Intracranial haemorrhage (ICH) accounts for 10–30% of strokes, being the form with the worst prognosis. The causes of cerebral haemorrhage can be both primary, mainly hypertensive and amyloid angiopathy, and secondary, such as tumours or vascular lesions.
Identifying the aetiology of bleeding is essential since it determines the treatment to be performed and the patient's prognosis. The main objective of this review is to review the main magnetic resonance imaging (MRI) findings of the primary and secondary causes of ICH, focusing on those radiological signs that help guide bleeding due to primary angiopathy or secondary to an underlying lesion. The indications for MRI in the event of non-traumatic intracranial haemorrhage will also be reviewed.
La hemorragia intracraneal (HIC) supone un 10–30% de los ictus, siendo la forma de peor pronóstico. Las causas de hemorragia cerebral pueden ser primarias, fundamentalmente la angiopatía hipertensiva y amiloide, o secundarias, como tumores o lesiones vasculares.
Identificar la etiología del sangrado es importante, ya que determina el tratamiento a realizar y el pronóstico del paciente. El objetivo principal de esta revisión es repasar los principales hallazgos por resonancia magnética (RM) de las causas de HIC primarias y secundarias, deteniéndonos en aquellos signos radiológicos que ayudan a orientar hacia un sangrado por una angiopatía primaria o bien secundario a una lesión subyacente. También se revisarán las indicaciones de RM ante una hemorragia intracraneal no traumática.
Intracranial haemorrhage (ICH) accounts for 10–30% of strokes,1,2 and is the worst form in terms of prognosis. Despite therapeutic efforts, prevalence has not decreased, but rather remained stable over the last four decades.3 Its incidence is 20–24/100,000 people/year4 and is much higher in some regions of the world, such as Southeast Asia.3,5
The fundamental role of the radiologist is to try to determine the cause of the bleeding, given that the patient's prognosis depends on how quickly treatment is started.4 Computed tomography (CT) is suitable for the diagnosis of intracranial haemorrhage,1,6,7 and should there be cause to suspect a vascular lesion in the acute phase, a CT angiography (CTA) should be the next test performed.8 Magnetic resonance imaging (MRI) is used later to establish the aetiology of the ICH, as this may provide evidence of small vessel disease or an underlying lesion.2,9
When presented with a brain haemorrhage, we need to know when it is appropriate to perform an MRI. MRI is very useful for determining the aetiology of a bleed, which can influence the initial diagnosis and treatment.2,9 If an ICH is diagnosed, an MRI is recommended with urgent priority,10 except for in the following situations11:
- □
Patients > 65 years old, with hypertension and haematomas in the thalamus or basal ganglia, in which case we assume the origin is hypertension.10
- □
