 5000 liver transplants per year from 2005 to 2010.2
There are multiple indications: uncompensated cirrhosis of the liver (of viral, alcoholic, autoimmune or metabolic origin) which is resistant to medical treatment, some stages of hepatocarcinoma, and cholestatic and congestive diseases.3
Doppler ultrasonography is the technique of choice in the immediate postoperative period, to evaluate the biliary tree, the hepatic parenchyma and its vascularization.3–5
Knowing the regular non-pathological ultrasonographic findings in the immediate postoperative period is of interest for radiologists, as this is a common examination at many hospitals. In the first month, the most common complications are vascular, particularly arterial or venous thrombosis, stenoses and fistulae.3,6
2 Anatomy and surgical techniqueClassification according to donor, graft and recipient:
- 1
Donor type: deceased (the most common), in asystole and living donor.
- 2
Graft type7–10:
- •
Whole: transplant of the entire graft.
- •
Lobular or segmental: transplant of one or several hepatic segments.
- •
Split-liver: two grafts (usually paediatric and adult) are obtained from the same liver.
- •
Domino: chosen patients undergoing liver transplantation can donate their native livers to be used as grafts in other patients (e.g. familial amyloid polyneuropathy).
- •
- 3
Recipient: adult or paediatric.
There are different scenarios involved (Fig. 1).
- A.)
In adults, orthotopic (in the same anatomical position as the native liver) transplantation is the most common. Anastomosis:
- 1
In the hepatic artery (HA) and portal vein (PV), they are end-to-end. The donor's HA, proper or common, is anastomosed to the recipient in variable locations according to his/her anatomy: bifurcation between right and left HA, bifurcation between proper and gastroduodenal HA, or confluence between common and gastroduodenal HA.11 Double anastomosis is performed if there is an accessory HA; they should both be examined. If flow through any of the anastomoses is insufficient or altered (by thrombosis, atheromatosis, prior Transjugular Intrahepatic Portosystemic Shunt [TIPS]) or the connection is short, vascular grafts are used and should also be evaluated by the radiologist. The most commonly used grafts come are the decreased-donor iliac artery or vein.6,10–12
- 2
The suprahepatic veins (SHV) are usually anastomosed using the piggyback technique, preserving the recipient's inferior vena cava (IVC): the suprahepatic portion of the donor's IVC is anastomosed with the confluence of the recipient's SHV, leaving a closed stump of the infrahepatic portion of the donor's IVC.6,12 The classic technique, now less common, consists of resecting a segment of the recipient's IVC and two end-to-end anastomoses.
- 3
Cholecystectomy is performed to prevent infectious or ischaemic complications. The ductal anastomosis is end-to-end (choledococholedochostomy or choledocojejunostomy; hepaticojejunostomy is less common).6,7,12–15
- 1
- B.)
A whole graft can be used in children, although split-liver or partial grafts from live donor are more common. For whole grafts, the vascular anastomoses are similar to orthotopic transplantation in adults. For partial grafts, the donor's right or left arterial or venous branches are anastomosed with the recipient's HA (common/proper) and PV. For the piggyback technique, the donor's right SHV is anastomosed with the confluence of the recipient's SHV, or the middle and left SHV together, depending on whether the graft is right/left. One of the most common indications of transplantation in children is bile duct atresia. In these cases, the bile duct is reconstructed by Y-de-Roux hepaticojejunostomy.10
- C.)
Partial hepatectomy (left or right lobe) is performed in living-donor transplantation. The right lobe is preferentially grafted in adult recipients (occasionally the left lobe is sufficient). The lateral part of the left lobe (segments II and III) or right trisegmentectomy (segments I, V and VIII) are used in paediatric recipients.10 The vascular and ductal anastomoses are similar to those of partial graft in children.10,11
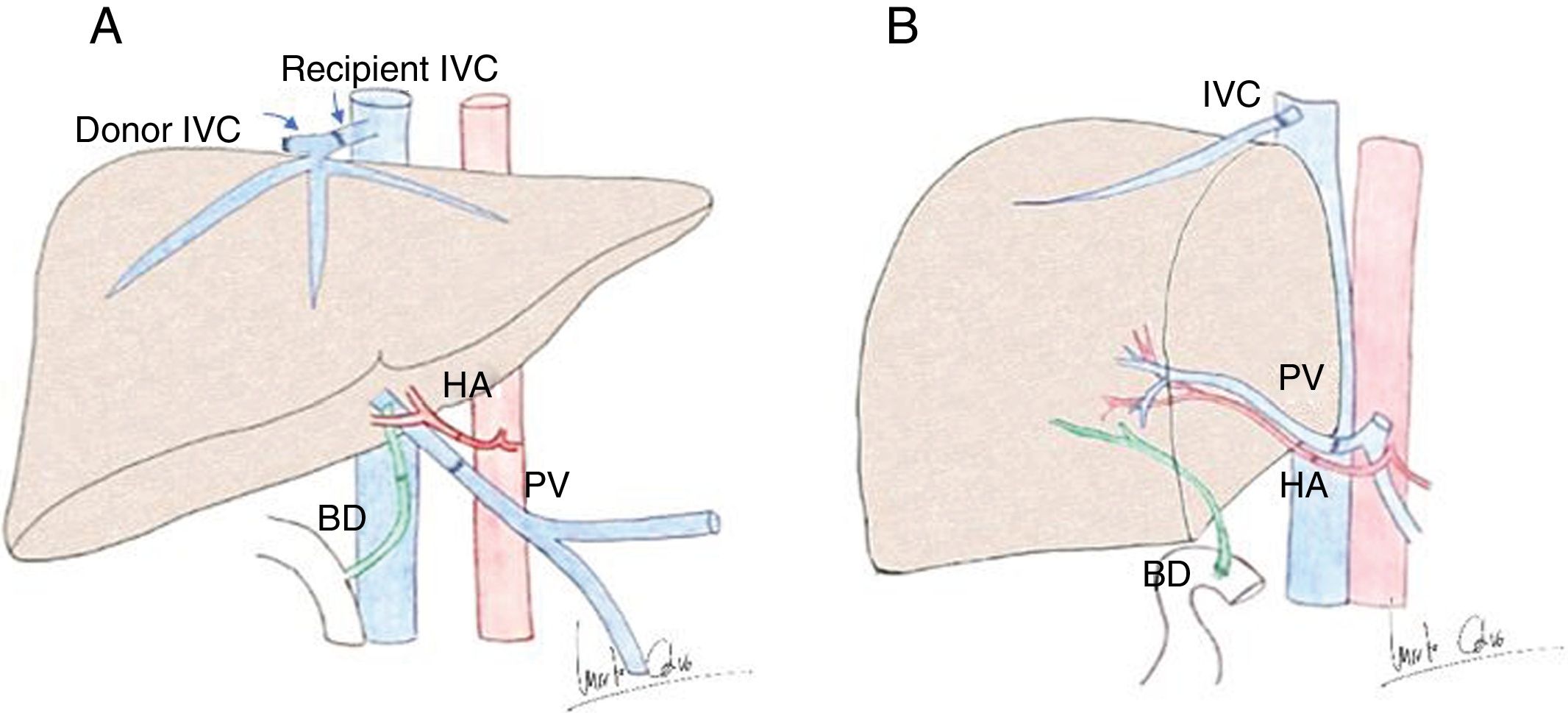
Anatomy of liver transplantation. A) Diagram of orthotopic liver transplant, with vascular anastomoses in the hepatic artery (HA), portal vein (PV), inferior vena cava (IVC) with piggyback technique and in the bile duct (BD). B) Liver transplant from live donor, in this case the right lobe, with anastomosis between right suprahepatic vein and IVC, HA, PV and Y-de-Roux reconstruction of the BD.
