Fungal lung co-infections associated with COVID-19 may occur in severely ill patients or those with underlying co-morbidities, and immunosuppression. The most common invasive fungal infections are caused by aspergillosis, mucormycosis, pneumocystis, cryptococcus, and candida. Radiologists integrate the clinical disease features with the CT pattern-based approach and play a crucial role in identifying these co-infections in COVID-19 to assist clinicians to make a confident diagnosis, initiate treatment and prevent complications.
Las coinfecciones pulmonares fúngicas asociadas a la COVID-19 pueden ocurrir en pacientes gravemente enfermos o con comorbilidades subyacentes e inmunosupresión. Las infecciones fúngicas invasivas más comunes son causadas por aspergilosis, mucormicosis, y las debidas a Pneumocystis, criptococo y cándida. Los radiólogos integran las características clínicas de la enfermedad con el enfoque basado en patrones de TAC y desempeñan un papel crucial en la identificación de estas coinfecciones en la COVID-19 para ayudar a los médicos a realizar un diagnóstico seguro, iniciar el tratamiento y prevenir complicaciones.
- •
Fungal co-infections in COVID-19 are the saprophytic type, commonly associated with immunocompromised status.
- •
Prolonged immunosuppression treatment and underlying co-morbidities predispose patients with COVID-19 to fungal lung infection.
- •
Clinical disease presentation, and CT pattern recognition can help identify and differentiate opportunistic fungal lung infections on a background of underlying COVID-19 pneumonia.
The COVID-19 pandemic caused by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-COV-2) has had significant clinical, social, and economic implications around the globe.1,2 The viral infection is accompanied by an aggressive immunological response and cytokine storm that results in an excessive inflammatory reaction.3 Immunosuppressive treatment including the use of steroids have been advocated in severely and critically ill patients to prevent morbidity and mortality associated with COVID-19.4 In some cases, indiscriminate use of immunosuppressive drugs or steroids in COVID-19 has led to complications including opportunistic infections. COVID-19 treatment-related immunosuppression, underlying comorbidity related to malignancies, diabetes mellitus, human immunodeficiency virus, indwelling prosthesis, burns, and neutropenia, etc., has led to fungal co-infections with increased morbidity and mortality. Fungal co-infections are difficult to diagnose on a background of severe parenchymal lung disease from COVID-19 peumonia.5,6 Radiological signs associated with fungal infections such as the halo sign and reverse halo sign are also seen with COVID-19 pneumonia.7–10
Radiologists may be the first to raise the suspicion of fungal infections when reviewing CT scans of patients under acute treatment for COVID-19 or in post-COVID-19 follow-up. In this pictorial essay, we systematically present the most common features and differential diagnosis related to fungal co-infections on Chest CT of COVID-19 patients.
Fungal infections and the host immunityThere is a wide spectrum of bacterial and fungal infections that can develop in a patient with COVID-19 infection, due to underlying co-morbidities, nosocomial infections, or ventilator-associated pneumonia. COVID-19 related fungal lung co-infections has been attributed to pathophysiologic processes of: (a) Danger-associated molecular patterns (DAMP) triggered by SARS-COV-2 infection through impaired immunological response culminating in acute pulmonary injury11–13; (b) Anti-viral immunity activation which creates a permissive environment for the proliferation of fungal organisms14 and; (c) Severe acute respiratory distress syndrome with a bed of interstitial and alveolar edema that serves as an environment for opportunistic lung infection to develop.15
Classification of pulmonary fungal infectionsFungal infection involving the lungs can be broadly classified into 2 types, either saprophytic, which predominantly infects immunocompromised individuals (Pneumocystis jirovecii, candidiasis, aspergillosis, mucormycosis, cryptococcus), or pathogenic which predominantly infects immunocompetent individuals (coccidiomycosis, blastomycosis, histoplasmosis).16
Fungal lung infections in COVID-19The fungal lung infections that occur in COVID-19 are the saprophytic type infecting immunocompromised individuals. These patients tend to be symptomatic, while the clinical picture is often confusing and difficult to determine the presence of a fungal infection on a background of COVID-19. The reported incidence of co-infections associated with COVID-19 varies from 4% to 35%, for patients admitted in intensive care unit with acute respiratory distress syndrome. Most common pathogens are invasive pulmonary aspergillosis, mucormycosis, P. jirovecii, cryptococcus and candida infections.17–23 The spectrum of infection caused due to these opportunistic organisms ranges from indolent to aggressive and also depends on the underlying co-morbidities (Table 1).