Patients > 85 years old with intracranial haematomas in any location, especially lobar haematomas that exhibit characteristic features of cerebral amyloid angiopathy on head CT, such as subarachnoid haemorrhage (SAH) or finger-like projections.12
Digital subtraction angiography is not routinely indicated due to its intrinsic risk being an invasive procedure. Its primary use is in cases where there is a high suspicion of an underlying vascular lesion, and when non-invasive diagnostic methods are inconclusive.4
The main objective of this article is to review the most significant MRI findings of primary and secondary causes of ICH, focusing on radiographic signs that help diagnose whether the bleed is due to a primary angiopathy or a secondary underlying lesion.
Haemorrhage on MRIFor intracranial haemorrhage, the recommended head MRI protocol should include the following sequences: T1-weighted (T1), T2-weighted (T2), Fluid-attenuated inversion recovery (FLAIR), Diffusion-weighted (DWI), T2-star (T2*), susceptibility-weighted imaging (SWI), and post-contrast T1 sequences. Additionally, if a vascular lesion is suspected, a dynamic MR angiography (MRA) should be performed, avoiding contrast-enhanced 3D Time-of-Flight (TOF) techniques because the hyperintense signal of the haematoma and contrast uptake overlap with the vascular structures.8
Haemorrhages pass through five stages depending on the time elapsed, and their appearance on MRI varies according to the stage (Table 1).2,13
Evolutionary stages of intracranial haemorrhages and their appearance on MRI on T1 and T2 sequences.
| State | T1WI | T2WI | Time |
|---|---|---|---|
| Hyperacute | Isointense | Hyperintense | Hours |
| Intracellular OxiHgb | |||
| Acute | Isointense | Hypointense | Days |
| Intracellular DeoxyHgb | |||
| Early subacute | Hyperintense | Hypointense | Days–1 week |
| Intracellular MetaHgb | |||
| Late subacute | Hyperintense | Hyperintense | 1 week–1 month |
| Extracellular MetaHbg | |||
| Chronic | Isointense | Hyperintense with hypointense rim | >Months |
In the hyperacute stage, they appear isointense on T1 sequences, hyperintense on T2 sequences, and characteristically show a hypointense halo on SWI sequences.14
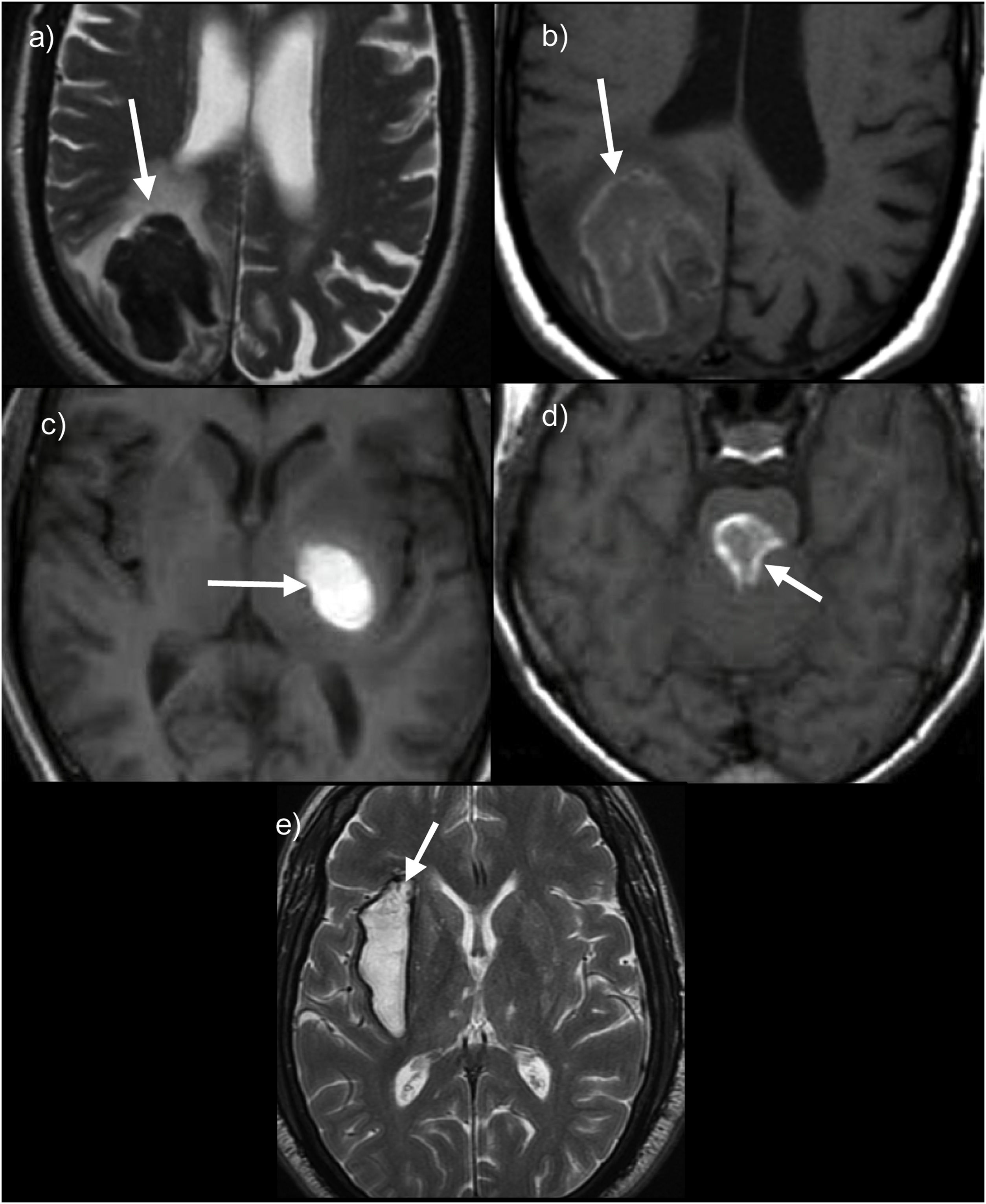
In acute phases, T2 sequences should be used, in which haemorrhage is markedly hypointense (Fig. 1).
Examples of the different evolutionary stages of intraparenchymal haematomas on MRI. (a and b) Right occipital lobar haematoma in acute phase showing marked signal hypointensity on T2WI due to deoxyhaemoglobin, while on T1 they are relatively isointense. (c and d) Examples of haematomas in subacute phase located in the left basal ganglia (c) with homogeneous hyperintense signal on T1 in pons (d) showing a hyperintense halo on T1 with a hypointense centre. (e) Chronic or old haematoma with hyperintense centre and hypointense rim on T2WI.
Subacute haemorrhages, both in the early and late phases, appear as strongly hyperintense lesions on T1 sequences (Fig. 1); early subacute haemorrhages are hypointense on T2 sequences, given their shorter evolution time, while late subacute haemorrhages are hyperintense on both T1 and T2 sequences.