Modified from: Singh et al.4
It is the technique of choice, as it evaluates flow rates in real time, enabling the detection and follow-up of complications; it can be performed at the bedside, has high availability and low cost and does not use ionising radiation. It is, however, operator-dependent and its performance is limited if there is a poor acoustic window.16–18
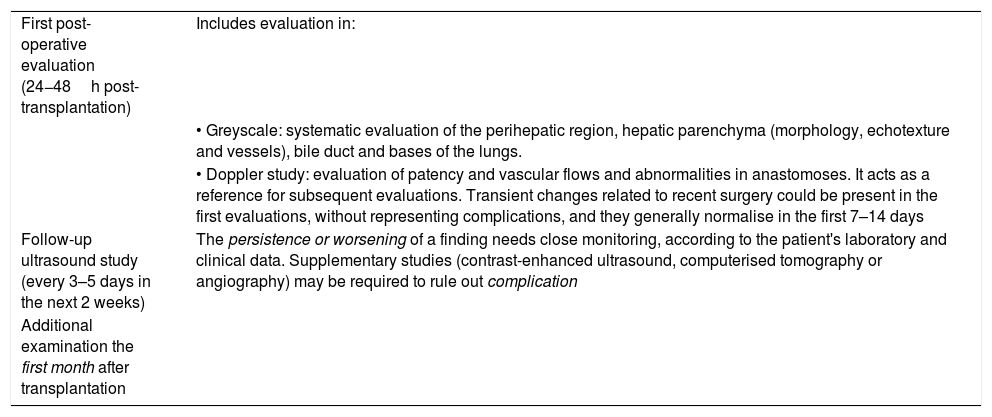
Table 1 shows the recommended protocol for ultrasound evaluation in the immediate postoperative period after transplantation.3,17,19
Protocol for ultrasound evaluation after liver transplantation.3,17,19
| First post-operative evaluation (24−48h post-transplantation) | Includes evaluation in: |
| • Greyscale: systematic evaluation of the perihepatic region, hepatic parenchyma (morphology, echotexture and vessels), bile duct and bases of the lungs. | |
| • Doppler study: evaluation of patency and vascular flows and abnormalities in anastomoses. It acts as a reference for subsequent evaluations. Transient changes related to recent surgery could be present in the first evaluations, without representing complications, and they generally normalise in the first 7–14 days | |
| Follow-up ultrasound study (every 3–5 days in the next 2 weeks) | The persistence or worsening of a finding needs close monitoring, according to the patient's laboratory and clinical data. Supplementary studies (contrast-enhanced ultrasound, computerised tomography or angiography) may be required to rule out complication |
| Additional examination the first month after transplantation |
A low frequency convex transducer is used. The linear transducer can be used in children to obtain higher resolution. The approach can be transabdominal, subcostal or intercostal.
The first evaluation serves as a reference. Some transient changes related to recent surgery can be present, without indicating a complication and normalising in 7–14 days. Any decline in successive studies requires supplementary studies to rule out complications.
Remember that recognition of normal and transient ultrasound findings in the immediate postoperative period after liver transplantation prevents diagnostic errors.
4 Findings in greyscale assessmentIt is performed before the Doppler study to evaluate the hepatic parenchyma, bile ducts, presence of collections and vascular structures17 (Figs. 2–4).
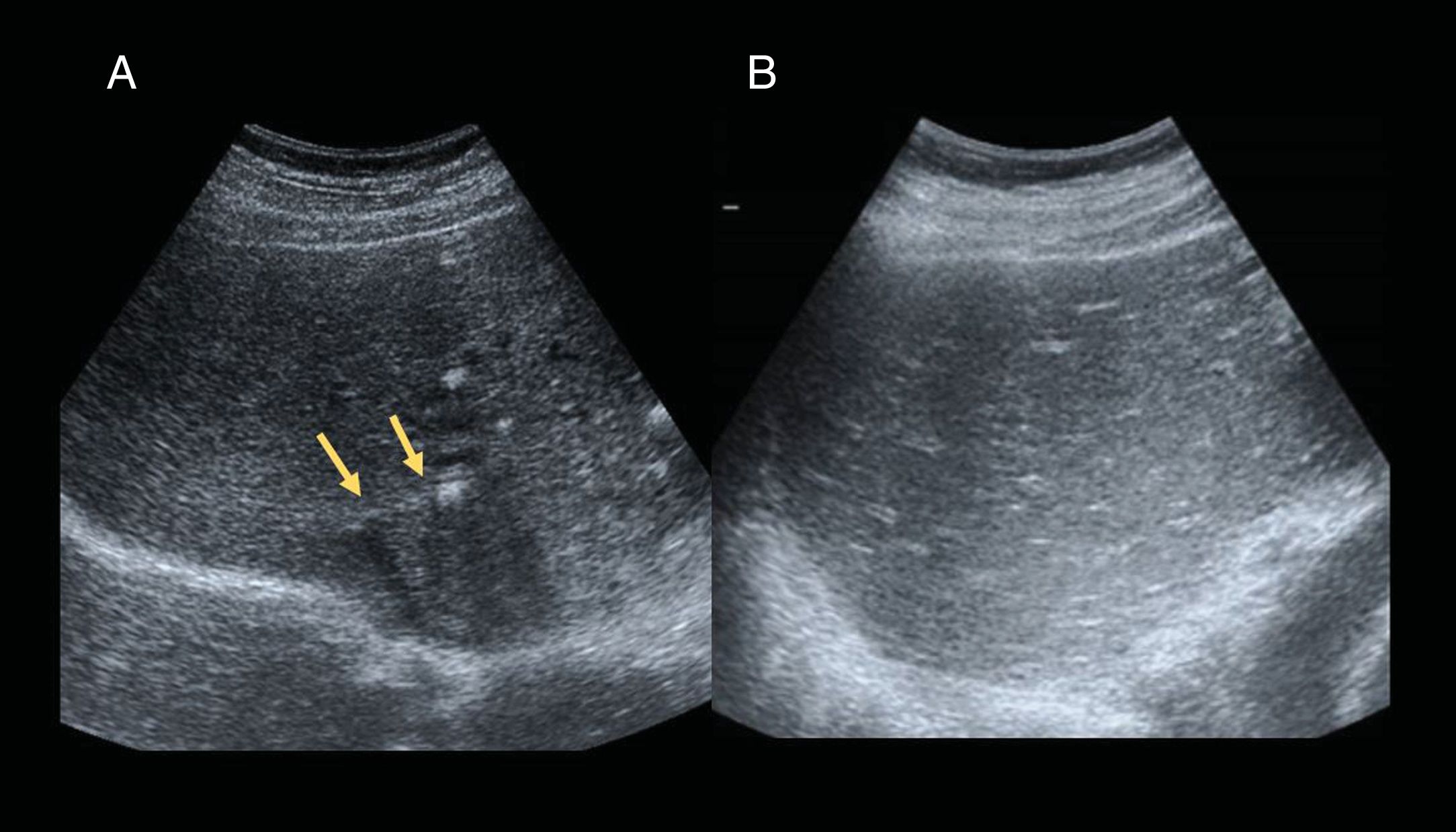
A) 53-year-old male, liver transplantation due to alcoholic cirrhosis and hepatitis C virus. The ultrasound on the first day after surgery shows small hyperechogenic interphases in the intraheptic bile duct (arrows) leaving a posterior acoustic shadow and representing the presence of gas (aerobilia), a finding to be expected with bilioenteric anastomoses. The bile duct is normal in size. B) 67-year-old woman transplanted due to cirrhosis secondary to primary biliary cholangitis. The ultrasound on the first day after transplantation shows periportal hyperechogenic reinforcement due to reperfusion oedema, known as a starry sky pattern.