Predisposing factors associated with fungal lung infections.
| Organism | Predisposing associated conditions |
|---|---|
| CAPA | |
| Airway-invasive aspergillosis | Diabetes mellitus |
| Angio-invasive aspergillosis | Diabetes mellitus, neutropenia, HSCT allografts or solid organ transplants, hematological malignancies, prolonged steroids. |
| CAM | Diabetes mellitus, hematological malignancies, solid organ transplants, chronic respiratory diseases, burns or local trauma. |
| Pneumocystis jirovecii | HIV, patients with lymphopenia, solid organ transplants, hematological malignancies. |
| Cryptococcus neoformans | Bird feces |
| Candida (White fungus) | Hospitalization, indwelling venous catheter, hematological malignancy, peritoneal dialysis, post thoracic surgery |
CAM-SARS-COV-2 associated mucormycosis, CAPA-SARS-COV-2 associated pulmonary aspergillosis, HIV — human immunodeficiency virus, HSCT— hematopoietic stem cell transplant.
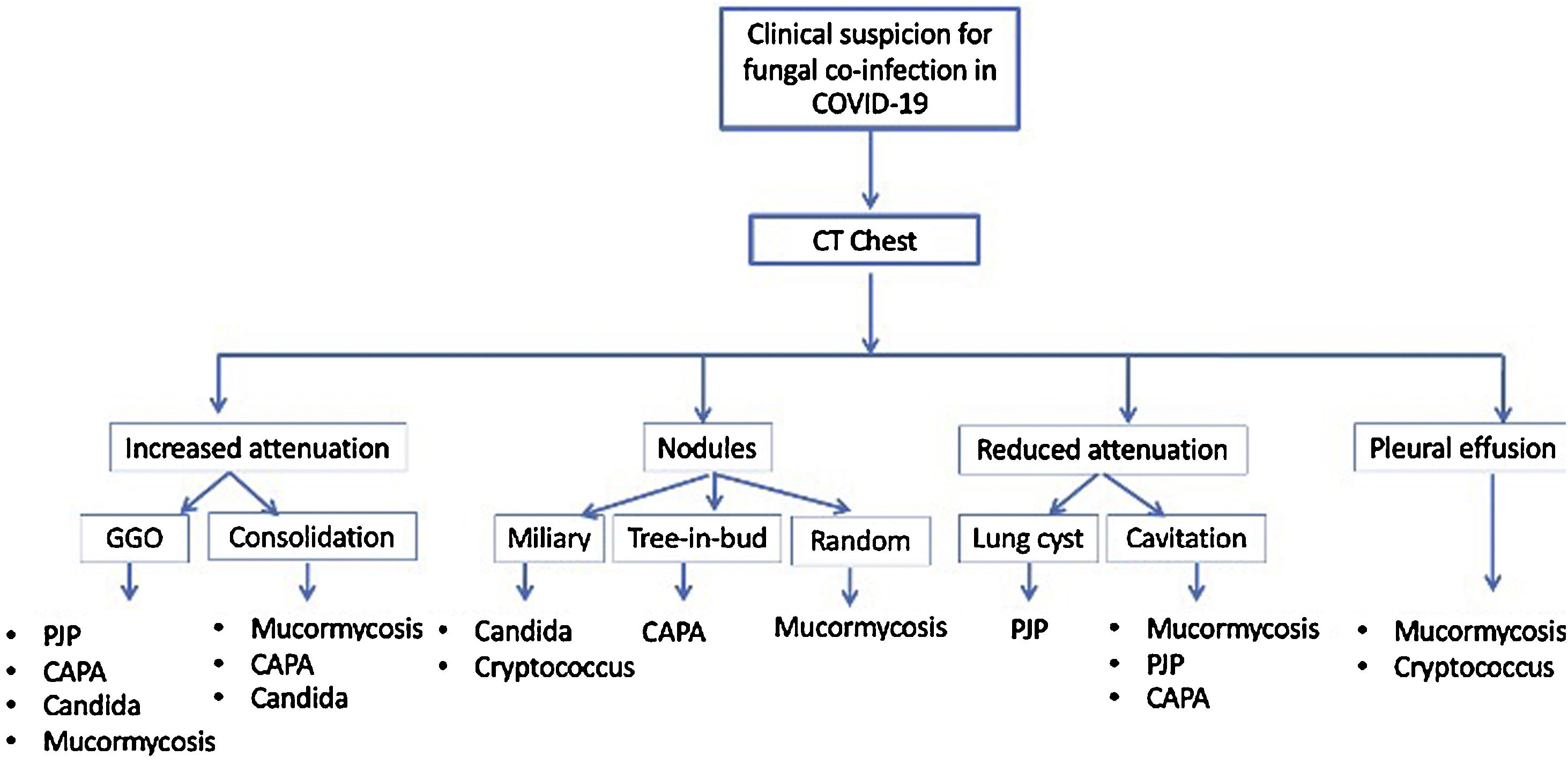
The saprophytic fungal infections have a wide spectrum of disease patterns on the Chest CT including ground-glass opacities, consolidation, nodules, cysts, and cavitations24 (Fig. 1). Ancillary features of tracheobronchial wall thickening, mediastinal lymphadenopathy and pleural effusion may be present. A high index of suspicion and knowledge of the characteristic and overlapping patterns can help determine the presence of fungal co-infections. Often it is the reporting radiologist who raises the possibility of opportunistic fungal co-infection based on the Chest CT findings. Early detection and clinical integration may allow for initiation of targeted treatment and improved care.
Chest CT findings in COVID-19The most common reported findings of COVID-19 lung infection include ground glass opacities (GGO), consolidation, crazy-paving pattern, bilateral lung abnormalities, lower lobe and posterior predilection, and vascular engorgement25–27 (Fig. 2). The pattern of abnormality changes over time depending on the stage of the disease process.27 In early stage GGO is the predominant manifestation, crazy pavement in the progressive stage, consolidation in the peak stage, the consolidation may be completely absorbed or replaced by fibrotic changes in recovery stage.27 In a suspicious clinical scenario, with a positive test, or in a COVID-19 endemic region the presence of these findings should raise the suspicion for COVID-19.
