This study was designed to determine predictors of pulmonary hypertension and signs of right heart dysfunction caused by pulmonary embolism (PE) that may lead to early detection of high-risk patients. So the predictive value of pulmonary artery obstruction index (PAOI), measured by pulmonary CT angiography (PCTA) in the acute setting, in predicting the patients susceptible to PE cardiac complications was evaluated. Also two other PCTA indices, pulmonary artery diameter (PAD), and right ventricle (RV) strain, in these patients were investigated and their predictive value for cardiac complications on follow up echocardiography were demonstrated.
Materials and methodsIn the study 120 patients with a definite diagnosis of PE were included. The PAOI, PAD and RV strain were measured using PCTA at the time of the initial diagnosis. Transthoracic echocardiography was done 6 months after the diagnosis of PE and RV echocardiographic indices were measured. Pearson correlation was used to investigate correlation between PAOI, PAD, RV strain and signs of right heart dysfunction.
ResultsPAOI was strongly correlated with systolic pulmonary artery pressure (SPAP) (r=0.83), RV systolic pressure (r=0.78) and RV wall thickness (r=0.61) in long-term follow up echocardiography. A higher rate of RV dysfunction and RV dilation was detected among the patients with higher PAOI (P<0.001). PAOI≥18 was strongly predictive for development of RV dysfunction. Also developments of pulmonary hypertension, RV systolic hypertension, RV dilation, RV dysfunction, and RV hypertrophy were significantly more common among patients with higher PAD and RV strain (P<0.001).
ConclusionsPAOI, PAD and RV strain are sensitive and specific PCTA indices that can predict the development of long-term complications such as pulmonary hypertension and right heart dysfunction, at the time of initial PE diagnosis.
Este estudio fue diseñado para determinar los predictores de la hipertensión pulmonar y los signos de disfunción cardíaca derecha causados por la embolia pulmonar (EP) que pueden conducir a la detección temprana de los pacientes de alto riesgo. Por lo tanto, se evaluó el valor predictivo del índice de obstrucción de la arteria pulmonar (IOAP), medido mediante angiografía pulmonar por TC (APTC) en el contexto agudo, para predecir los pacientes susceptibles de sufrir complicaciones cardíacas por EP. También se investigaron otros dos índices de APTC, el diámetro de la arteria pulmonar (DAP) y el strain del ventrículo derecho (VD), en estos pacientes y se demostró su valor predictivo de las complicaciones cardíacas en la ecocardiografía de seguimiento.
Materiales y métodosEn el estudio fueron incluidos 120 pacientes con diagnóstico definitivo de EP. El IOAP, el DAP y el strain del VD se midieron mediante APTC en el momento del diagnóstico inicial. Se realizó una ecocardiografía transtorácica 6 meses después del diagnóstico de EP y se midieron los índices ecocardiográficos del VD. Se utilizó la correlación de Pearson para investigar la correlación entre IOAP, DAP, strain del VD y los signos de disfunción del hemicardio derecho.
ResultadosEl IOAP estaba fuertemente correlacionado con la presión arterial pulmonar sistólica (PAPS) (r=0,83), la presión sistólica del VD (r=0,78) y el grosor de la pared del VD (r=0,61) en la ecocardiografía de seguimiento a largo plazo. Se detectó una mayor tasa de disfunción del VD y de dilatación del VD entre los pacientes con mayor IOAP (p<0,001). Un IOAP≥18 fue claramente predictivo del desarrollo de la disfunción del VD. También la evolución de la hipertensión pulmonar, la hipertensión sistólica del VD, la dilatación del VD, la disfunción del VD y la hipertrofia del VD fueron significativamente más frecuentes entre los pacientes con mayor DAP y strain del VD (p<0,001).
ConclusionesEl IOAP, el DAP y el strain del VD son índices de APTC sensibles y específicos que pueden predecir el desarrollo de complicaciones a largo plazo, como la hipertensión pulmonar y la disfunción cardíaca derecha, en el momento del diagnóstico inicial de la EP.
Pulmonary embolism (PE) is a life-threatening condition that may lead to chronic thromboembolic pulmonary hypertension (CTEPH) due to an incomplete resolution of vascular obstruction. Patients with CTEPH may develop right ventricle (RV) dilation, dysfunction, and hypertrophy due to cardiac remodeling.1 Identifying the predictors of CTEPH and RV remodeling can help physicians in performing a more targeted follow up of the patients and detecting the high-risk patients for developing right heart dysfunction. Pulmonary CT angiography (PCTA) is the more commonly used and gold standard study for diagnosis of acute PE because its favorable accuracy, predictive value, sensitivity and specificity.2 It is also easily available and can show intraluminal thrombosis or other causes of patient's symptoms.3 Pulmonary artery obstruction index (PAOI) is a score used to assess the severity of acute PE using PCTA. There are some studies conducted on the association among PAOI and the development of RV dysfunction and short term mortality in acute PE.4,5 Also, the Pulmonary artery diameter (PAD) was reported as an independent predictor of RV dysfunction.4 Although PAOI and PAD could predict the severity of acute PE, there are only few studies focused on the predictive value of these indices on the development of CTEPH or RV dysfunction in the patients with PE.6
To detect the patients who have developed CTEPH, European guidelines recommended 6 months follow up echocardiography in the patients with PE and signs of pulmonary hypertension or RV dysfunction.7 However, in some cases, the follow-up echocardiography is not performed due to various reasons, and may lead to undiagnosed CTEPH and right-sided heart failure.8
In this study, we aimed to evaluate the relationship between PCTA indices (including PAOI measured using Qanadli score, PAD, RV strain) and the development of pulmonary hypertension, RV systolic hypertension, RV dilation, RV dysfunction and RV hypertrophy in the follow-up echocardiography.9 We also aimed to measure the diagnostic accuracy of the PAOI and PAD in detecting CTEPH and right-sided heart dysfunction. Therefore, we introduced the cutoff point for PAOI, so that physicians can predict the high-risk patients for the development of CTEPH and heart problems using PCTA in the acute settings.
Material and methodsPatient selectionThis prospective observational study was performed between December 2018 and December 2019. The study was approved by the ethical board of the Radiology department, Isfahan University of Medical Sciences, Isfahan, Iran. The aim of this study was explained to the patients and the informed consent was signed by them. Patients were identified using patient records and radiologic information systems. The patients referred to Alzahra and Noor hospitals with positive PCTA for PE, within the last 6 months who survived from the acute PE, entered into the study. Patients who died, those with a history of cardiac disease (heart failure or heart valvular disease), a history of previous PE, and those with the signs of chronic obstructive pulmonary disease in the CT were excluded from the study. All patients received anticoagulant therapy for at least three months. In our institution warfarin is preferred treatment, adequate treatment consists of administration of warfarin while INR is preserved between 2–3. Occasionally novel oral anticoagulants such as Rivaroxaban was administrated with dose of 15mg per oral every 12h without checking INR. None of the patients were received Interventional thrombectomy or thrombolytic therapy.
From December 2018 to December 2019, 3360 PCTA studies were performed at our institution, 466 cases verified as positive for PE. Forty-one patients did not survive six months after diagnosis of PE. In 42 cases images showed poor quality secondary to poor vessel enhancement, motion (i.e., respiratory or pulsation) artifacts. Patients with comorbidity (221) were excluded from our study. Forty-two patients did not cooperate for follow up echocardiography. So 120 patients entered in this study.
CT techniquePCTA studies were performed with GE LIGHT SPEED VCT 64 detector row 128 slices CT scanner by using a standard PCTA protocol for PE with the following imaging parameters: detector width 40mm, detector row 64, section thickness 0.625mm, rotation time 0.4sec, 120kvp (in thin patients 100kvp) and mAs according to thickness setting by mAs modulation. Images were obtained after intravenous administration of 100 cc of nonionic iso-osmolar iodinated contrast media (iodixanol, VISIPAQUE™ 320mg/ml) at a rate of 5ml/s.