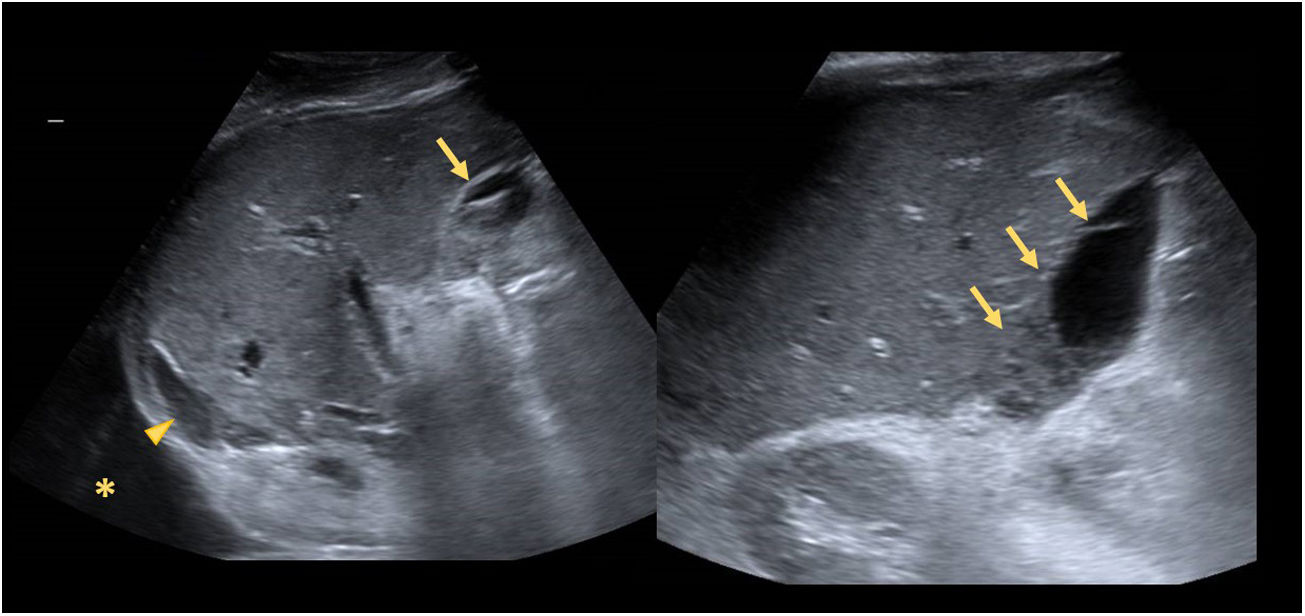
67-year-old male, liver transplant due to hepatocarcinoma (single nodule) secondary to alcoholic cirrhosis. 72h post-transplantation, the ultrasound shows right pleural effusion (asterisk), a small subhepatic collection that is suggestive of haematoma with anechoic areas and hyperechogenic clots in the lower portion (arrows) and minimal perihepatic preoperative mildly hyperechogenic ascites (arrowhead) that could be related to a blood clot. The patient was haemodynamically stable, with no clinical signs or blood tests showing active bleeding, so it appears to be residual to the surgery.
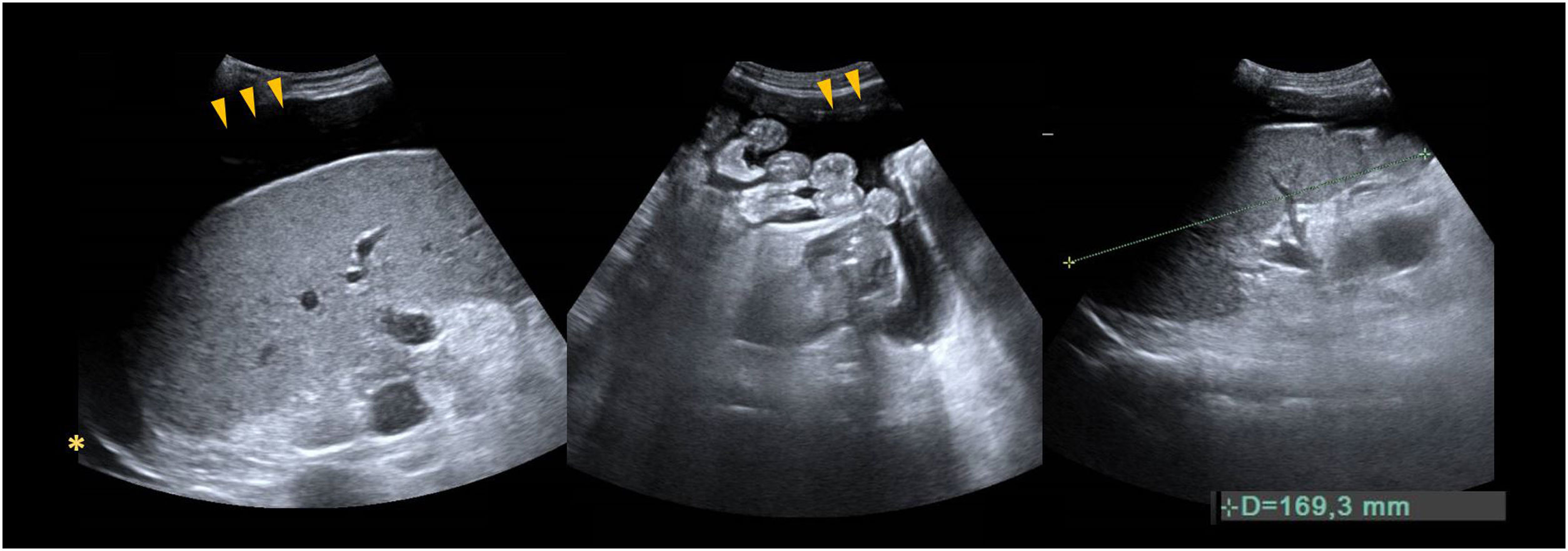
48-year-old woman, transplanted due to cirrhosis from hepatitis B virus. The first ultrasound 4h after transplantation shows a homogeneous graft and the presence of residual ascites (arrowheads), and minimal right pleural effusion (asterisk). Enlarged spleen, 16.9cm craniocaudal diameter, in relation to splenomegaly.
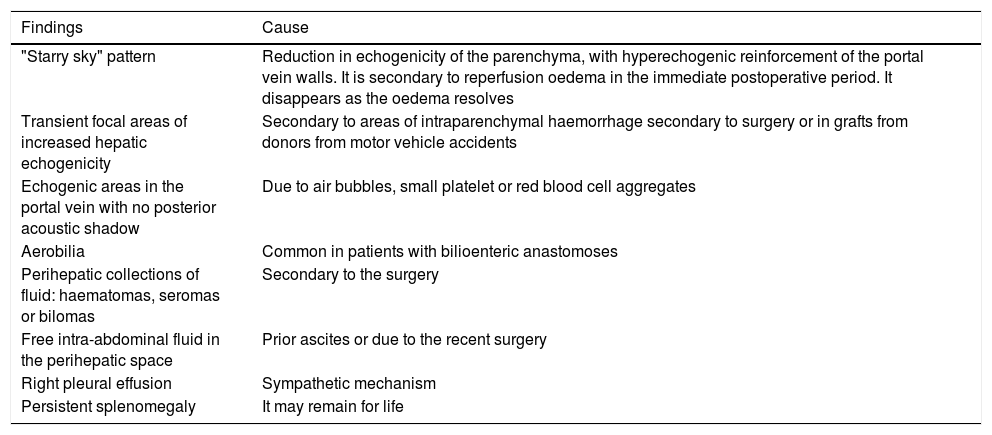
A normal graft has a homogeneous appearance. Table 2 summarises some findings secondary to reperfusion oedema that can be seen in the immediate postoperative period and spontaneously resolve (first few weeks).17–19
Common transient findings in greyscale ultrasound evaluation.17–19.
| Findings | Cause |
|---|---|
| "Starry sky" pattern | Reduction in echogenicity of the parenchyma, with hyperechogenic reinforcement of the portal vein walls. It is secondary to reperfusion oedema in the immediate postoperative period. It disappears as the oedema resolves |
| Transient focal areas of increased hepatic echogenicity | Secondary to areas of intraparenchymal haemorrhage secondary to surgery or in grafts from donors from motor vehicle accidents |
| Echogenic areas in the portal vein with no posterior acoustic shadow | Due to air bubbles, small platelet or red blood cell aggregates |
| Aerobilia | Common in patients with bilioenteric anastomoses |
| Perihepatic collections of fluid: haematomas, seromas or bilomas | Secondary to the surgery |
| Free intra-abdominal fluid in the perihepatic space | Prior ascites or due to the recent surgery |
| Right pleural effusion | Sympathetic mechanism |
| Persistent splenomegaly | It may remain for life |
The intrahepatic bile duct must be normal in size. Its dilation suggests stenosis in the anastomosis.