Chest CT findings in fungal co-infections with COVID-19Aspergillus fumigatusAspergillus fumigatus infects immunocompetent and immunocompromised individuals. SARS-COV-2-associated pulmonary aspergillosis (CAPA) has been one of the predominant fungal infections in COVID-19 patients with acute respiratory distress syndrome (ARDS).28,29 CAPA manifests as either airway invasive aspergillosis, or angio-invasive aspergillosis (ANIA). The reported incidence of CAPA may vary depending on the treatment undertaken for severe or critically ill COVID-19 patients, especially with corticosteroids and anti-interleukin 6 (IL-6) like tocilizumab.30,31 CAPA is difficult to diagnose when the radiological findings related to aspergillosis resemble severe pulmonary manifestations of COVID-19.32,33 CAPA in critically ill or intubated patients is reported to have a mortality rate reaching up to 40%.34–38
- (a)
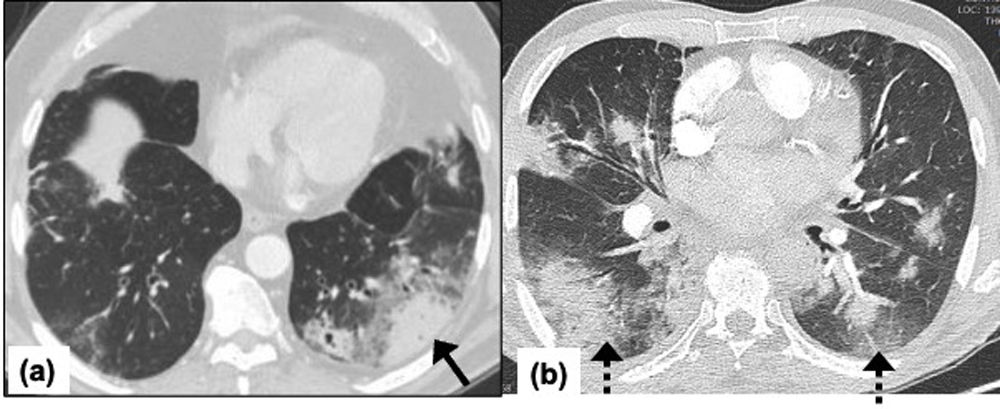
Angio-invasive aspergillosis (ANIA), occurs exclusively in immunocompromised patients (Fig. 3). The organism invades small to medium-sized arteries and leads to solitary or multiple necrotic and hemorrhagic nodules, or infarcts. Chest CT demonstrates solitary or multiple pulmonary nodules and masses. The halo of hemorrhage may be seen as an area of ground-glass opacity surrounding the nodule referred as the halo sign. A reverse halo sign (defined as central ground-glass opacity surrounded by a crescentic or circumferential denser consolidation) may also be encountered. CT angiography may demonstrate vascular invasion.39 Lymph node enlargement or pleural effusion is usually absent. Confident diagnosis may be difficult because the halo sign and the reverse halo sign are also seen commonly with COVID-19 pneumonia. The presence of an obvious vascular invasion would prompt the radiologist to suspect ANIA co-infection on a background of COVID-19.