For determining the optimal timing of contrast material bolus, in our institution, timing bolus technique was performed as following: a small bolus of contrast material (10ml) diluted with 40 cc normal saline was injected at the rate of 5ml/s, followed by a dynamic scan with a ROI over the pulmonary artery. A time-attenuation curve is generated, the time-to-peak opacification is determined and the scan delay time is calculated by adding a few seconds to account for the larger amount of contrast material that will be utilized in the diagnostic phase of the study and the time taken for scanner to move to the start position. Then CT scanning was performed with time delay that was measured by bolus test, in caudocranial direction.
The images were displayed using three different gray scales, with the following values of window width and window level, expressed in Hounsfield units: pulmonary (1500/−600), mediastinal (400/40) and for PE (700/100).
Image analysisPositive PCTA images, which fulfilled inclusion criteria were de-identified and transferred from our picture archiving and communication system to a dedicated image processing workstation (GE AW 4.7). Image analysis was performed by consensus between two radiologists with four years of experience each in thoracic CT. Any disagreement was resolved by the senior investigator, with 15 years of experience in the interpretation of chest CT images. Readers were blinded to the patient's condition at the time of presentation, short term and final clinical outcome.
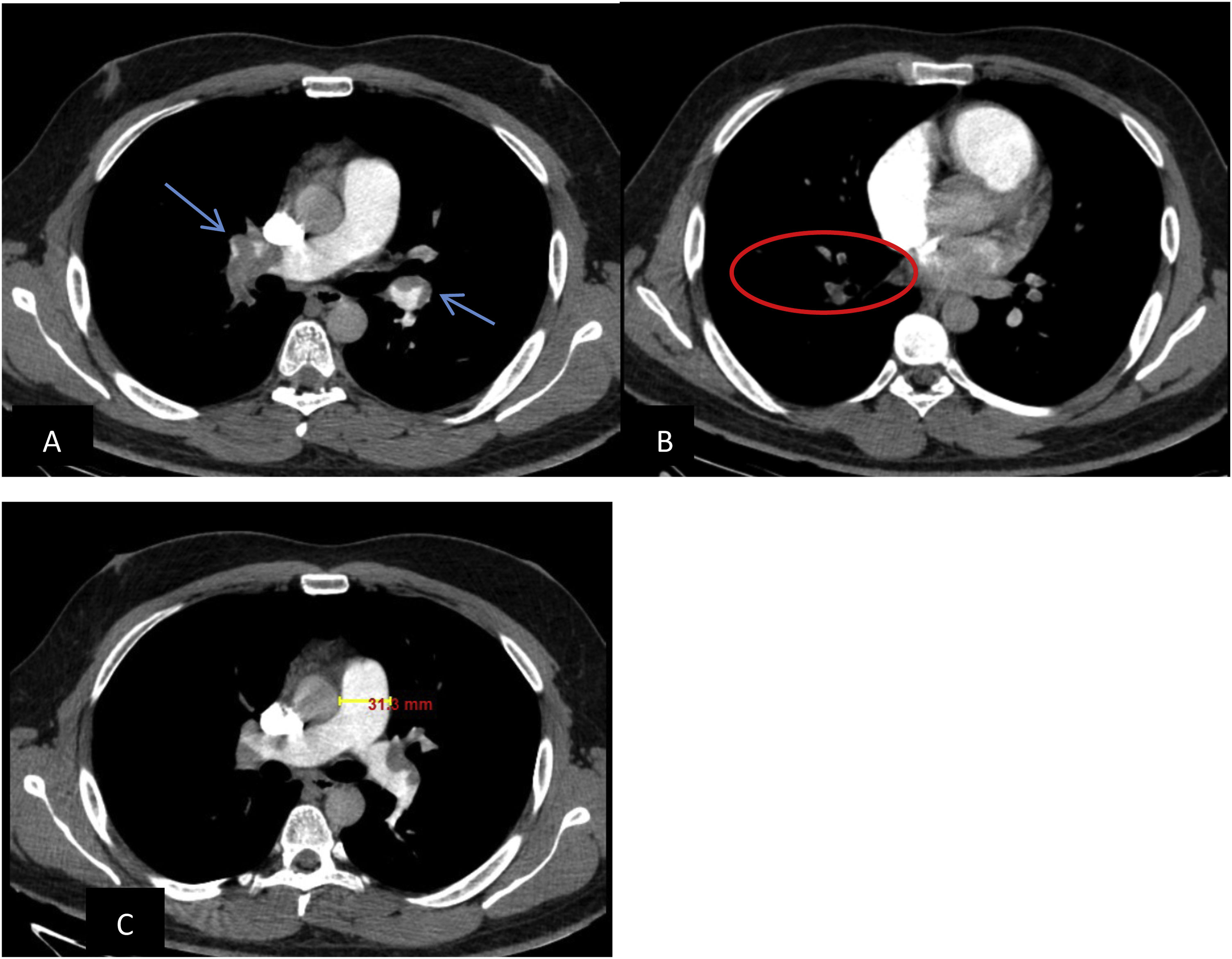
Presence, location and degree of obstruction of clots can be calculated at PCTA by applying angiographic scores. In this study dedicated CT score of Qanadli score was used. Moreover, to calculate this score, the arterial tree of each lung was regarded as having 10 segmental pulmonary arteries (three to the upper lobes, two to the middle lobes and