5 Findings in the Doppler ultrasound evaluationIt enables the dynamic real-time evaluation of the graft's vascularisation. The primary HA and its intrahepatic branches, the primary PV and its branches, and SHV and ICV are evaluated.
- •
Colour Doppler ultrasonography: it determines the patency and direction of flow (towards the liver: hepatopetal; away from the liver: hepatofugal).
- •
Spectral Doppler ultrasonography: it evaluates the morphology of the flow wave.20
Vascular complications are the most common and dangerous in liver transplantation. They are most common in partial grafts because the anastomoses are more complex. They should be evaluated by both greyscale and Doppler ultrasound.
6 Hepatic arteryIn the Doppler study, colour is identified in the hilum (adjacent to the primary PV) and its intrahepatic branches.
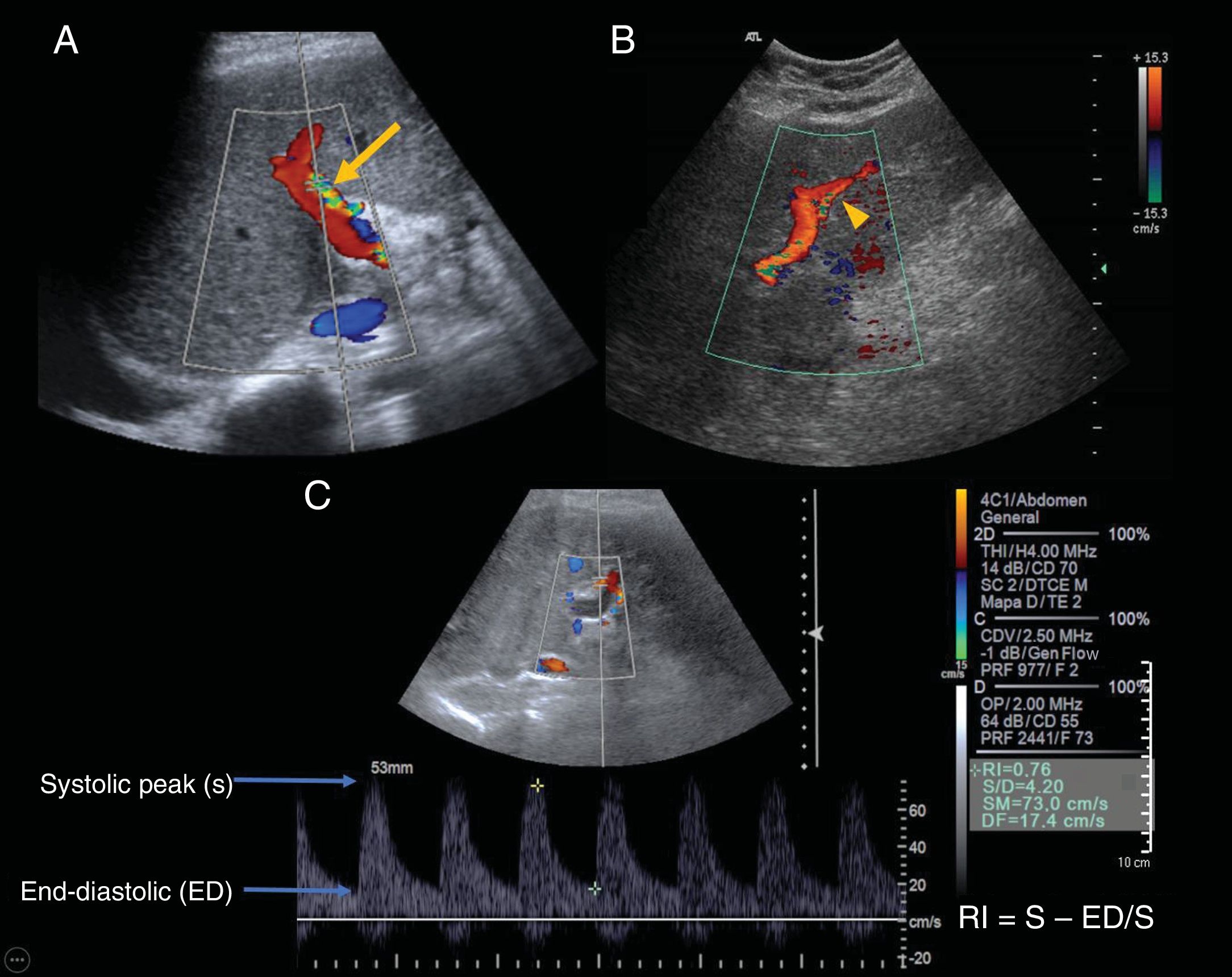
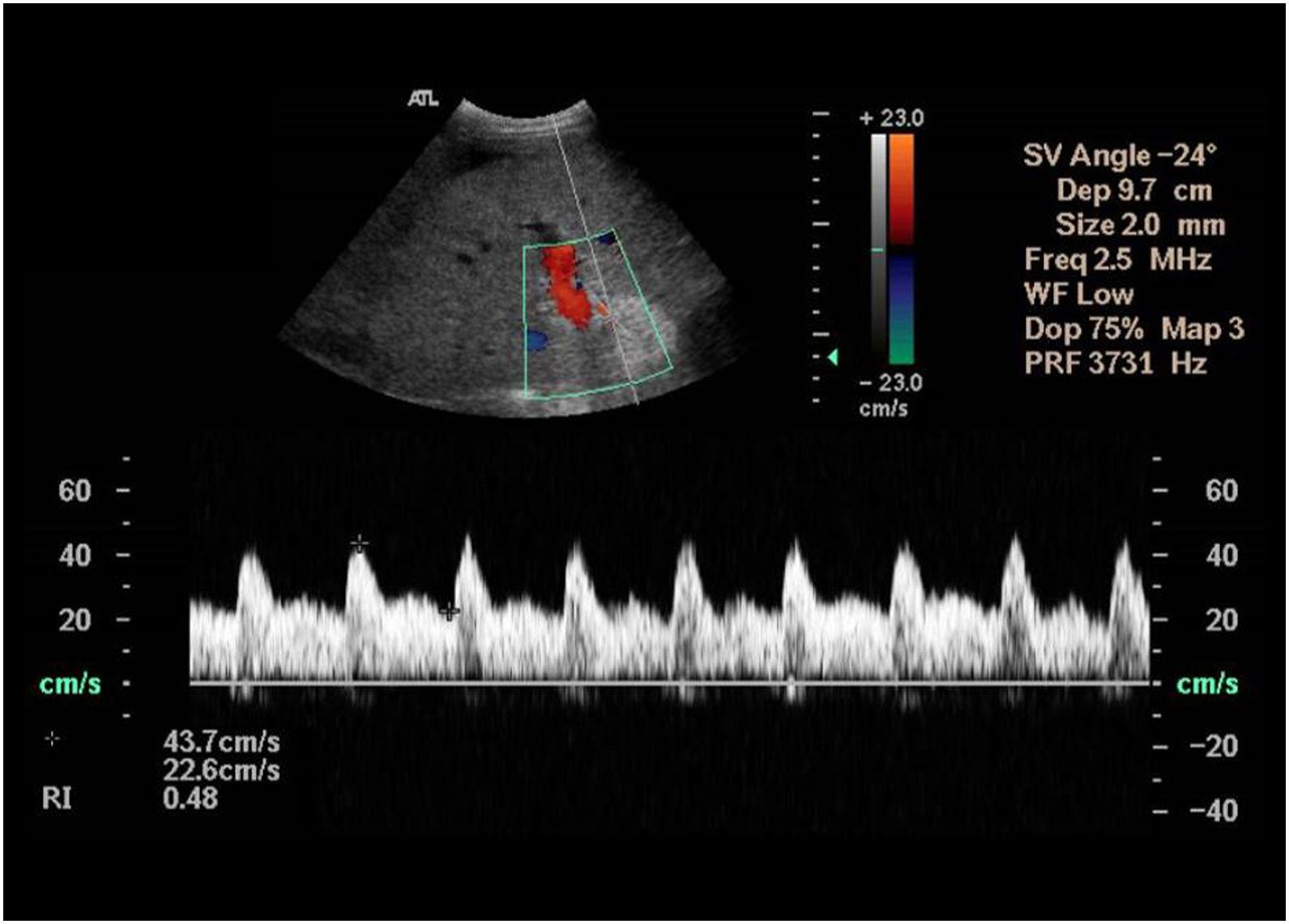
The following must be evaluated: flow morphology, acceleration time (AT), resistance index (RI) and peak-systolic velocity (PSV) in the right and left primary HA, which should have similar values16 (Fig. 5). Determination of RI and AT are mainly of post-anastomotic interest, while PSV is important to detect possible stenosis in the anastomosis:
- •
The morphology of hepatic arterial flow is that of a low-resistance pulsating wave, with a rapidly ascending systolic peak and continuous anterograde diastolic flow.