Finally, haematomas in the chronic phase exhibit a strongly hypointense halo on T2 sequences or simply appear as a hypointense line (Fig. 1), which may remain indefinitely. It should be noted that the change from one phase to the next is first seen at the periphery and spreads towards the centre.
Susceptibility-weighted imagingIn addition to conventional T1- and T2-weighted images, SWI sequences are an essential part of the study of brain haemorrhages. These are 3D gradient-echo images which make use of both magnitude and phase information.15
They make it possible to visualise materials which cause focal distortion of the magnetic field and generate high susceptibility. In our case, we are interested in blood degradation products, but air, metal and calcium also have high susceptibility.
Deoxyhaemoglobin, ferritin and haemosiderin are blood degradation products that are paramagnetic and produce the ‘blooming’ artefact (increased hypointense signal with respect to their appearance on T2).
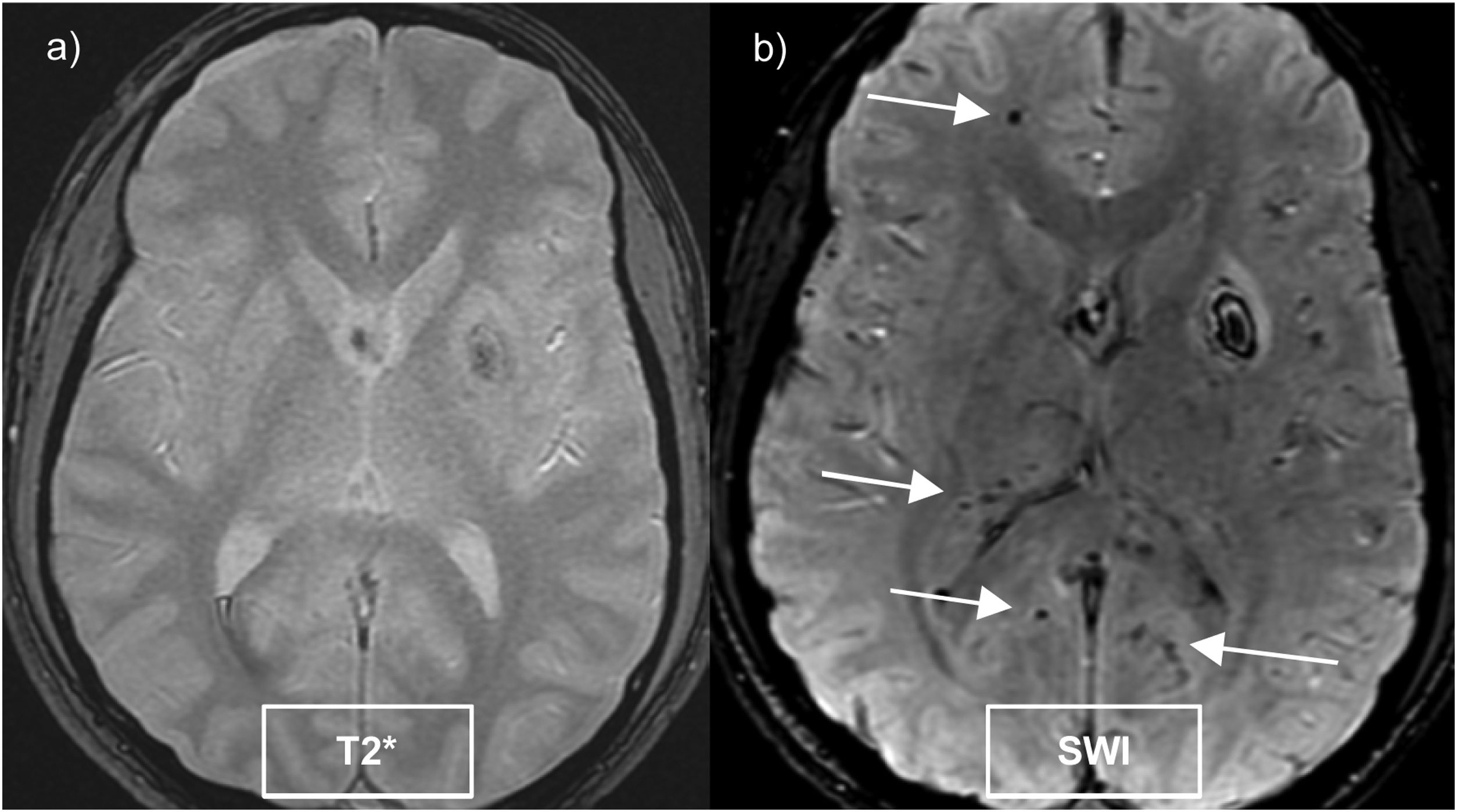
These sequences double the detection of microhaemorrhages compared to gradient echo sequences (Fig. 2) and are reliant on the magnetic field strength.16
Cerebral microhaemorrhages are small hypointense foci (5–10 mm) detected on SWI sequences, which histologically correspond to accumulations of haemosiderin in macrophages. They are mainly caused by cerebral small vessel disease, the most frequent being sporadic cerebral amyloid angiopathy and hypertensive angiopathy.16
The frequency of microhaemorrhages is related to age, since approximately 40% of patients over 80 years of age experience them.17 However, there is an even stronger association with vascular risk factors such as hypertension, leukoaraiosis, lacunar strokes and even genetic factors.17
Most importantly, microhaemorrhages are a marker of ongoing cerebrovascular disease and therefore represent an increased risk of ischaemic stroke,17 as well as recurrence of ICH and de novo ICH.5,18 It should be noted that the presence of microhaemorrhages is not currently a contraindication for anticoagulant, antiplatelet or thrombolysis treatment.15
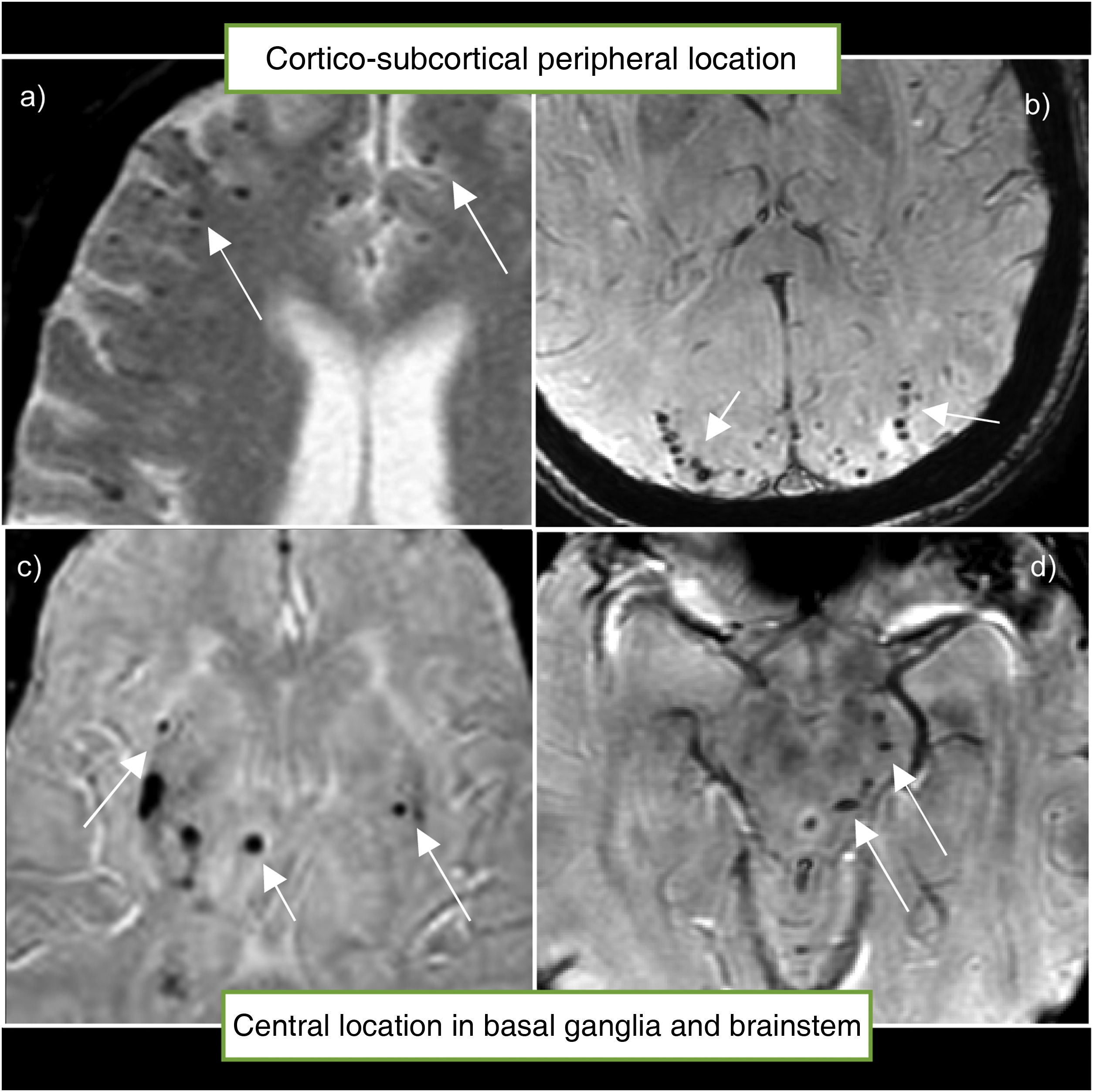
The location of the microhaemorrhage correlates with its cause. Thus, lobar microhaemorrhages located in the region of the cerebral cortex or cerebellar cortex have a very strong association with amyloid angiopathy; while those located in deep brain areas such as the basal ganglia, brainstem or deep cerebellar nuclei are more closely associated with hypertensive angiopathy (Fig. 3).17,19
Location of microhaemorrhages. (a and b) microhaemorrhages in the typical peripheral or cortico-subcortical location, associated with cerebral amyloid angiopathy. (c and d) microhaemorrhages in the typical central location in the basal ganglia and brainstem, associated with hypertensive angiopathy.