lingual, and five to the lower lobes). Score 1 was given to the presence of embolus in a segmental artery. A score equal to the number of segmental arteries arising distally was given to the presence of embolus at the most proximal arterial level. The zero score was used when no vascular obstruction was detected. The presence of partial or total occlusion were scored as 1 and 2 points, respectively. The final index was the sum of the obtained scores with a maximum value of 409 (Figs. 1 and 2).
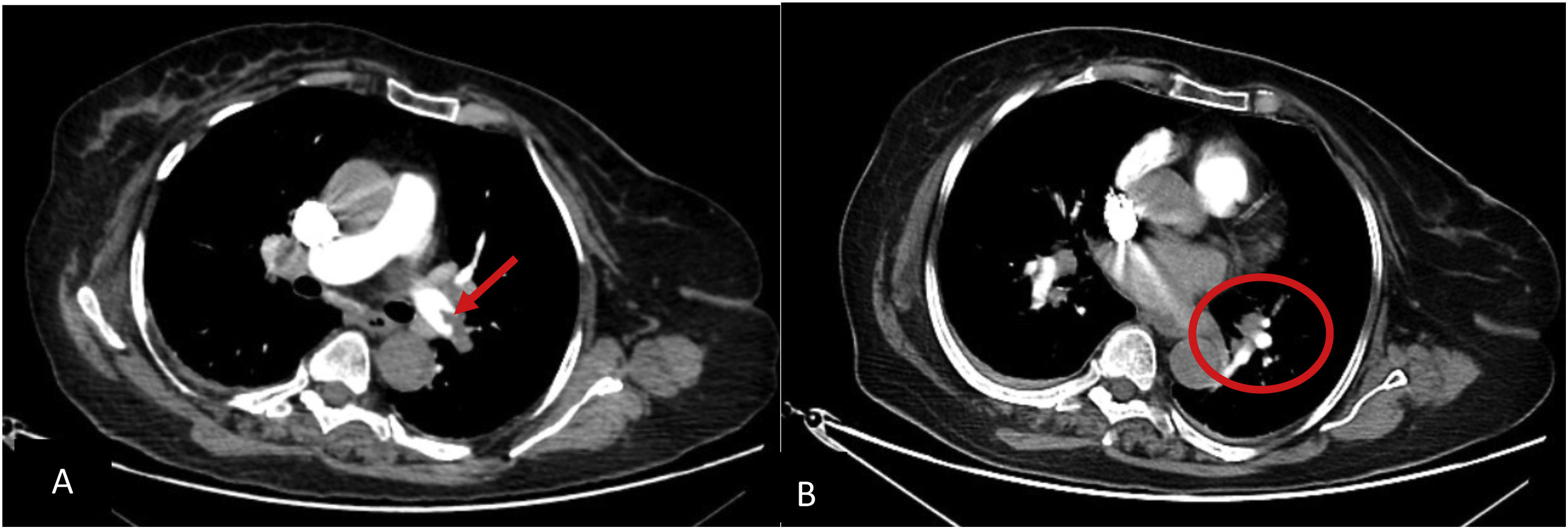
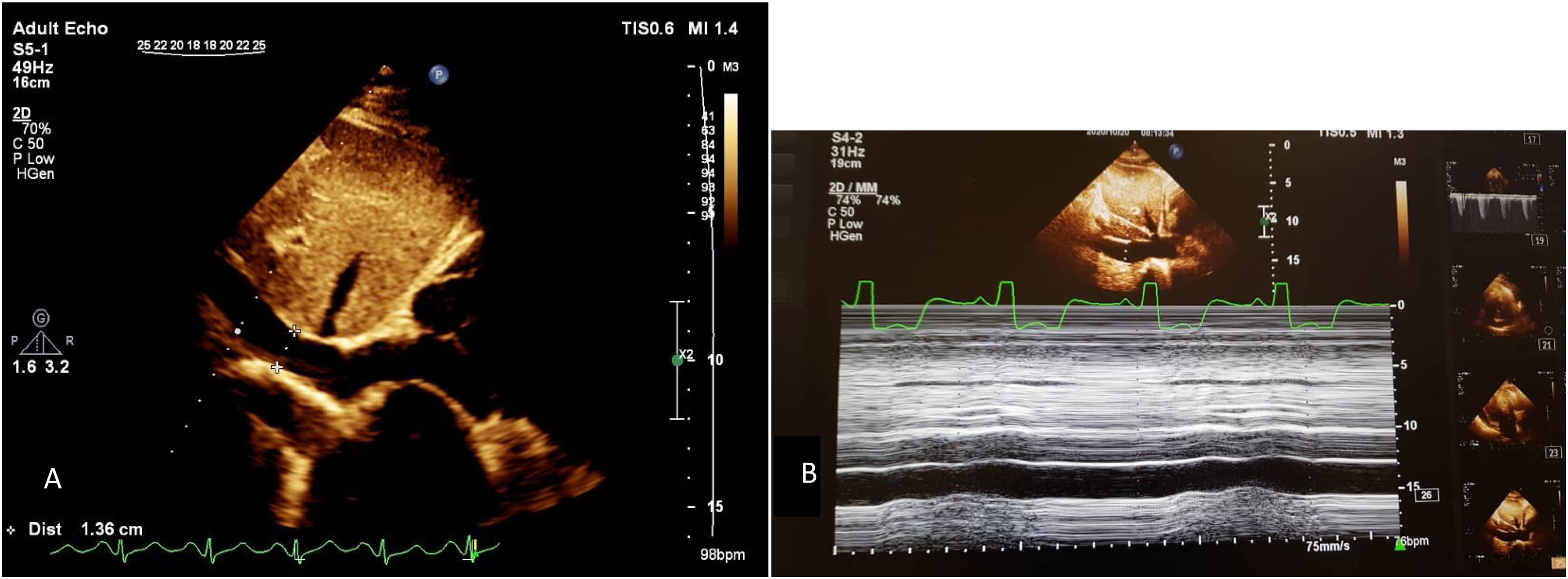
Axial PCTA of 45 years old man with acute PE: A) filling defect in distal part of both main pulmonary arteries (blue arrows) is discerned. B) segmental branches in RLL are not opacified (within the circle). Also segmental branches of RML are not opacified (not shown). PAOI in this patient is 27. C) main pulmonary artery is measured in 31 millimeters at the level of its bifurcation.
On transverse images, main pulmonary artery is measured in millimeter at the level of its bifurcation, orthogonal to its long axis10 (Fig. 2).
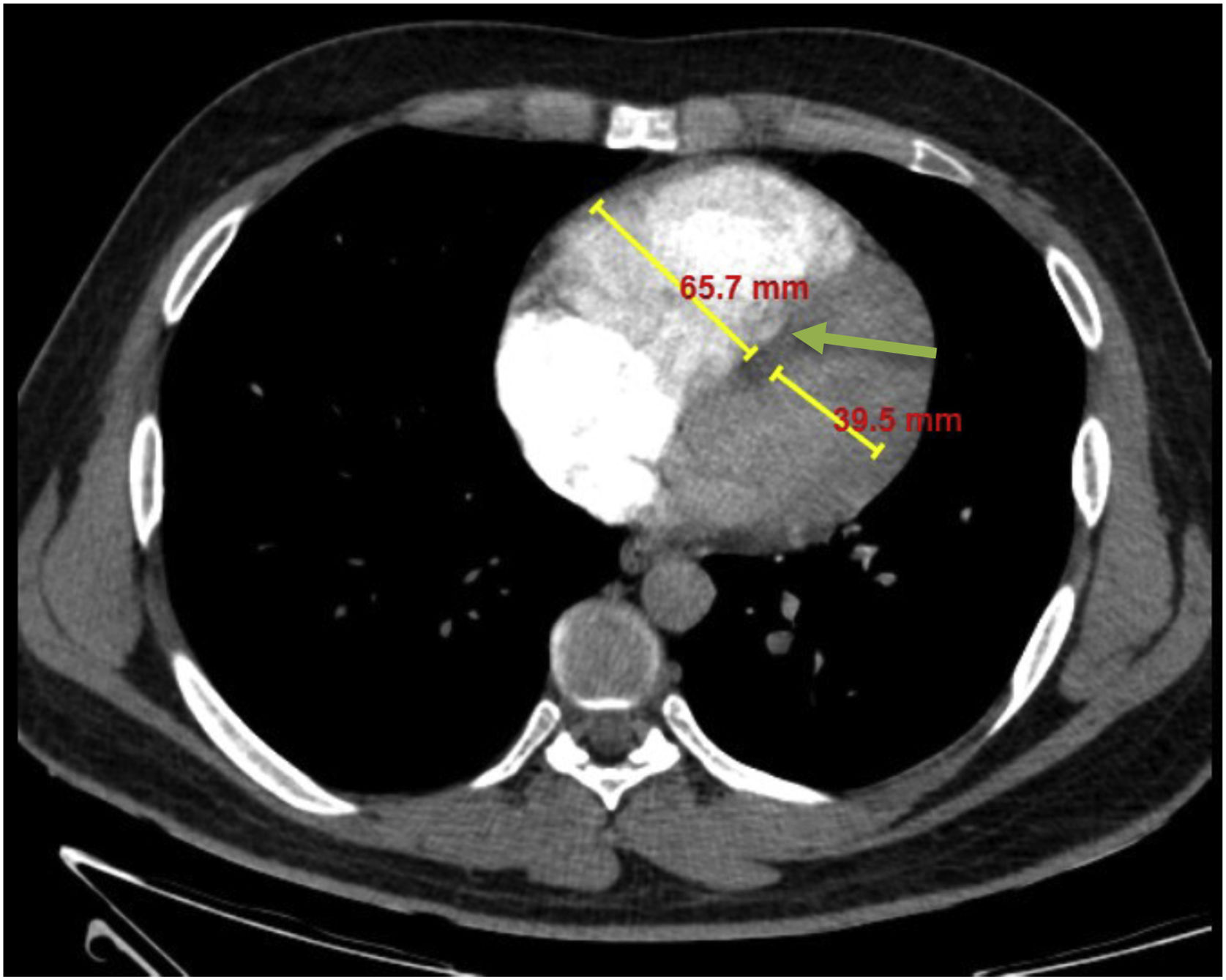
RV strain was determined as right ventricle to left ventricle diameter ratio of more than 1:1 at mid-ventricular level on axial images or straightening or leftward bowing of interventricular septum10,11 (Fig. 3).
Axial PCTA of 40 years old man with acute PE and evidence of RV strain: The short-axis diameters of the RV and left ventricle at mid-ventricular level were 66 mm and 40 mm respectively, and the RV/left ventricle diameter ratio was 1.65. Also note the leftward bowing of the interventricular septum (green arrow).
All patients underwent two-dimensional transthoracic echocardiography using Philips device, Affiniti 50C with a 3MHz transducer 6 months after diagnosing the acute PE by an expert echo-cardiographer with 8 years’ experience who was blinded to the PAOI.