- •
Acceleration time measures the gradient of the ascending peak, in seconds. It should be less than 0.08s.
- •
RI reflects resistance to arterial flow in the microvascular bed that is distal to the measurement point. Normal values range from 0.55 to 0.8. The RI increases as diastolic flow diminishes. Absent or inverted diastolic flow represents an RI of 1.0.18,20–22
- •
The PSV of the HA ranges from 30 to 70cm/s.
61-year-old woman transplanted due to incoercible pruritus secondary to primary biliary cholangitis. Images A and B show the hepatic artery with colour Doppler ultrasound, and image C shows the wave morphology determined with spectral Doppler. A) The hepatic artery (HA) in the hepatic hilum (arrow) adjacent to the portal vein with an intercostal approach. Aliasing is seen in the artery; at this stage it is not necessarily pathological. B) The left branch of the HA (arrowhead), with an epigastric approach. C) We can see the normal wave morphology of the hepatic artery: pulsating, with a high systolic peak (S) and a continuous antegrade end-diastolic flow (ED).
Contrast-enhanced ultrasound (CEUS) makes it possible to confirm the presence of flow in some patients in whom it is difficult to study the artery in the first few hours, ruling out arterial thrombosis, and preventing unnecessary additional studies.23,24
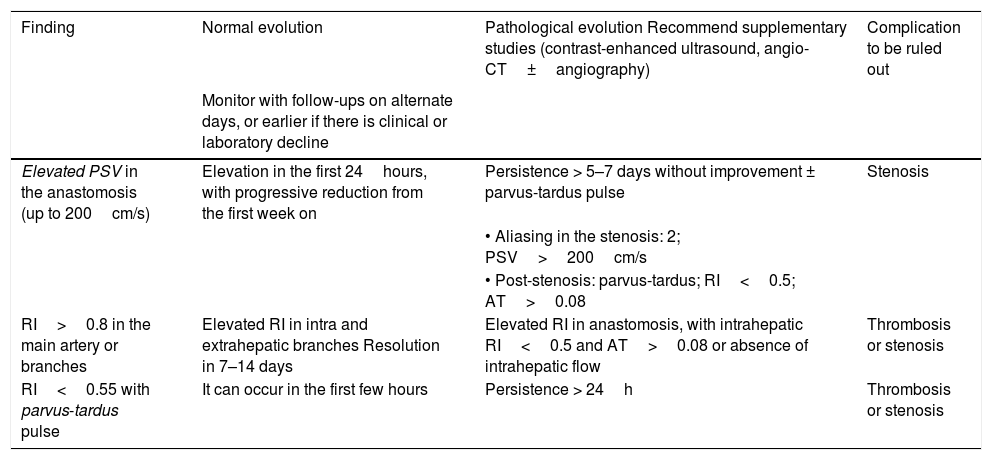
6.1 Reversible transient findings in the hepatic artery (Table 3)There are some transient changes in the HA in the immediate postoperative period. Other than the absence of arterial flow, the diagnosis of a hepatic arterial complication is rarely established from a single ultrasound. They require close monitoring and correlation with other clinical and analytical data (liver function tests)18:
- 1
Increased PSV in the anastomosis (broad range: up to 200cm/s)19,25:
- •
Secondary to persistent high pre-operative arterial flow due to portal hypertension, peri-anastomotic oedema or vascular fold (in case of redundant HA). It increases in the first 24−48h and diminishes during the first week as haemodynamic status/oedema normalizes.6
- •
Its persistence for more than a week, or elevation, should lead to the suspicion of an arterial stenosis, especially when associated with a parvus-tardus intrahepatic wave (Fig. 6).
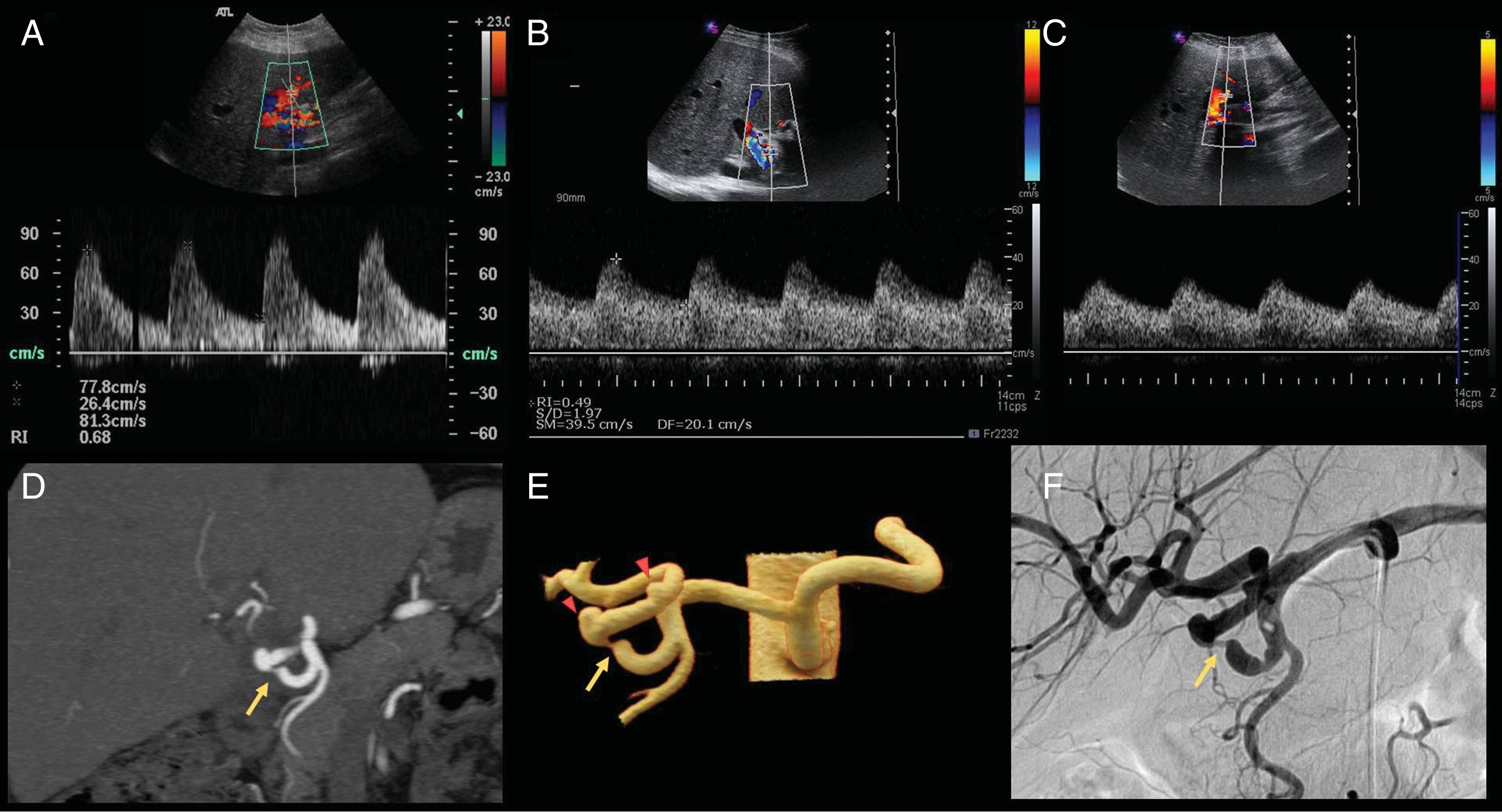
Figure 6.63-year-old male, transplanted due to alcoholic cirrhosis with frequent asctitic-edematous decompensations. A) Doppler ultrasound of the hepatic artery (HA) the first day after liver transplantation, showing an arterial flow with normal morphology, a resistance index (RI) of 0.68 and mild increase of peak systolic velocity (PSV) of 77.8cm/s, normal findings in the immediate postoperative period. B and C) Follow-up of the same patient 7 days post-transplantation. The HA is patent with diminished RI (0.49) in both the intrahepatic (C) and extrahepatic (B) branches, so it is indicated to rule out stenosis in the artery. D and E) The angio-CT and cinematic reconstruction of the branches of the celiac trunk show stenosis of approximately 70% of the span in the recipient's HA proper (arrow), approximately 2cm from the gastroduodenal artery. The anastomosis is immediately located distal to the stenosis, and a vascular graft can be seen between the donor and recipient's hepatic artery (arrowheads). F) Angiography through the celiac trunk showing the stenosis (arrow) proximal to the surgical anastomosis. It was treated by dilation with angioplasty balloon with a satisfactory outcome.