There are many other rare causes of microhaemorrhages, including holo-cranial radiotherapy treatment, disseminated intravascular coagulation, septic emboli, diffuse axonal injury from head trauma, the use of extracorporeal membrane oxygenation (ECMO) machines, and cardiac surgery.
Aetiology of brain haemorrhagesIntracranial haemorrhages can have primary or secondary aetiology (Table 2). Primary causes are the most frequent, accounting for almost 90% of cases,15 and include in particular hypertensive angiopathy and cerebral amyloid angiopathy.
Primary and secondary aetiologies of non-traumatic intracranial haemorrhages.
| Primary aetiology |
| Hypertensive angiopathy |
| Cerebral amyloid angiopathy |
| Secondary aetiology |
| Vascular lesions: |
| Arteriovenous malformation (AVM) |
| Dural arteriovenous fistula |
| Cavernous hemangioma |
| Vascular causes: |
| Venous haemorrhagic infarction |
| Haemorrhagic transformation of arterial infarction |
| Anticoagulation/coagulopathy |
| Sympathomimetic drugs |
| Structural lesions: Tumours |
Location is one of the factors that can help us establish the aetiology of a cerebral haemorrhage:
- □
Lobar haematomas: located superficially in the cerebral hemispheres. In elderly patients, the main cause is amyloid angiopathy.
- □
Non-lobar haematomas: located in deep structures such as the basal ganglia, thalamus, pons and cerebellum. The main cause of this type of haematoma is chronic hypertension.
Another important factor to take into account is age, since the possibility of an underlying lesion that is causing the bleeding should be suspected in young patients.
Primary intracranial haemorrhagesCerebral amyloid angiopathyCerebral amyloid angiopathy (CAA) is highly prevalent, occurring in up to half of elderly patients, and is strongly associated with Alzheimer's disease.20 It is characterised by a progressive accumulation of amyloid-beta in the walls of leptomeningeal and cortical vessels,21 and their rupture produces lobar haemorrhages, most frequently in the posterior regions (occipital lobe and posterior temporal lobe),22,23 and generally in older individuals.
There are three clinical features that suggest the possibility of CAA: spontaneous lobar haemorrhage with a tendency to recur,24,25 cognitive impairment and dementia,26 and transient neurological episodes (‘amyloid spells’).21
Five biomarkers of diagnostic significance have been described in MRI (Fig. 4)27:
- 1.
Multiple microhaemorrhages located in the periphery of the lobes, which have a high predictive value for the diagnosis of cerebral amyloid angiopathy, even in patients with no lobar haematoma.24
- 2.
Leukoaraiosis or white matter hyperintensities, favouring posterior regions24 or with ‘multispot pattern’ defined as ≥ 10 circular or ovoid hyperintense foci on T2/FLAIR sequences in the subcortical white matter.28
- 3.
Cortical superficial siderosis. Amyloid deposition in leptomeningeal vessels makes them fragile and prone to a convexity subarachnoid haemorrhage (cSAH) in the sulci. In patients > 60 years old, amyloid angiopathy is the most frequent cause of cSAH in the sulci, typically in the pericentral region and associated with ‘amyloid spells’.10,23 However, in young people, the most common cause of cSAH in the sulci is reversible cerebral vasoconstriction syndrome.29 In the subacute and chronic phases, blood degradation products (haemosiderin-containing macrophages) are deposited in the superficial layers of the cortex and in the subpial region, resulting in superficial cortical siderosis, which is visualised on SWI and T2* sequences as a hypointense bilinear image surrounding one or more sulci. Patients with superficial siderosis are at higher risk of lobar haemorrhages (16%)29 and more frequent transient neurological episodes (‘amyloid spells’).30,31
- 4.
Small cerebral microinfarcts most commonly peripherally distributed.
- 5.
Dilatation of Virchow-Robin spaces which is considered a neuroimaging marker of small vessel disease. Specifically, dilated perivascular spaces in the centrum semiovale or convexity are more frequent in intracranial haemorrhages that are caused by amyloid angiopathy; whereas in the basal ganglia region, Virchow-Robin dilatation and lacunar strokes are more characteristic of hypertensive angiopathy.
MRI image biomarkers associated with cerebral amyloid angiopathy. (a) Axial non-contrast T1WI with subacute lobar haematoma showing signal hyperintensity due to methaemoglobin. (b) SWI showing multiple peripherally distributed microhaemorrhages. (c) SWI showing cortical siderosis in multiple sulci. (d) Lobar haematoma with associated SAH on CT. (e) Isolated SAH in a left superior frontal sulcus in an elderly patient on CT. (f) FLAIR sequence showing multiple hyperintense foci in the white matter with multi-spot pattern. (g) T2WI showing Virchow-Robin spaces in the centrum semiovale. (h) DWI sequence showing microinfarct periphery.
The Boston criteria were developed to facilitate and standardise the non-invasive diagnosis of CAA,32 which were then replaced by the modified Boston criteria, and recently updated to the Boston criteria 2.0 (Table 3),33 to incorporate these emerging MRI biomarkers for amyloid angiopathy.
Boston Criteria 2.0 for the diagnosis of cerebral amyloid angiopathy.
| Definite CAA | post-mortem diagnosis |
| Probable CAA with supporting pathological evidence | Diagnosis supported by tissue biopsy |
| Clinical | MRIa criteria | |
|---|---|---|
| Probable CAA | • >50 years old | • At least two of the following haemorrhagic lesions in any combination: lobar haemorrhage, lobar microhaemorrhage, focal superficial cortical siderosis or convexity SAH; or |
| • Spontaneous lobar haemorrhage, episodes of focal neurological deficits, convexity SAH or cognitive decline or dementia. | • Unifocal lobar haemorrhage with at least one characteristic white matter finding (including multiple dilated perivascular spaces in the centrum semiovale or signal hyperintensities with a multi-spot pattern) | |
| Possible CAA | • At least one haemorrhagic lesion: lobar haemorrhage, lobar microhaemorrhage, focal superficial cortical siderosis or convexity SAH; or | |
| • Characteristic white matter findings (including multiple dilated perivascular spaces in the centrum semiovale or signal hyperintensities with a multi-spot pattern). |
CAA: cerebral amyloid angiopathy; SAH: subarachnoid haemorrhage.