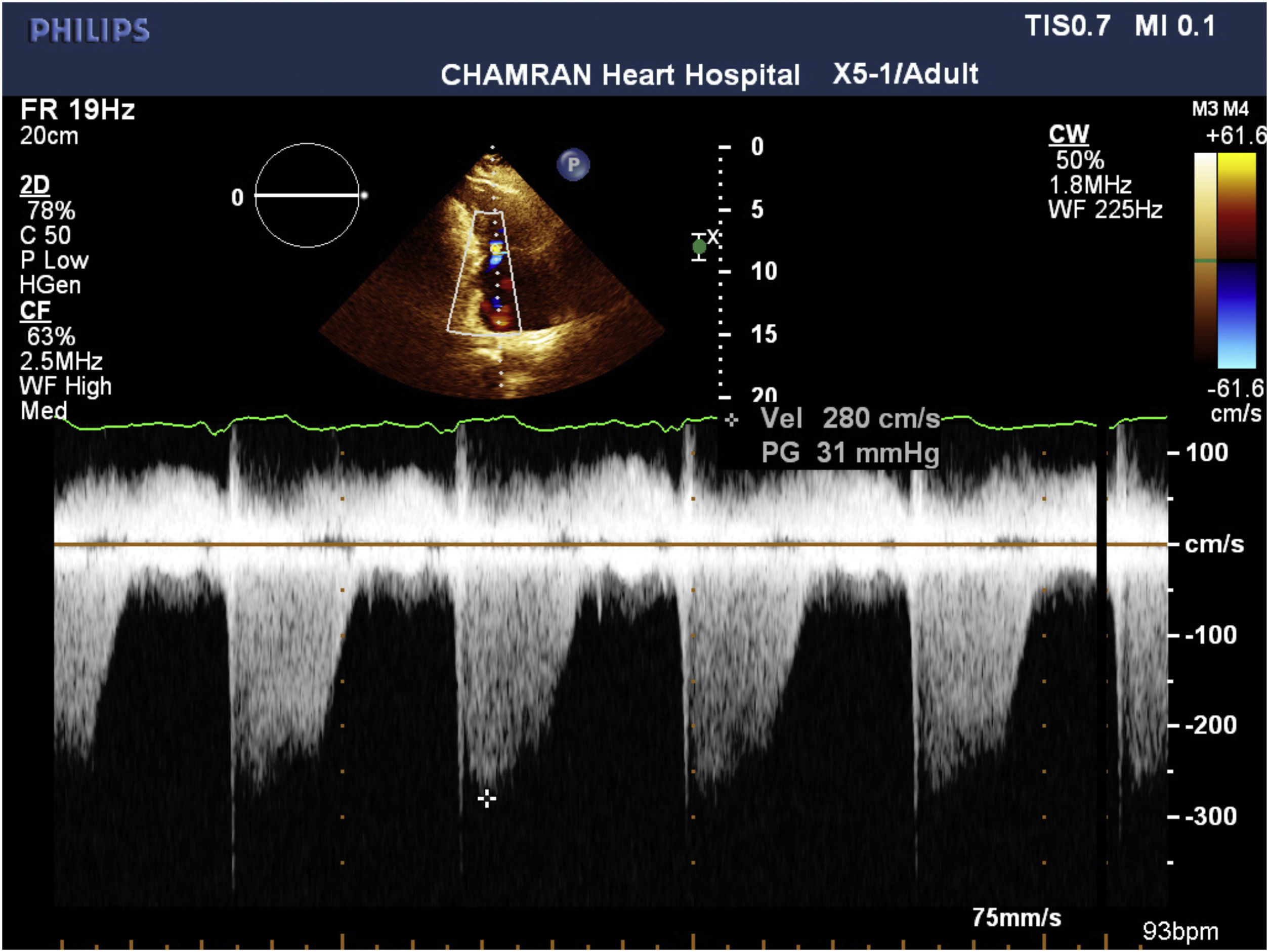
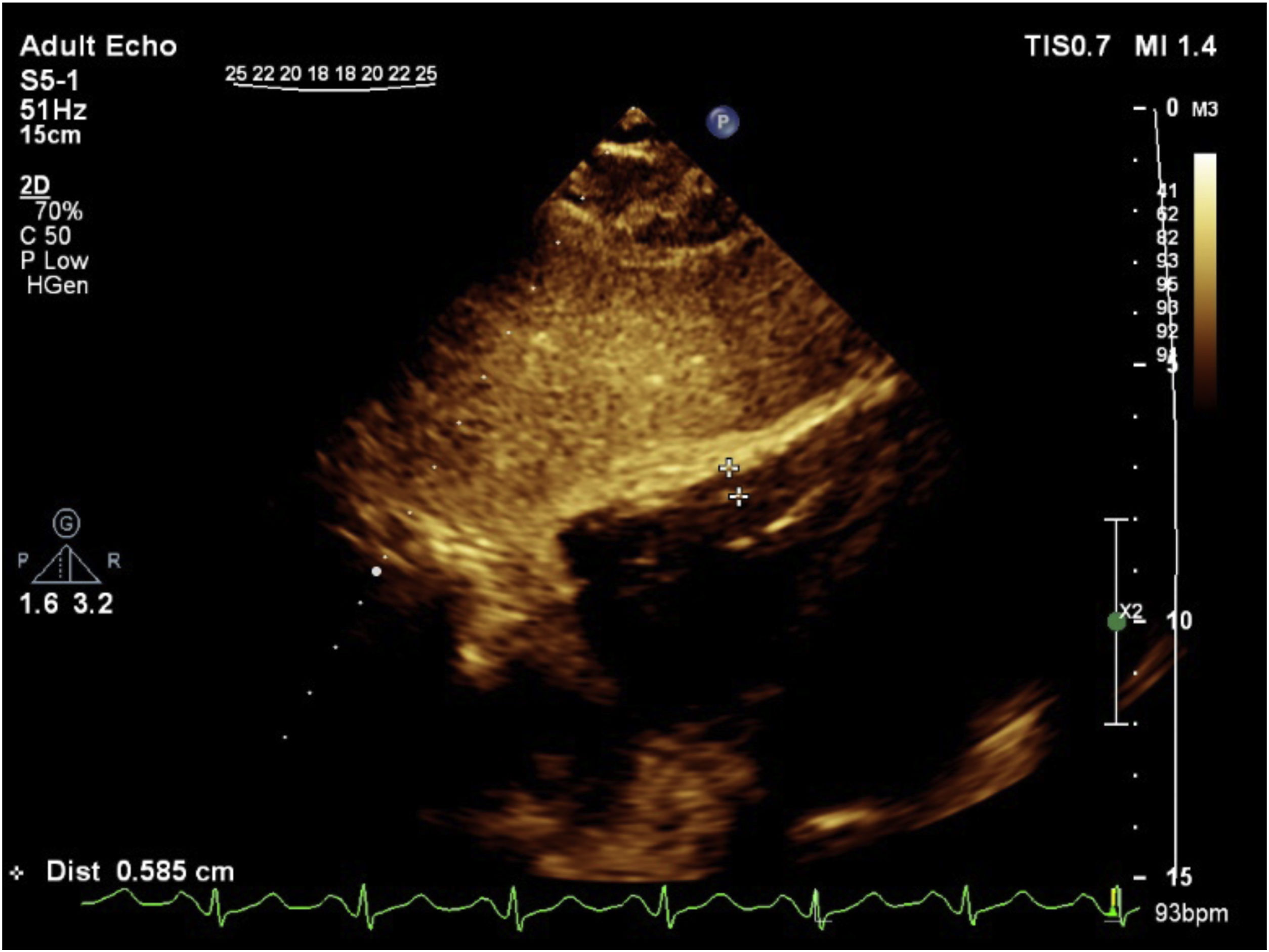
Systolic pulmonary artey pressure (SPAP) was measured using tricuspid regurgitation velocity (Fig. 4) in combination with an estimation of the right atrial pressure, using right atrial pressure based on inferior vena cava diameter at end of diastole and its collapse. The value of less than 35mm Hg was considered as normal SPAP.