(0.38MB).
- •
- 2
Elevation of RI>0.8: most common transient anomaly (nearly 50% of patients):
- •
Due to reduced, absence or inverted diastolic flow (Fig. 7).
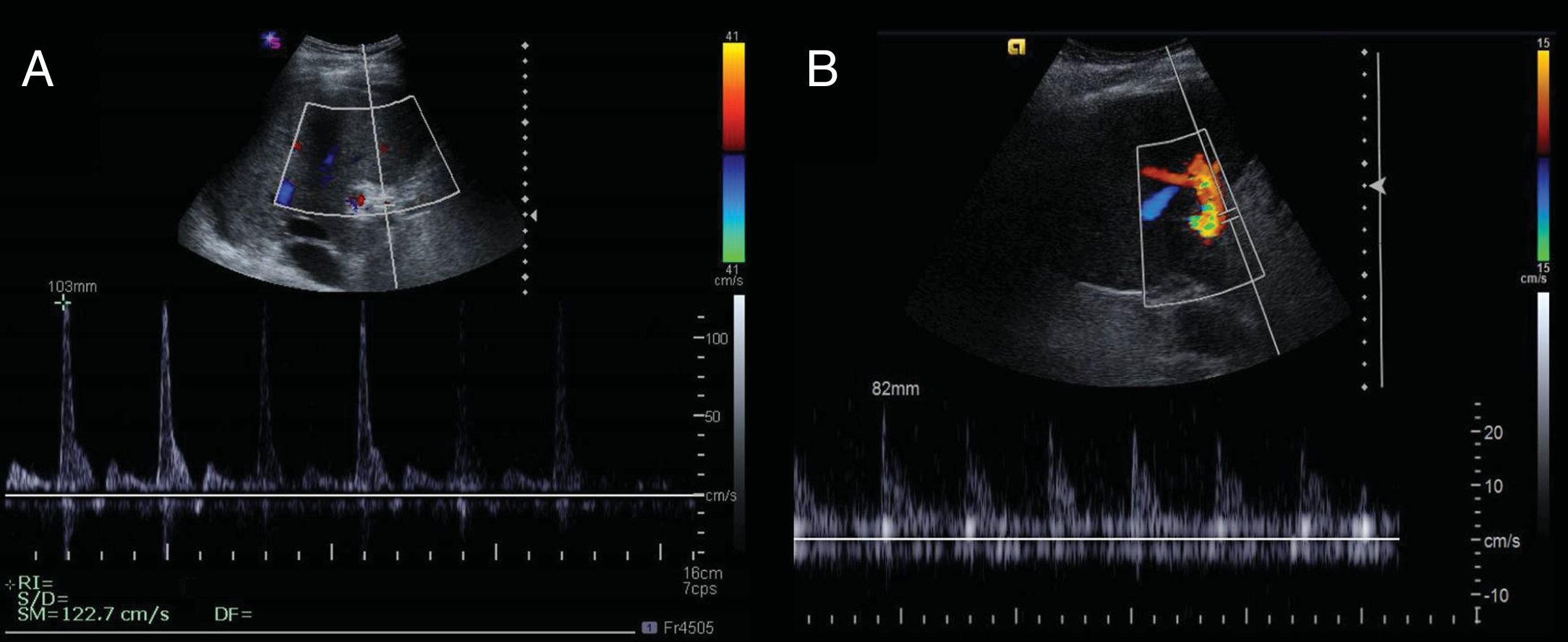
Figure 7.67-year-old woman, transplanted due to cirrhosis from hepatitis C virus. The first day after surgery, the Doppler ultrasound shows an increase in peak systolic velocity (PSV) of 122.7cm/s and increased resistance index (RI) with a value of 1 due to inverted or missing diastolic flow. The findings normalised in subsequent evaluations. B) 62-year-old male, transplanted due to cirrhosis of the liver of alcoholic origin. On the first day after surgery, the Doppler ultrasound shows absent diastolic flow, with an RI of 1.
(0.13MB). - •
Secondary to graft oedema, increased cold ischaemia time, increased portal flow or vasospasm. It normalises in a few days and is not associated with poor graft function19,22,26:
- •
Common transient findings in the hepatic artery.3,16–19,22,23,26.
| Finding | Normal evolution | Pathological evolution Recommend supplementary studies (contrast-enhanced ultrasound, angio-CT±angiography) | Complication to be ruled out |
|---|---|---|---|
| Monitor with follow-ups on alternate days, or earlier if there is clinical or laboratory decline | |||
| Elevated PSV in the anastomosis (up to 200cm/s) | Elevation in the first 24hours, with progressive reduction from the first week on | Persistence > 5–7 days without improvement ± parvus-tardus pulse | Stenosis |
| • Aliasing in the stenosis: 2; PSV>200cm/s | |||
| • Post-stenosis: parvus-tardus; RI<0.5; AT>0.08 | |||
| RI>0.8 in the main artery or branches | Elevated RI in intra and extrahepatic branches Resolution in 7–14 days | Elevated RI in anastomosis, with intrahepatic RI<0.5 and AT>0.08 or absence of intrahepatic flow | Thrombosis or stenosis |
| RI<0.55 with parvus-tardus pulse | It can occur in the first few hours | Persistence > 24h | Thrombosis or stenosis |
RI: resistance index; AT: acceleration time; CT: computerised tomography; PSV: peak systolic velocity.
If RI is elevated in the intra and extrahepatic branches, it is more likely to be transient.
If RI is elevated in the anastomosis and presents low-resistance wave morphology or absence of flow in the intrahepatic branches, arterial thrombosis/stenosis should be suspected. This requires urgent confirmation through CEUS, CT or angiography.3,23,24
- 3
Drop in RI<0.55 and parvus-tardus pulse (Fig. 8):
- •
Due to increased diastolic flow.