If MRI is not available, the Edinburgh criteria, published in 2018, include CT findings that can be used to identify spontaneous haematomas associated with CAA. The criteria are based on three variables: one is APOE4 which cannot be determined radiologically, but the other two are image-based markers, namely SAH and finger-like projections, which greatly increase the specificity.34
Cerebral amyloid angiopathy-related inflammationCerebral amyloid angiopathy-related inflammation (CAA-ri) is a rare reversible encephalopathy syndrome that has been diagnosed and recognised in recent years. It affects a subgroup of patients with amyloid angiopathy, in whom an immune-mediated inflammatory response occurs35 secondary to amyloid deposition in the vessel wall, coupled with perivascular or transmural inflammatory infiltration.20
In 2014, Danve et al.36 published clinical and imaging criteria for the diagnosis of CAA-ri, which were subsequently modified and validated in 2016.37 For its diagnosis, the patient should meet all the following criteria:
- □
Over 40 years old.
- □
Acute or sub-acute symptoms (at least one): headache, decrease in consciousness, behavioural change, focal neurological symptoms or seizures; not attributable to acute intracranial haemorrhage.
- □
Hyperintense T2/FLAIR lesions of the white matter with patchy or confluent distribution (Fig. 5), usually asymmetric and extending into the subcortical white matter.
Figure 5.Cerebral amyloid angiopathy-related inflammation in a 72-year-old male patient with seizures. The images in the top row show T2 signal hyperintensity (a) in the left white matter with no diffusion restriction (b) also accompanied by leptomeningeal enhancement. (c) On suspicion of an inflammatory amyloid angiopathy, the patient was treated with steroids and progressed very well. He presented with a new outbreak two years later, with recurrence in a different location (d and e). In the gradient echo sequences (f), he also presented with multiple cortical microhaemorrhages characteristic of amyloid angiopathy.
(0.53MB). - □
Presence of ≥ 1 of the following cortical or subcortical haemorrhagic lesions: cerebral macrohaemorrhage, microhaemorrhage or superficial cortical siderosis.
- □
No neoplasia or infection.
Although not included in the criteria, some reports suggest that T2/FLAIR hyperintense lesions may be associated with leptomeningeal or cortical enhancement,37,38 and there have even been isolated cases in the literature in which this has been the only radiological finding.39
In cases with atypical imaging findings, brain biopsy—showing perivascular or transmural inflammation and amyloid deposits in cortical vessels—is important for diagnosis.35
Unlike conventional amyloid angiopathy, CAA-ri is rarely associated with haemorrhage40 or superficial siderosis. Initial treatment is with corticosteroids41 and a good response to this therapy supports the diagnosis.37 However, some patients suffer recurrence or are resistant to treatment, in which case they require immunosuppressive therapy.
Hypertensive angiopathyDespite significant improvements in the management of blood pressure, hypertensive vascular disease remains the most common cause of non-traumatic ICH in patients aged 40–70 years old, accounting for up to 55% of cases.10,42 Thus, the most important modifiable risk factor in ICH is high blood pressure.43
Chronic hypertension causes fibrinoid necrosis and changes in the small-to-medium sized arteries, leading to subendothelial proliferation which produces focal dilatations of the arterioles (Charcot-Bouchard microaneurysms), which may rupture, leading to haemorrhages, or obstruct leading to lacunar strokes.10
The perforating lenticulostriate arteries of the middle cerebral artery (MCA) are particularly affected and therefore 60–65% of these haemorrhages are located in the basal ganglia (putamen and caudate nucleus) and 20–25% in the thalamus. Less frequently, the paramedian perforating branches of the basilar artery or perforating branches of the cerebellar arteries may also be involved, resulting in brainstem or cerebellar haemorrhages in approximately 10% of cases (Fig. 6).
Other imaging findings associated with hypertensive angiopathy are lacunar strokes, linear signal hyperintensity on T2/FLAIR sequences surrounding the basal ganglia and central enlarged perivascular spaces,24,28 as well as the presence of microhaemorrhages located deep in the basal ganglia and in the brainstem (Fig. 7).
MRI findings associated with hypertensive angiopathy. (a) Central microhaemorrhages in basal ganglia and thalamus. (b and c) T2WI and FLAIR sequences with chronic lacunar strokes in the right centrum semiovale. (d) T2WI showing dilated perivascular spaces in the basal ganglia (BG) region and an old left thalamic lacunar infarct. (e) FLAIR sequence showing signal hyperintensities of central/peri-BG region.
The growth of haematomas of hypertensive aetiology occurs in about one third of patients within the first six hours. The ‘spot-sign’, which manifests as contrast extravasation within the haematoma, visible on both CTA and MRA, indicates active bleeding and is considered to be an independent growth factor with poorer prognosis.42,44 In fact, the presence of the ‘spot-sign’ is rare in haematomas of secondary aetiology and is a specific sign of haematomas of primary aetiology.45
Other findings such as the presence of fluid levels, heterogeneous internal density and irregular margins are also associated with poor prognosis and haematoma expansion.46
Secondary haemorrhagesThe causes of secondary intracranial haemorrhage include:
- □
Structural brain lesions: both primary and secondary brain tumours.
- □
Vascular lesions including arteriovenous malformations, dural fistulas and cavernous hemangiomas.
- □
Vascular causes: haemorrhagic infarctions of both arterial and venous causes, as well as haemorrhages caused by coagulopathies, including anticoagulation, and the use of sympathomimetic drugs.
Location is extremely important in helping us determine the aetiology of secondary haemorrhages:
- □
Intraparenchymal haemorrhage (in young, non-hypertensive patients) is not helpful in this respect because it can result from all the possible causes.
- □
Brainstem haemorrhage is more specific and is caused by cavernous hemangiomas and less commonly by arteriovenous malformations (AVMs).
- □
Cerebellar haemorrhage is most commonly caused by vascular malformations, both arteriovenous malformations and dural fistulas.
- □
Intraventricular haemorrhage occurs in patients with coagulopathies, with anterior communicating aneurysms and can also be secondary to AVMs.
- □
Multiple haemorrhages can be caused by coagulopathies, metastases or venous haemorrhagic infarction.
Malignant tumours are the cause of 10% of secondary haemorrhages.47 Fifteen per cent of metastases are haemorrhagic, and the tumours that bleed most commonly are choriocarcinoma, thyroid, kidney and melanoma.48 Most melanoma metastases are melanocytic, and due to the melanin content, they exhibit intrinsic hypersignal on non-contrast T1 sequences and low susceptibility. Haemorrhagic metastases appear as signal hyperintensity on T1 sequences, and have a strong susceptibility effect.49
Approximately 5% of primary tumours of the central nervous system are haemorrhagic, and these include glioblastomas, oligodendrogliomas and ependymomas.