RV systolic pressure was determined from peak tricuspid regurgitation jet velocity, and by using simplified Bernoulli equation and estimation of the right atrial pressure by inferior vena cava diameter and respiratory variation: RV systolic pressure=4(V) 2+right atrial pressure, where V is the peak velocity(in meters per second) of the tricuspid valve regurgitant jet. In the absence of a gradient across the pulmonic valve or RV outflow tract, SPAP is equal to RV systolic pressure. For evaluation of right atrial pressure, we used inferior vena cava. It was evaluated in subcostal view, at end-expiration and just proximal to the hepatic veines’ junction to inferior vena cava when it was seen in its long axis position. Inferior vena cava diameter ≤2.1cm that collapses >50% during respiration suggests a normal right atrial pressure of 3mm Hg (range, 0–5mm Hg), on the other hand, an inferior vena cava diameter >2.1cm that collapses <50% with a respiration suggests a high right atrial pressure of 15mm Hg (range, 10–20mm Hg). When inferior vena cava diameter and collapse do not fit this ranges, a value of 8mm Hg (range, 5–10mm Hg) may be used12 (Fig. 5).
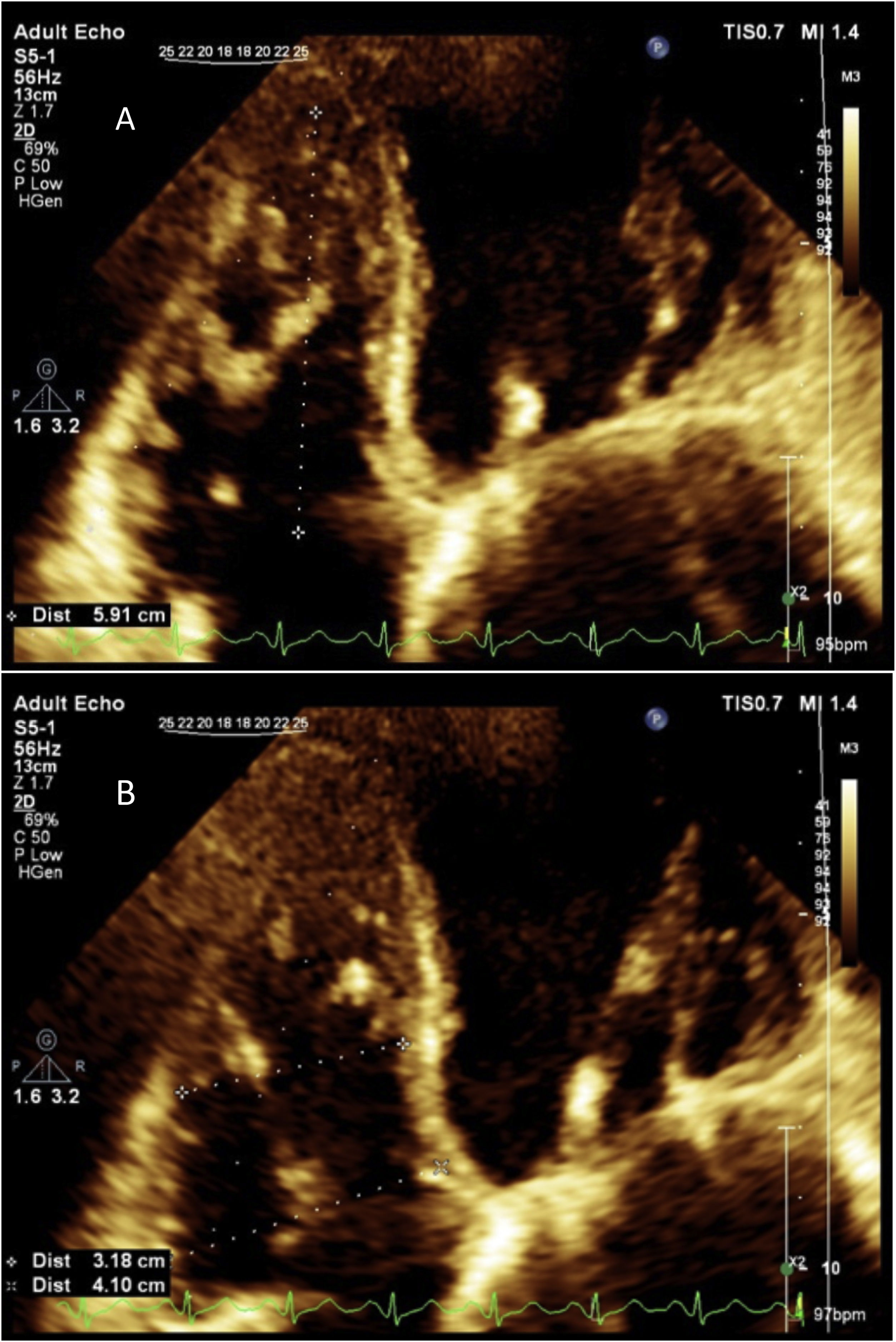
For evaluation of RV dilation and to see the free wall of the RV wall better, we used right ventricle–focused view. The basal and mid RV cavity and longitudinal diameter of RV, were obtained in this view. The basal diameter was measured in the basal one third of the RV. The midcavity diameter was measured in the middle third of the RV at the level of the left ventricle papillary muscles. The longitudinal dimension was determined from the tricuspid annulus plane to the RV apex12 (Fig. 6). RV dimension was estimated at end-diastole and the values >41mm at the base, >35mm at the mid-level and RV longitudinal diameter >83mm were considered as RV dilation.
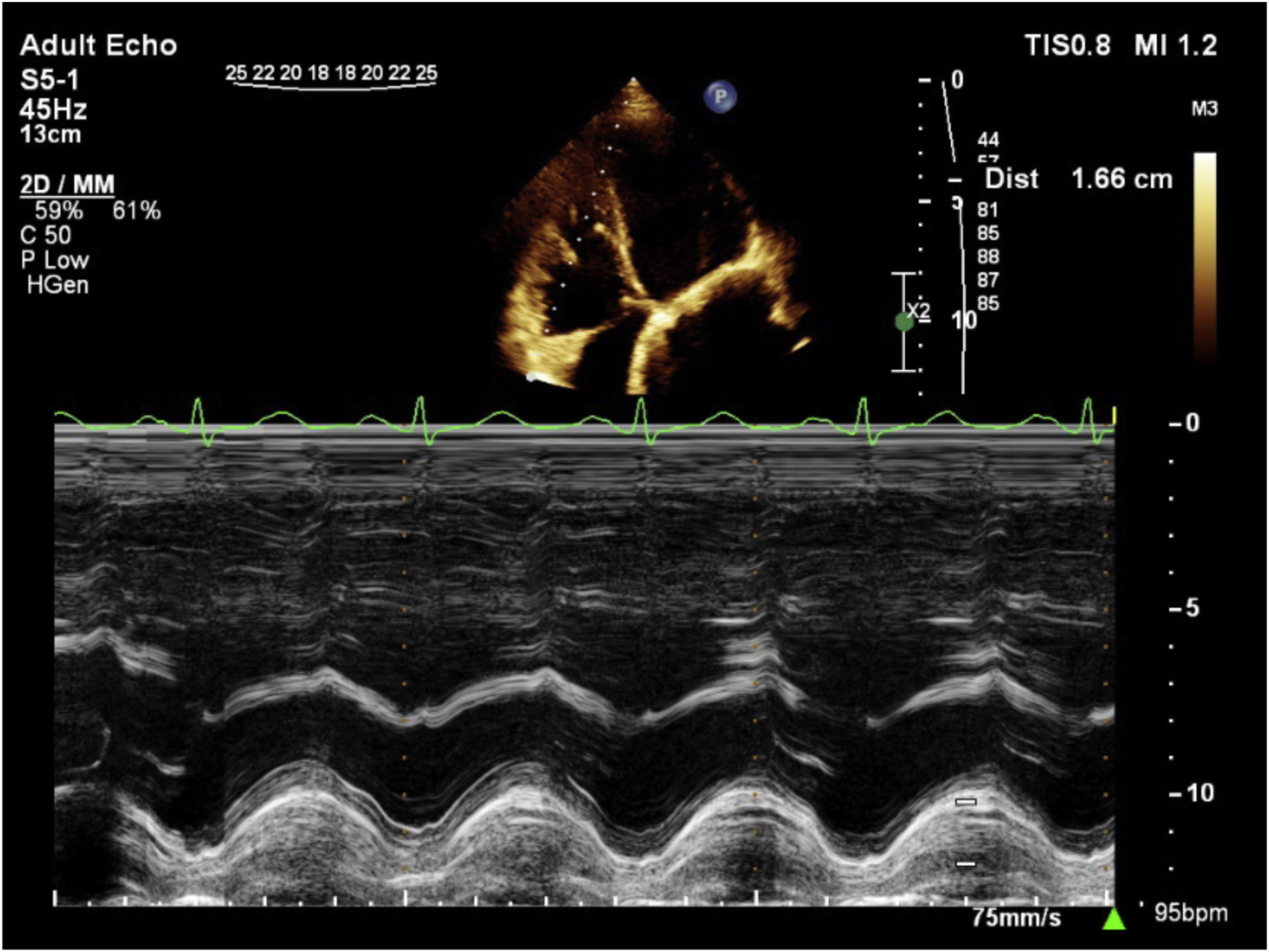
Evaluation of RV function by transthoracic echocardiography remains challenging because the RV anatomy is complex. There is several parameters for evaluation of RV by echocardiography. The simplest and most commonly used parameter is visual assessment.13 For transthoracic echocardiography assessment of the RV, the use of at least one quantitative parameter in addition to visual assessment is mandatory, so we used tricuspid annular plane systolic excursion.13 Pulsed Doppler wave <9.5cm/s and tricuspid annular plane systolic excursion <17mm considered as RV dysfunction. Visual assessment (“eyeballing”) for RV function also was used. The base of the RV free wall moves in systole and It is obvious in normal echocardiography. Tricuspid annular plane systolic excursion is measured in standard 4-chamber view and define by measuring the distance of systolic excursion of the RV annular segment along its longitudinal plane. The greater the movement of the base toward the apex in systole, the better the RV systolic function. Tricuspid annular plane systolic excursion was acquired by placing M-mode cursor through the tricuspid annulus to measure the amount of longitudinal motion of the annulus at peak systole (Fig. 7).