- •
Secondary to peri-anastomosis oedema. It should be interpreted with caution, especially in patients with good liver function. It requires close monitoring. It generally resolves in 24−48h. If it persists, stenosis or dissection should be ruled out.16,22–26
Figure 8.70-year-old male, transplanted due to acute liver failure secondary to hepatitis B virus. On the first day after surgery, the Doppler ultrasound shows arterial flow with normal wave morphology, with a resistance index of 0.48 (diminished). This finding requires close ultrasound monitoring to establish normalisation (it could indicate arterial stenosis if it does not normalise in 24−48h).
(0.12MB). - •
Most complications arise in the first few days or weeks after transplantation. Those related to the anastomosis of the HA are the most serious and require prompt diagnosis and treatment to prevent graft dysfunction.
It must be remembered that there is a post-operative adjustment period in which HA wave morphology can present transient findings that do not necessary involve complication.
7 Portal veinThe confluence of the PV is better identified with a transverse approach on the subcostal midline. The intercostal approach is preferred to evaluate the right intrahepatic branches.
The wave morphology shows a continuous hepatopetal pattern with moderate changes due to respiratory movement or heartbeat. The flow has low velocity, 15−40cm/s.16,20
7.1 Reversible transient findings in the PV (Fig. 9)- 1
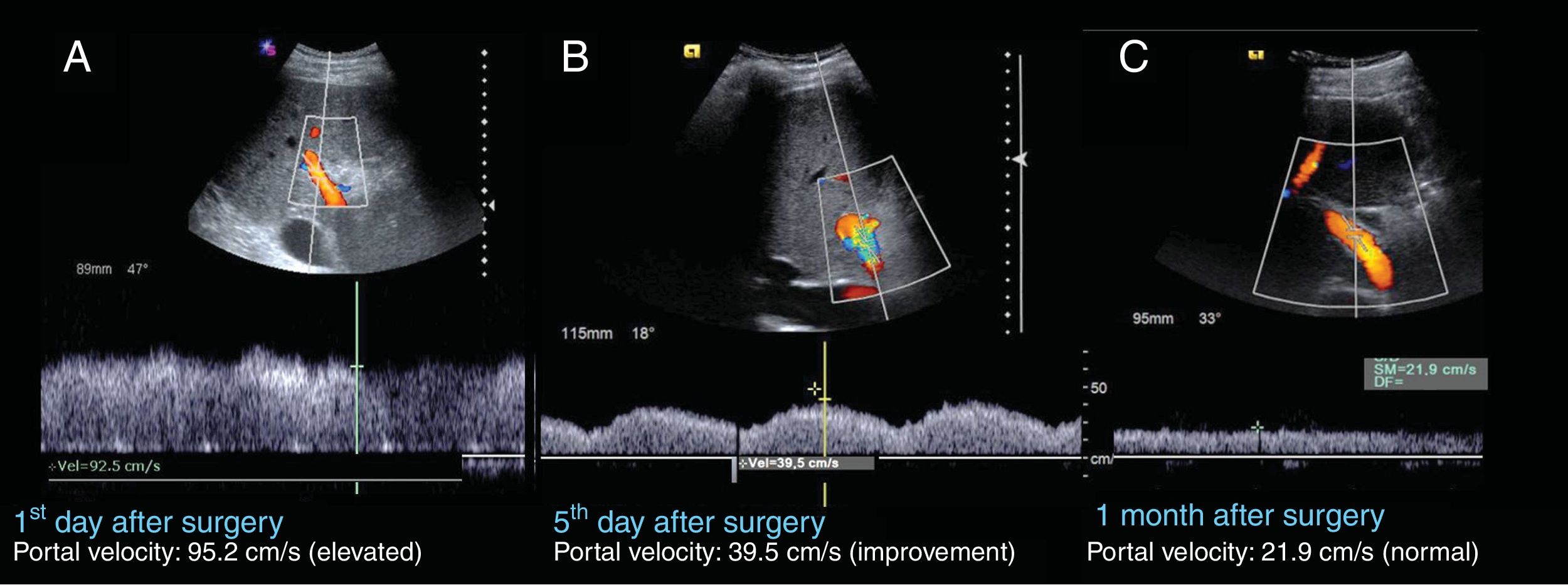
Increased portal velocity in the anastomosis and post-anastomotic: some haemodynamic changes, such as reduced portal resistance and increased splanchnic flow due to prior hypertension, increase velocity. It increases in the first 24h and progressively decreases after the first week, for months.6 It is occasionally accompanied by turbulent or aliasing flow, which improves as velocity normalizes.6
The differential diagnosis should be with portal stenosis (very rare) when increased velocity is only in the anastomosis, especially if the anastomosis/pre-anastomosis velocity is greater than 3:1.6,18
- 2
Transient pulsating flow: common finding in patients with congestive heart failure and in the postoperative period after transplantation due to haemodynamic changes.19
- 3
Helicoidal flow (two-directional pre-anastomotic hepatopetal and post-anastomotic hepatofugal). It should not be mistaken for reverse portal flow.15 It can be seen when the donor's PV is larger than that of the recipient. It occasionally indicates stenosis.6
They are seen by a transverse approach on the midline or intercostal line. In the piggyback technique, the stump of the donor's IVC should not be mistaken for collections.
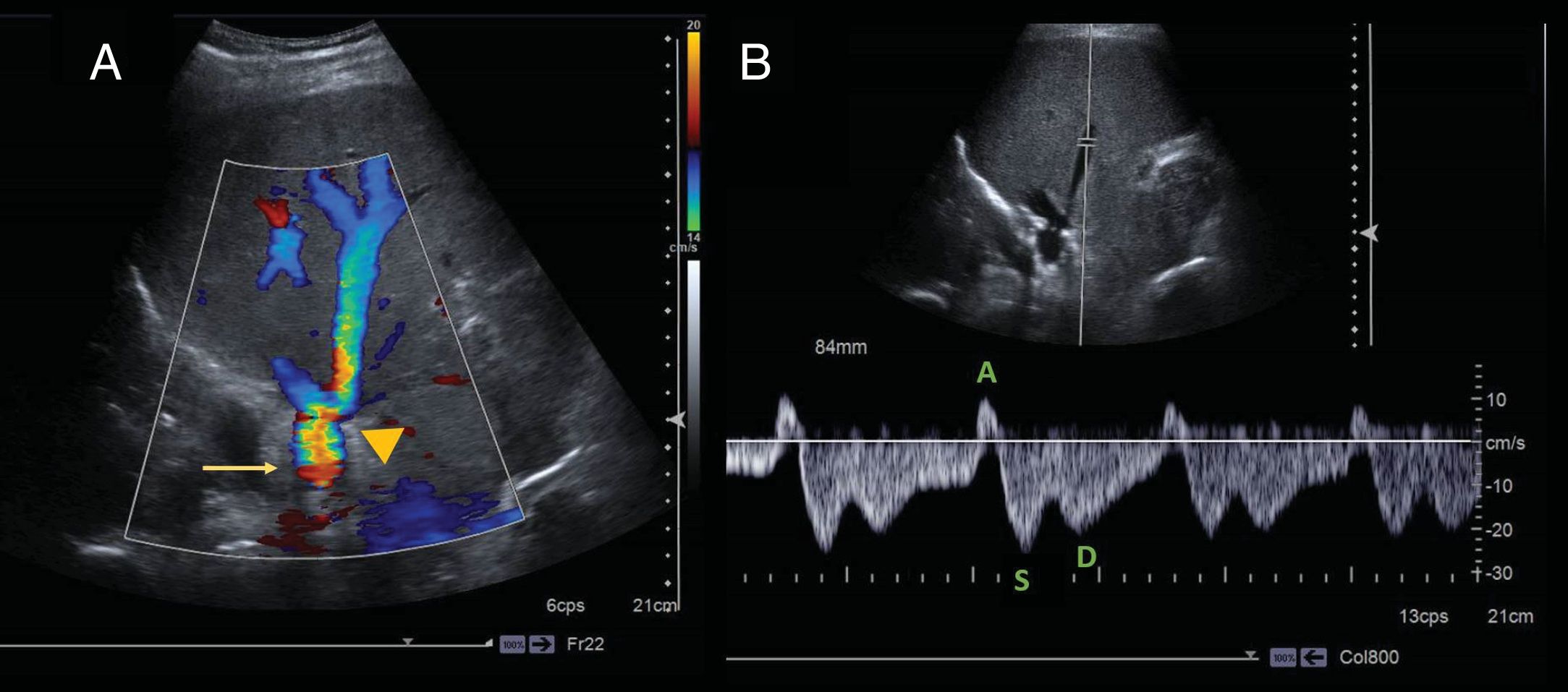
Wave morphology depends on the cardiac cycle, with predominantly three-phase hepatofugal flow due to the transmission of heart beats27,28 (Fig. 10).