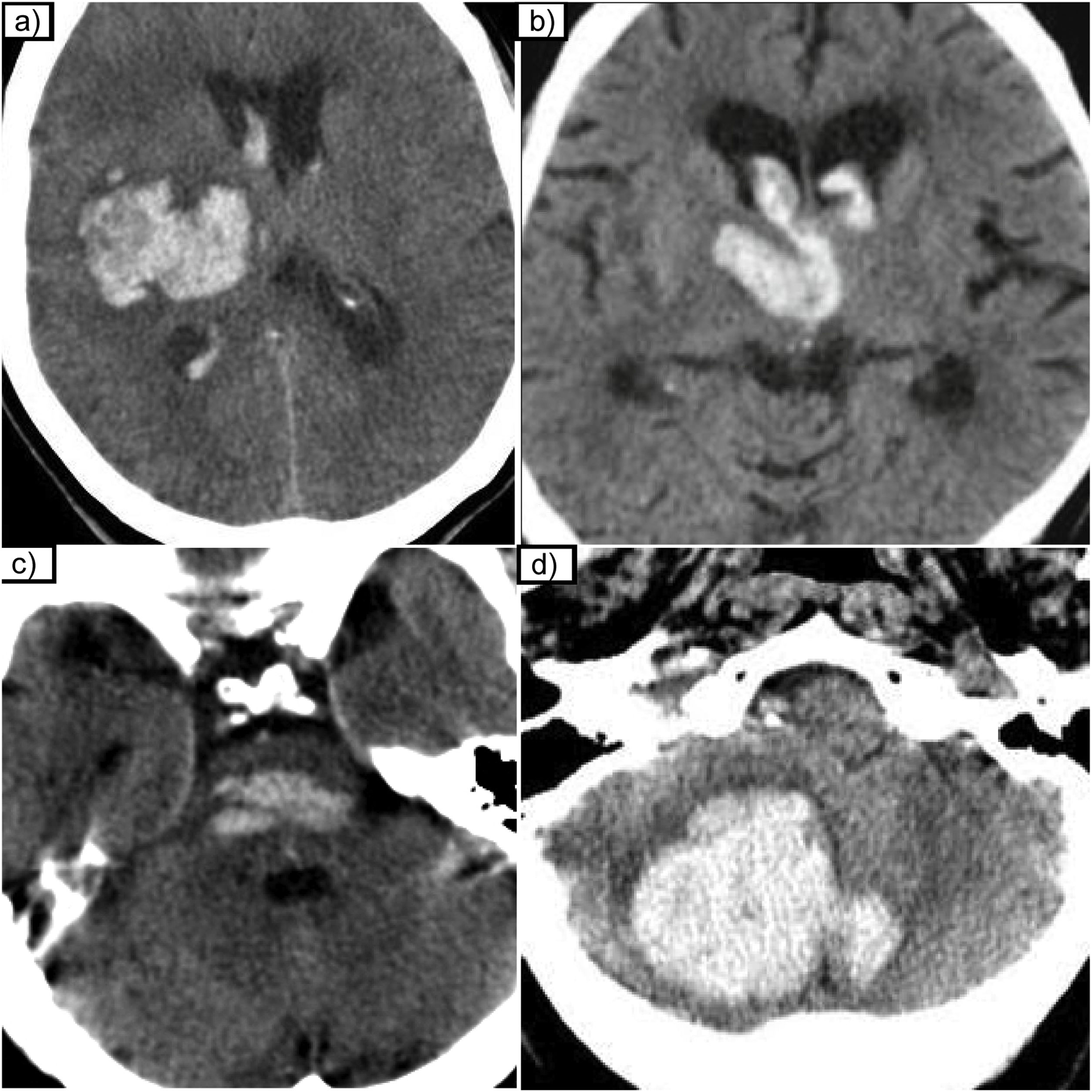
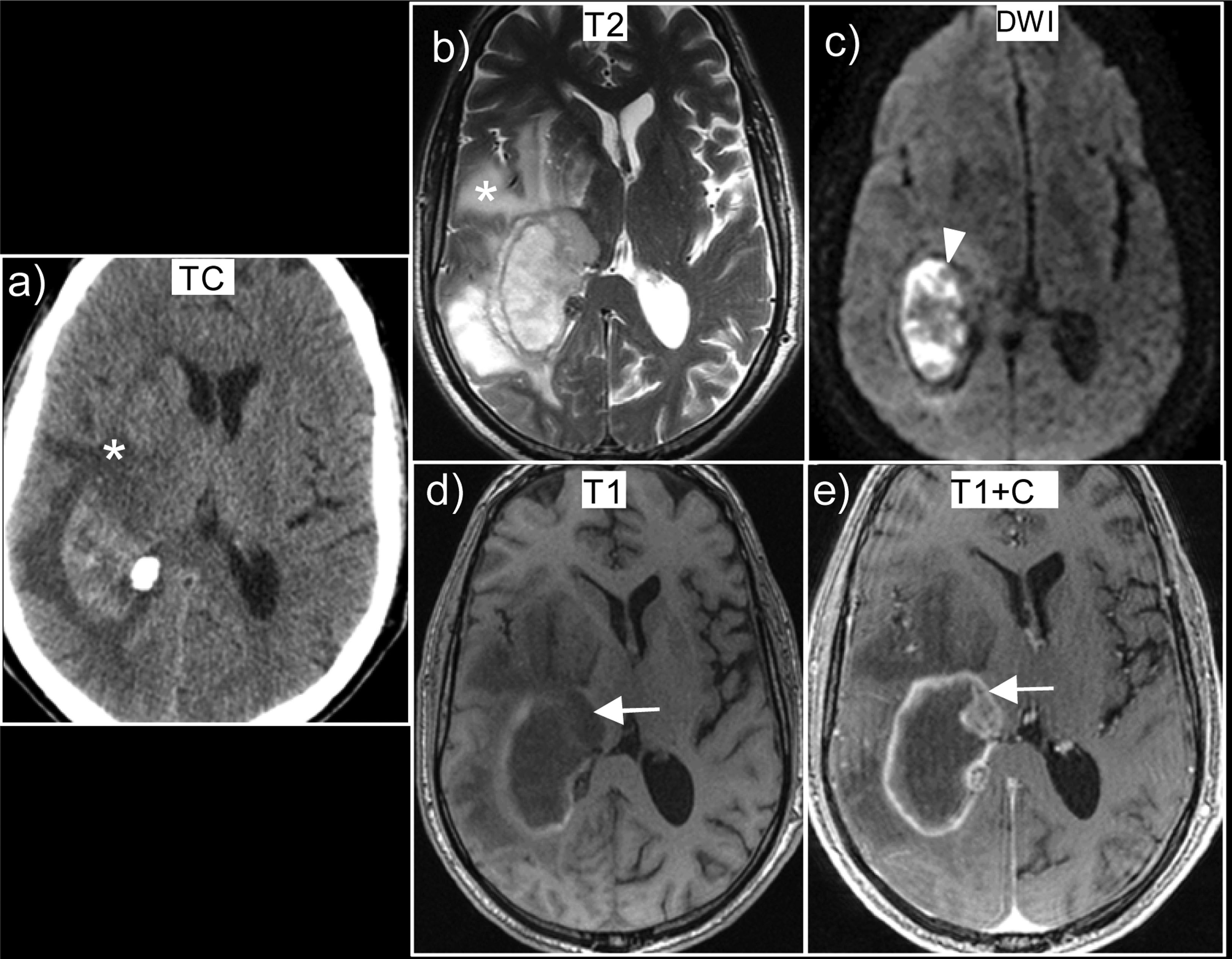
The following MRI data suggest tumour haemorrhage50,51 (Fig. 8): heterogeneous and complex haemorrhages with blood at different stages of evolution; identification of non-haemorrhagic tumour tissue with contrast enhancement; persistent oedema and mass effect in later stages; multiple haemorrhages (suggesting the possibility of haemorrhagic metastases); presence of an irregular or discontinuous haemosiderin halo with persistent or increasing oedema.