In subcostal view, aligned to the ultrasound beam and perpendicular to the RV free wall, RV wall thickness was measured. RV trabeculations and papillary muscle was excluded from RV endocardial border to accurately measuring the RV wall thickness. By decreasing the depth and moving the focus to the RV wall region we improved the endocardial border definition.12 The values >5mm indicated the abnormal RV wall thickness as well as RV hypertrophy12 (Fig. 8).
Statistical analysisCollected data were analyzed using mean±standard deviation (SD) for quantitative variables. Frequencies and percentages were used for qualitative indices. The independent t-test was used to compare the means. The association between variables was measured using Pearson correlation. ROC curve analysis was used to detect the cutoff points for PAOI, PAD and RV strain, sensitivity and specificity of the thresholds in detecting SPAP, RV systolic hypertension, RV dilation, RV dysfunction and RV hypertrophy in follow up echocardiography. The Odds ratio (OR) was calculated for each variable. The analysis was done by the use of SPSS software, version 21 (SPSS Inc., Chicago, IL, USA). Also, P values <0.05 were considered as the statistical significance level.
ResultsIn this study, 120 patients were enrolled. There were 49 (40.9%) women and 71 (59.1%) men. The mean±SD of age was 53.49±12.78 in male and 52.18±17.25 in female.
Unilateral PE was detected in 64 (53.3%) patients. In other 56 (46.7%) of participants, PE was located bilaterally. Segmental arteries were involved in 49 (40.8%) patients. PE was detected in lobar arteries, interlobar arteries, and main arteries with a frequency of 23 (19.2%), 16 (13.3%), 22 (18.3%), respectively. Saddle PE was seen in 10 patients (8.3%).
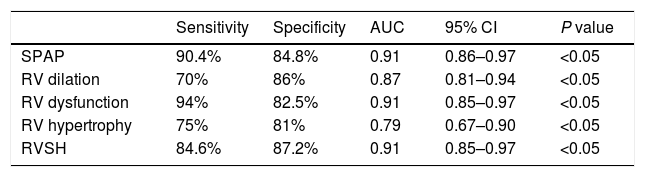
The relationship between pulmonary artery obstruction index and the follow-up echocardiographic indicesThe measured PAOI in the acute setting were ranged from 1 to 36 with a mean±SD of 12.52±8.69. SPAP was ranged from 13 to 80mm Hg with a mean±SD of 27.87±9.64. An abnormal SPAP was detected in 28 (23.1%) patients. A strongly significant positive correlation was detected between SPAP and PAOI (P<0.001, r=0.83). ROC curve analysis showed that PAOI ≥18 could predict the development of pulmonary hypertension with a sensitivity of 90.4% and specificity of 84.8% (Table 1).
Predictive value of the cutoff point of 18 for PAOI in detecting patients susceptible to long-term pulmonary embolism complications.
| Sensitivity | Specificity | AUC | 95% CI | P value | |
|---|---|---|---|---|---|
| SPAP | 90.4% | 84.8% | 0.91 | 0.86–0.97 | <0.05 |
| RV dilation | 70% | 86% | 0.87 | 0.81–0.94 | <0.05 |
| RV dysfunction | 94% | 82.5% | 0.91 | 0.85–0.97 | <0.05 |
| RV hypertrophy | 75% | 81% | 0.79 | 0.67–0.90 | <0.05 |
| RVSH | 84.6% | 87.2% | 0.91 | 0.85–0.97 | <0.05 |
PAOI: Pulmonary artery obstruction index; SPAP: Systolic pulmonary artery pressure; RV: Right ventricle; RVSH: Right ventricular systolic hypertension; AUC: Area under the curve; CI: Confidence interval.
RV systolic pressure was ranged from 10mm Hg to 55mm Hg with a mean±SD of 20.82±8.38mm Hg. RV systolic hypertension was detected in 26 (21.5%) patients. However, other 94 (77.7%) subjects had normal RV systolic pressure. Also, a positive correlation was observed between PAOI and RV systolic pressure (P<0.001, r=0.78). Using ROC curve analysis, a cutoff point of 18 with a sensitivity of 84.6% and a specificity of 87.2% was detected to predict the susceptible subjects to RV systolic hypertension (Table 1).
Thirty patients (24.8%) had RV dilation. However, 90 (74.4%) of participants indicated no signs of RV dilation. Signs of RV dysfunction and RV strain were detected in 17 (14%) and 44 (36.4%) participants, respectively. The PAOI was significantly higher in the patients with signs of RV dilation, RV dysfunction, or RV strain (P<0.001) (Table 2). PAOI ≥18 had a sensitivity of 70% and specificity of 86% in predicting RV dilation development (Table 1). Patients with a PAOI ≥18 were susceptible to develop RV dysfunction with a sensitivity of 94% and specificity of 82.5% (Table 1).
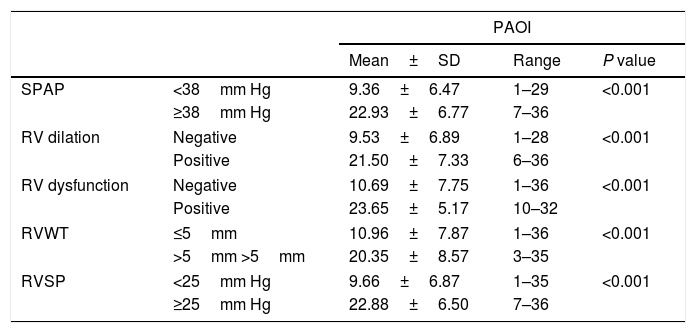
The mean±SD of PAOI in different follow-up echocardiographic indices.
| PAOI | ||||
|---|---|---|---|---|
| Mean±SD | Range | P value | ||
| SPAP | <38mm Hg | 9.36±6.47 | 1–29 | <0.001 |
| ≥38mm Hg | 22.93±6.77 | 7–36 | ||
| RV dilation | Negative | 9.53±6.89 | 1–28 | <0.001 |
| Positive | 21.50±7.33 | 6–36 | ||
| RV dysfunction | Negative | 10.69±7.75 | 1–36 | <0.001 |
| Positive | 23.65±5.17 | 10–32 | ||
| RVWT | ≤5mm | 10.96±7.87 | 1–36 | <0.001 |
| >5mm >5mm | 20.35±8.57 | 3–35 | ||
| RVSP | <25mm Hg | 9.66±6.87 | 1–35 | <0.001 |
| ≥25mm Hg | 22.88±6.50 | 7–36 | ||
PAOI: Pulmonary artery obstruction index; SPAP: Systolic pulmonary artery pressure; RV: Right ventricle; RVWT: Right ventricular wall thickness; RVSP: Right ventricular systolic pressure.