56-year-old male, transplanted due to cryptogenetic cirrhosis of the liver. A) The first ultrasound evaluation shows hepatopetal flow with elevated portal velocity of around 95.2cm/s, and mild modulation due to breathing. B) The ultrasound on the fifth day after surgery, shows improved portal velocity, 39.5cm/s. Some aliasing and pulsatility is also seen. C) One month post-transplantation, the ultrasound shows normalisation of portal velocity, 21.9cm/s, and absence of pulsatility.
68-year-old woman, transplanted due to cirrhosis secondary to metabolic steatohepatitis. Ultrasound evaluation of the suprahepatic veins with pulsed colour Dopplier with a subcostal approach. A) Colour Doppler in the anastomosis region between the donor's (arrowhead) and the recipient's (arrow) inferior vena cava with the piggyback technique, showing the anastomosis intact with some aliasing and normal hepatofugal flow in the suprahepatic veins (SHV). B) The spectral Doppler in the mid SHV shows the characteristic wave morphology of the SHV, with a three-phase wave distinguishing its three cardiac cycle-dependent components: atrial systole (A) ventricular systole (S) and atrial diastole (D). The S wave is deeper than the D wave.
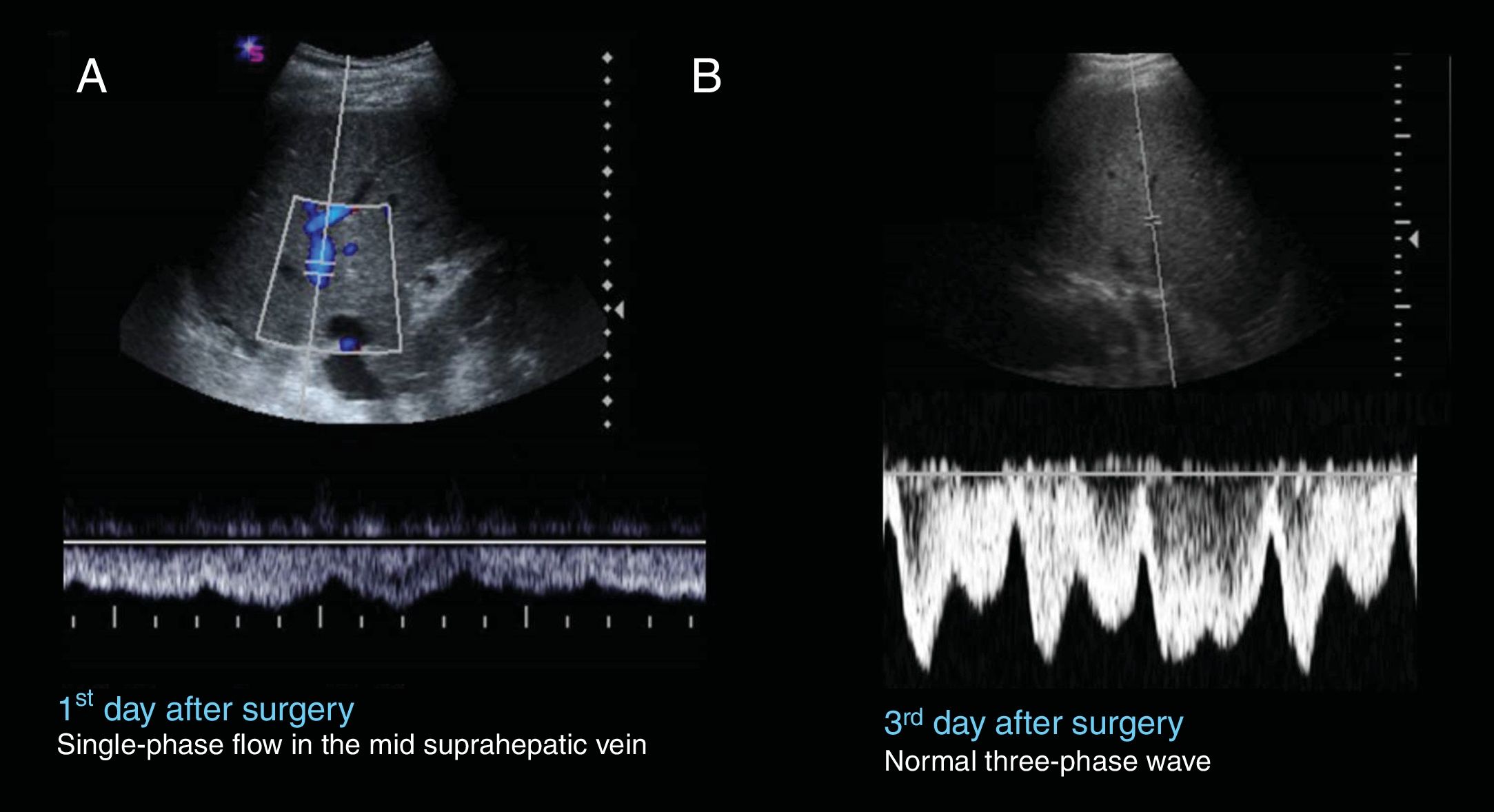
- 1
One-phase or two-phase flow (Fig. 11): it is also generally secondary to oedema or compression by an adjacent haematoma/collection. It normalises in successive follow-up imaging.
Figure 11.73-year-old male, transplanted due to uncompensated cirrhosis of the liver of alcoholic origin. A) On the first day after surgery, the Doppler ultrasound shows loss of normal phasicity in the middle SHV. B) On the third day after surgery, the normal three-phase morphology of the supraheptic veins has been recovered.
(0.15MB). - 2
Turbulence in the anastomosis: secondary to oedema.
Anastomotic stenoses also manifest as loss of phasicity or turbulent flow maintained over time, associated with ascites. It is a rare, generally late (weeks-month) complication.16,18,19
10 ConclusionEarly complications of liver transplantation represent a threat to the graft or the patient. Ultrasonography, including a Doppler study, plays a critical role in graft evaluation. It is not uncommon to encounter findings other than the usual findings, which are not necessary pathological; familiarity with them is key to detecting or ruling out complications. Any of these findings, maintained over time, could be a sign of complications.
Authorship- 1
Responsible for the integrity of the study: MCI, DCR.
- 2
Study conception: MCI, DCR.
- 3
Study design: MCI, ISA.
- 4
Data acquisition: MCI, DCR, ISA.
- 5
Data analysis and interpretation: MCI, AEC, ISA, ABB, DCR.
- 6
Statistical processing: NA.
- 7
Literature search: MCI, ISA.
- 8
Writing the article: MCI, AEC, ISA.
- 9
Critical review of the manuscript with intellectually relevant contributions: MCI, ABB, DCR.
- 10
Approval of the final version: MCI, AEC, ISA, ABB, DCR.
The authors declare that they have no conflicts of interest.
Please cite this article as: Calvo-Imirizaldu M, Ezponda Casajús A, Soriano Aguadero I, Benito Boillos A, Cano Rafart D. Hallazgos ecográficos transitorios y normales en el postoperatorio inmediato del trasplante hepático. Radiología. 2020;62:112–121.