Glioblastoma, IDH-wildtype, presented as an intracranial haemorrhage. (a) Axial non-contrast CT scan shows a right parietal haematoma with vasogenic oedema (*). The MRI performed at seven days showed a heterogeneous haematoma with blood at different stages of evolution, due to the heterogeneous signal on T2 sequences (b) and on T1 (d). There is tumour tissue uptake (arrow) which can be seen on T1 contrast-enhanced images (e). Extensive oedema (*) visible both in CT (a) and T2 sequences (b). Note that as the haemorrhage is in the subacute phase (methaemoglobin) it displays restriction (arrowhead) on diffusion sequences (c).
Twenty per cent of secondary intracranial haemorrhages are caused by vascular malformations,10 accounting for up to 70% of spontaneous haemorrhages in young people (<40 years).
Vascular malformations that cause haemorrhage are arteriovenous malformations, dural fistulas and cavernous hemangiomas.
The following data suggest an intracranial haemorrhage with an underlying vascular malformation:52,53 a) patient under 45 years old; b) lobar or posterior fossa haematoma; c) regular haematoma shape; d) absence of small vessel disease; and e) patient who is not hypertensive or on anticoagulant therapy.
Arteriovenous malformationsArteriovenous malformations (AVMs) are high-flow vascular lesions composed of anomalous vessels consisting of a tangle of dysplastic vessels called a nidus, with feeding arteries and draining veins but no capillary involvement. A major risk factor for bleeding from an AVM is a history of previous bleeding, with a risk of recurrence of 7–18% in the first year and 2–4% in each subsequent year.54
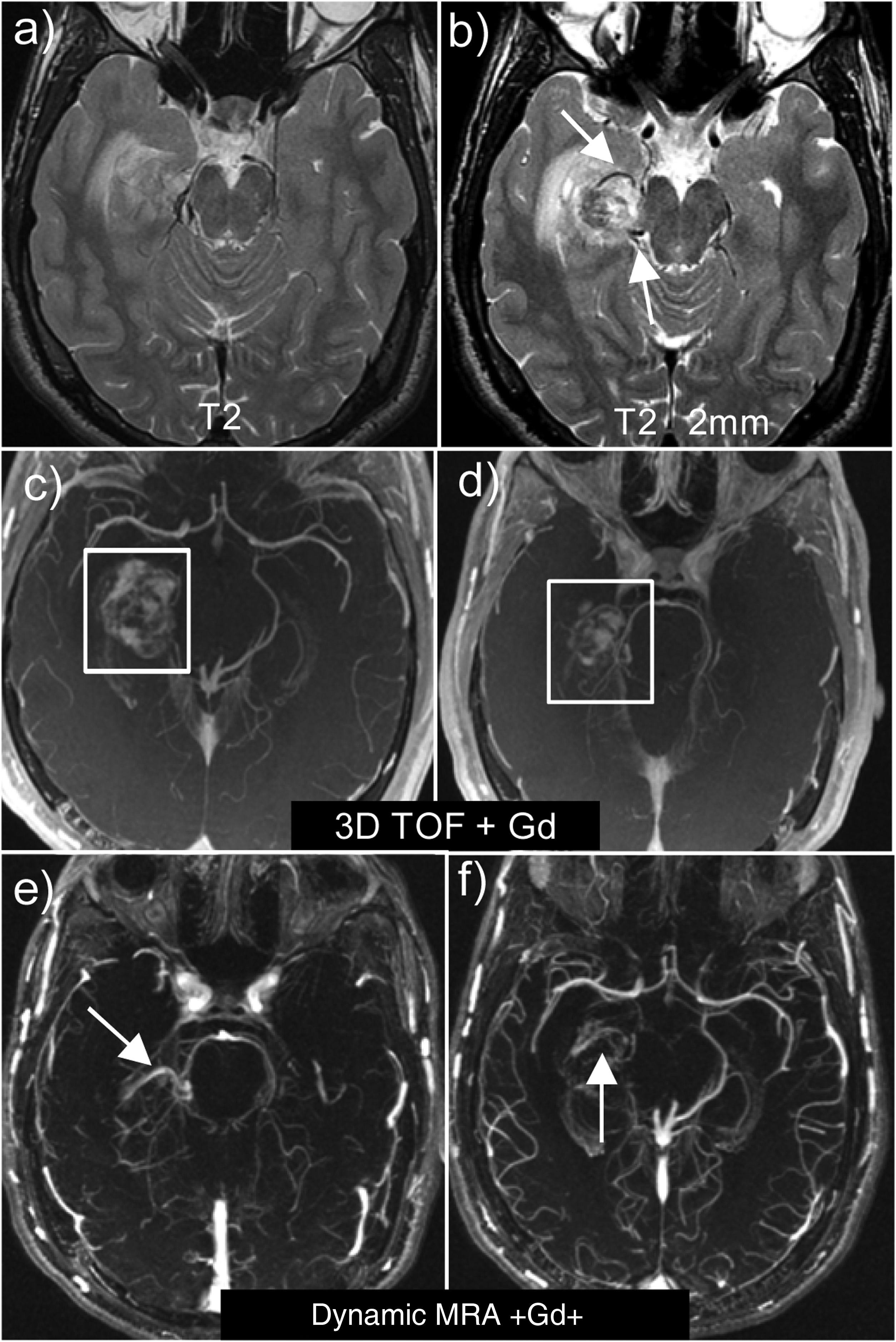
Two important technical details should be noted when studying a haematoma with a possible underlying vascular malformation (Fig. 9):
- □
First, it is advisable to use high-resolution T2-weighted sequences to easily identify the pathological vessels and the nidus, which are visualised as tortuous structures with flow voids. AVMs should be sought at the edges of the haematoma and not in the centre.