The mean±SD of RV wall thickness was 4.00±1.01mm with a minimum of 2 and a maximum of 8mm. 20 (16.5%) patients had RV hypertrophy. Patients with higher PAOI had significantly higher RV wall thickness (P<0.001, r=0.61). Also, PAOI ≥18 could predict the RV hypertrophy development with a sensitivity of 75% and specificity of 81% (Table 1).
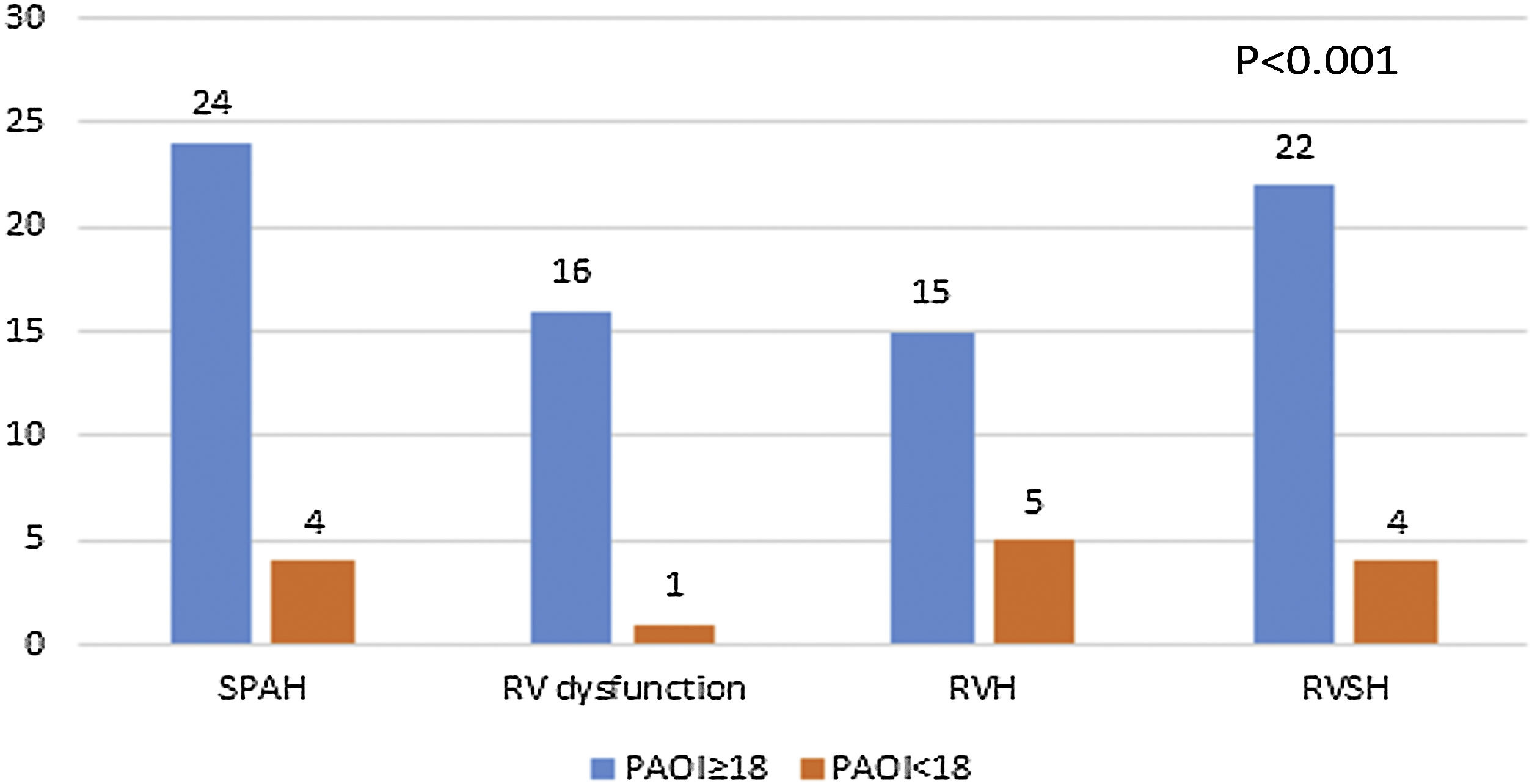
So the patients with PAOI ≥18 had significantly higher rates of pulmonary hypertension, RV systolic hypertension, RV dilation, RV dysfunction and RV hypertrophy compared to those with PAOI<18 (Fig. 9).
The relationship between pulmonary artery diameter and the follow-up echocardiographic indicesThe minimum and maximum values of PAD were 20mm and 37mm with a mean±SD of 28.14±3.35mm, respectively. Sixty-two subjects (51.2%) had a normal PAD. However, PAD was increased in 58 (47.9%) participants. A significant increase was detected in PAD along with the increase of PAOI. A statistically significant positive correlation was detected between PAD and SPAP (P<0.001, r=0.49), RV systolic pressure (P<0.001, r=0.49) and RV wall thickness (P<0.001, r=0.39).
Patients with dilated pulmonary artery had significantly higher rates of development of pulmonary hypertension, RV systolic hypertension, RV dilation, RV dysfunction and RV hypertrophy (Table 3). Analysis revealed the PAD at the initial PCTA as a sensitive index for predicting patients at risk of pulmonary hypertension, RV systolic hypertension, RV dilation, RV dysfunction and RV hypertrophy (Table 3).
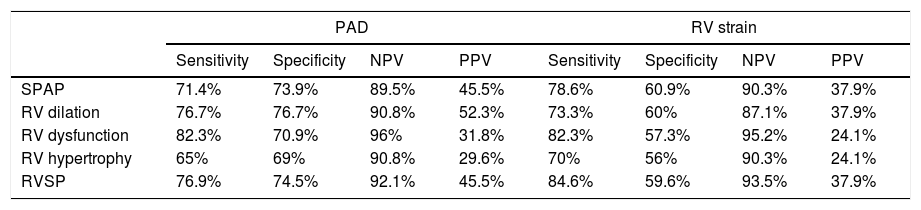
Sensitivity, specificity and predictive value of RV strain and PAD in detecting patients susceptible to long-term pulmonary embolism complications.
| PAD | RV strain | |||||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | NPV | PPV | Sensitivity | Specificity | NPV | PPV | |
| SPAP | 71.4% | 73.9% | 89.5% | 45.5% | 78.6% | 60.9% | 90.3% | 37.9% |
| RV dilation | 76.7% | 76.7% | 90.8% | 52.3% | 73.3% | 60% | 87.1% | 37.9% |
| RV dysfunction | 82.3% | 70.9% | 96% | 31.8% | 82.3% | 57.3% | 95.2% | 24.1% |
| RV hypertrophy | 65% | 69% | 90.8% | 29.6% | 70% | 56% | 90.3% | 24.1% |
| RVSP | 76.9% | 74.5% | 92.1% | 45.5% | 84.6% | 59.6% | 93.5% | 37.9% |
RV: Right ventricular; PAD: Pulmonary artery diameter; SPAP: Systolic pulmonary artery pressure; RVSP: Right ventricular systolic pressure; NPV: Negative predictive value; PPV: Positive predictive value.