- □
The second important technical point is the choice of MRA sequence. Contrast-enhanced 3D TOF sequences should be avoided because, being T1-weighted sequences, the hyperintensity of the haematoma overlaps with the contrast enhancement. Thus, in the case of a haematoma, it is advisable to perform dynamic or 4D sequences, because they are quicker and suppress stationary tissue better.
Right parahippocampal AVM. Note the usefulness of obtaining high-resolution, thin-slice (2 mm) T2 sequences (b), which allow us to detect the pathological vessels of the AVM, which are not visible on normal T2 sequences (a). Contrast-enhanced 3D TOF sequences (c and d) where the hyperintensity of the haematoma overlaps with the contrast in the AVM vessels. Dynamic MRA sequences with 4D contrast (e and f), where the signal of the stationary tissue is suppressed, allowing us to visualise both the afferent arterioles and the venous drainage.
Micro-AVMs are those AVMs with a nidus < 1 cm or with no nidus, but with early venous filling. They account for less than 10% of AVMs and occur in young adults. They frequently present in the form of bleeding. In these cases, as in cases where the nidus is hidden by the haematoma, the important thing is to detect the early venous filling of a dilated vein in vascular studies.55
Dural fistulasIntracranial arteriovenous dural fistulas are pathological anastomoses involving meningeal arteries and venous sinuses and/or cortical veins. Haemorrhage is present in approximately 25% of dural fistulas.
Imaging findings suggestive of dural fistulas are: absence of vascular nidus, early venous drainage and increased number of deep medullary and/or leptomeningeal veins secondary to cortical venous drainage.10
Susceptibility sequences are very useful for revealing both the anatomy and venous haemodynamics of a dural fistula. They allow us to see dilated veins that reflect venous congestion and should we detect signal hyperintensity in the veins, we can deduce retrograde cortical venous drainage (Fig. 10) and the signal can help us locate the fistula point.56 The radiological findings that indicate an increased risk of bleeding in fistulas are oedema, microhaemorrhages or the presence of a hypointense area on SWI.
Usefulness of SWI in the study of dural arteriovenous fistulas. The veins of the fistula appear as signal hyperintensity (arrows) on SWI sequences (c and d), which reflects arterialised flow secondary to retrograde cortical venous drainage and helps us locate the fistula point. They correlate well with angiographic sequences (a and b).
Dural fistulas located in the tentorium, frontal lobe and basal ganglia region, foramen magnum or convexity are also at increased risk of bleeding.
The Borden classification is the simplest and is based on the pattern of venous drainage, so fistulas with cortical venous drainage (types II and III) present a higher risk than type I fistulas that lack this drainage and are difficult to diagnose on MRI, unless 4D angiography sequences or conventional arteriography are used.57
Cavernous hemangiomasCavernous hemangiomas are low-flow vascular malformations composed of thin-walled vascular spaces. They account for 10–15% of intracranial vascular malformations and have a prevalence of 0.4–0.8%.58
Haemorrhage occurs in 35.6% of cavernous hemangiomas,58 but the most common form of presentation is seizures.59 In children, the risk of bleeding in a cavernous hemangioma is much higher, up to 60%. The cavernous hemangiomas with the highest risk of bleeding are infratentorial cavernous malformations,60 large cavernous hemangiomas (>10 mm), cavernous hemangiomas with previous bleeding,10 and those associated with a developmental venous anomaly in young patients (<45 years).61 There is considerable uncertainty and controversy over whether having multiple lesions or being pregnant increase the risk of haemorrhage.62
Fortunately, bleeding caused by cavernous hemangiomas is not extensive and does not cause major deficits, unlike bleeding caused by arteriovenous malformations. On MRI, a cavernous hemangioma appears as a popcorn-like lesion with a hypointense halo on T2 and SWI sequences due to haemosiderin deposition; the core exhibits a variable signal, and there is no mass effect or oedema. When they bleed, they exhibit a ‘fried egg’ appearance which corresponds to the cavernous hemangioma with acute/subacute haemorrhage adjacent to and outside the haemosiderin rim with oedema and mass effect (Fig. 11). There may also be a pooling of blood within the cavernous hemangioma.63,64
Haemorrhagic cavernous hemangioma in the right cerebellar hemisphere with ‘fried egg’ appearance, where we can see the cavernous hemangioma in the right lateral margin which is markedly hypointense on T2WI (b) with strong susceptibility effect on SWI (a). This cavernous hemangioma shows recent adjacent bleeding (arrow) which appears as signal hypointensity on T2WI (b) and signal hyperintensity on T1WI (c). In addition, it exhibits associated vasogenic oedema (arrowhead) visible on T2WI (b), as well as mass effect on the fourth ventricle.
Cavernous hemangiomas are categorised according to the Zabramski classification,65 which consists of four types: a type I exhibits a hyperintense core on T1 due to methaemoglobin in subacute haemorrhage; type II has a reticulated core of mixed signal on T1 and T2; type III has a core that is iso- or hypointense on T1 and hypointense on T2; and type IV appears as punctate foci of signal loss on SWI. Nikoubashman et al.62 added a type V which consists of cavernous hemangiomas with extralesional haemorrhage. In this classification, the risk of bleeding from a cavernous hemangioma is associated with certain types, with types I, II and V having a higher risk of bleeding—almost 30%—compared to types III and IV (3.4% and 1.3%, respectively).
ConclusionIntracranial haemorrhage (ICH) accounts for 10–30% of strokes, and it has the poorest prognosis. CT allows us to detect and diagnose intracranial haemorrhage, but MRI allows us to determine the cause of the bleeding, which is fundamental, as both treatment and prognosis depend on the underlying cause.
Contribution of authors- 1.
Research coordinators: ZHCZ, ARG.
- 2.
Development of study concept: ESÁ, AMCdC.
- 3.
Study design: ARG, AHB.
- 4.
Data collection: ESÁ, JRC.
- 5.
Literature search: ARG, ZHCZ, AMCdC, JRC.
- 6.
Writing of article: ZHCZ, ARG.
- 7.
Critical review of and contributions to the manuscript: ARG, AHB, ESÁ.
- 8.
Approval of the final version: ZHCZ, ARG.
This study has not received any funding.
Conflict of interestThe authors declare that they have no conflict of interest.