RV strain was detected in 44 (36.4%) patients. The rates of development of pulmonary hypertension, RV systolic hypertension, RV dilation, RV dysfunction and RV hypertrophy were significantly different among the patients with and without RV strain (P<0.01). The sensitivity, specificity, positive predictive value, and negative predictive value of RV strain are summarized in Table 3.
The relationship between pulmonary artery obstruction index and right ventricular strainThe mean±SD of PAOI was 19.3±7.9 and 8.6±6.5 in the patients with and without signs of RV strain, respectively. PAOI was significantly higher in the patients with RV strain compared to those with no RV strain (P<0.001). PAOI ≥11 was a predictor of RV strain with a sensitivity of 81% and specificity of 70% (P<0.05, AUC=0.85, 95% CI=0.78–0.92).
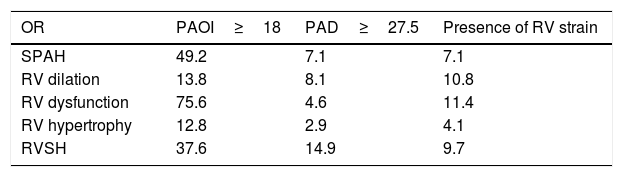
Correlation between pulmonary CT angiography indices and outcomeTable 4 shows the OR of the PCTA indices including PAOI, PAD, and presence of RV strain in the prediction of RV echocardiographic abnormalities development after a 6 month follow up using echocardiography. PAOI ≥18 had the highest OR for the development of right heart echocardiographic abnormalities in comparison with a dilated pulmonary artery and presence of RV strain.
OR for CTA indices in prediction of right heart echocardiographic abnormalities development in echocardiography.
| OR | PAOI≥18 | PAD≥27.5 | Presence of RV strain |
|---|---|---|---|
| SPAH | 49.2 | 7.1 | 7.1 |
| RV dilation | 13.8 | 8.1 | 10.8 |
| RV dysfunction | 75.6 | 4.6 | 11.4 |
| RV hypertrophy | 12.8 | 2.9 | 4.1 |
| RVSH | 37.6 | 14.9 | 9.7 |
OR: Odds ratio; RV; Right ventricle; SPAH: Systolic pulmonary artery hypertension; RVSH: Right ventricular systolic hypertension.
This study was conducted on 120 patients with a proven PE in PCTA who underwent an echocardiography by passing six months from the diagnosis. In this study, PAOI was measured using PCTA, and RV indices including SPAP, RV systolic pressure, RV dilation, RV dysfunction and RV wall thickness were measured using echocardiography. A significant positive correlation was detected among PAOI and SPAP, RV systolic pressure and RV wall thickness. In addition, it was detected that, the patients with a higher PAOI, had significantly higher rates of RV dilation and RV dysfunction. The results of this study showed that PAOI ≥18 could predict the development of pulmonary hypertension, RV systolic hypertension, RV dilation, RV dysfunction and RV hypertrophy with high sensitivity and specificity. Up to the best of our knowledge, this study was one of the first studies introducing cutoff points for PAOI to predict the development of PE related cardiac complications.
Kumar et al. reported that Qanadli PE index >60% could predict the risk of RV dysfunction with a sensitivity of 100% and specificity of 87%.5 Also, in a study done by Rodrigues et al., a cutoff point 18 was reported for PAOI as a predictor of RV dysfunction development. They reported a sensitivity of 78.4% and specificity of 79% for the threshold of 18.14 However, in the present study, a similar cutoff point was introduced to predict RV dysfunction development with higher sensitivity and specificity (94% and 82.5%, respectively). Although Rodrigues reported the similar cutoff point to our study, there are some differences between the methods of these studies. In previous studies, the diagnosis of RV dysfunction was made by PCTA at the time of admission. However, we detected the cutoff point using echocardiography for diagnosis of RV dysfunction in the patients with chronic PE and after a 6 months follow up, which makes it more reliable.
There are some reports in the literature suggesting a strongly positive correlation between PAOI and CT signs of right heart dysfunction, right heart dilation, RV/left ventricle ratio, flattening and bowing of interventricular septum, and short-term mortality.15–17 In a study conducted on 82 patients with acute PE, the association between PAOI obtained from PCTA and RV dysfunction obtained from echocardiography, was evaluated. An increase in the PAOI was more prevalent in the patients with RV enlargement and dysfunction.18 Also, in another study conducted on 85 patients with acute PE, the impact of PE on RV performance was evaluated using echocardiography, showing an inverse correlation between CT clot burden score and RV function (r=−0.57), and a direct correlation between PAOI and SPAP (r=0.51).19 The results of the previous studies and the present study indicate that the PAOI is not only able to predict the risk of RV dysfunction development in the acute setting, but can also predict the high-risk patients for cardiac problems in a long term follow up.
In the present study, we also found a statistically significant difference in the developments of pulmonary hypertension, RV systolic hypertension, RV dilation, RV dysfunction and RV hypertrophy between the patients with a dilated pulmonary artery and the patients with normal pulmonary artery at initial PCTA. There is also a relationship between PAD and pulmonary artery pressure and the two parameters may simultaneously increase.20,21 Alashram et al. also reported a significant correlation among PAD and RV systolic pressure, RV dilation, and RV systolic dysfunction. In addition, they reported that PAD>30mm was related to the development of RV dysfunction in the patients with acute PE.22 An increase in pulmonary artery pressure for a long time can cause RV remodeling and may lead to a decline in RV function, which can be detected using echocardiography. The results of the present study suggested that the patients with an initial dilated pulmonary artery may develop RV problems. A dilated pulmonary artery can predict the patients at the risk of PE complications with high sensitivity. In addition to PAOI and PAD, we found RV strain as a predictor of right heart echocardiographic abnormalities development. In a study done by Dudzinski et al., the RV strain was associated with higher risk of clinical deterioration in the patients with acute PE.23 Also Plasencia-Martinez reported that absence of RV dysfunction is a good prognostic factor to identify low risk patients after acute PE and RV dysfunction is accurately evaluated with RV ratio (RV/left ventricle diameter).24
In this study, the strongest predictor of the right heart echocardiographic abnormalities development was PAOI. Although the PAD and presence of RV strain were the predictors of RV echocardiographic abnormalities, there were not considered as predictive as PAOI. PAD was reported as a weak predictor of acute PE complications in some studies, which is consistent with our findings.25–27
An increased risk of CTEPH was detected among the patients with younger age and idiopathic manifestation of PE.28 However, in the present study, we did not investigate the cause of the PE in participants. In addition, the participants of this study were older, which can be considered as a limitation in this study. Also, we did not have a control group and focused on follow up of one group of patients and calculation of CTA and echocardiographic indices in them. So more studies with considering a control group should be done.
In conclusion, in this study, we found cutoff point of 18 for PAOI with high sensitivity and specificity to predict the long-term probability of pulmonary hypertension, RV systolic hypertension, RV dilation, RV dysfunction and RV hypertrophy development. In addition, we found dilated pulmonary artery and presence of RV strain as the predictors of right heart echocardiographic abnormalities development. However, they were weaker predictors compared to PAOI. These values can suggest the use of PAOI in therapeutic management of patients with acute PE to reduce the risk of long-term morbidity and heart problems. Also it can be used to predict risk of CTEPH which is a serious complication in PE. However, performing some more studies with a higher sample size are needed to prove this theory.
Author contribution statementSomayeh Hajiahmadi: Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Supervision; Validation; Visualization.
Faezeh Tabesh: Data curation; Methodology; Project Administration; Resources; Software; Validation; Visualization; Writing – review & editing.
Azin Shayganfar: Formal analysis; Investigation; Methodology; Project Administration; Resources; Software; Roles/Writing – original draft; Writing – review & editing.
Fattane shirani: Conceptualization; Data curation; Investigation; Methodology; Project Administration; Resources; Visualization; Roles/Writing – original draft; Writing – review & editing.
Shadi Ebrahimian: Supervision; Validation; Visualization; Roles/Writing – original draft; Writing – review & editing.
Conflict of interestThe authors declare no conflict of interest