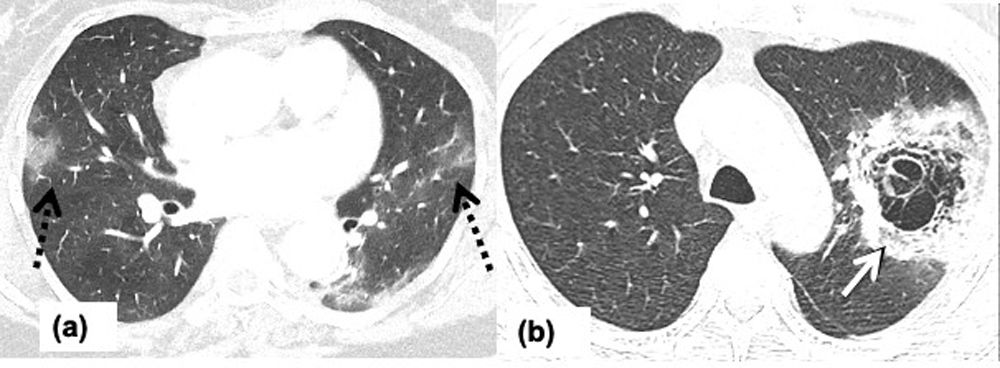
Figure 3.Angio-invasive aspergillosis in a 69-year-old man with COVID-19 pneumonia requiring non-invasive ventilation and ICU admission. Chest CT on day-10 since the symptom onset showing, (a) axial image showing left lower lobe mass like peripheral wedge-shaped consolidation with surrounding ground glass opacity keeping with a pulmonary infarct and, surrounding halo sign due to pulmonary hemorrhage (arrow), (b) axial image showing bilateral peripheral subpleural multifocal consolidations (dashed arrow) consistent with underlying COVID-19 pneumonia.
(0.07MB). - (b)
Airway invasive aspergillosis (AIA), manifestations depend on the site of involvement within the airway. It may present as tracheobronchitis, bronchiolitis, or bronchopneumonia. Chest CT findings in tracheobronchitis include tracheal and bronchial wall thickening, bronchiolitis findings include centrilobular nodules with ‘tree-in-bud pattern’, while bronchopneumonia type of involvement seen as lobar or perihilar consolidation.40 When there is AIA co-infection in COVID-19, the tracheobronchitis and bronchiolitis type of involvement can be distinctly identified from the pattern of COVID-19 pneumonia, while the bronchopneumonia type might be masked in severe or critical COVID-19 making the diagnosis difficult.
- (c)
CAPA is difficult to diagnose on routine imaging in critical patients with COVID-19, as the imaging features are non-specific and, the typical features like halo sign or cavitation are rarely observed.41 Blood tests may be negative in AIA, in the non-neutropenic patient.42 Bronchoalveolar lavage (BAL) is rarely performed due to risk of transmission.43 Standard screening for fungal infections with serum galactomannan (GM) has low sensitivity of only 21% for detection of CAPA.44
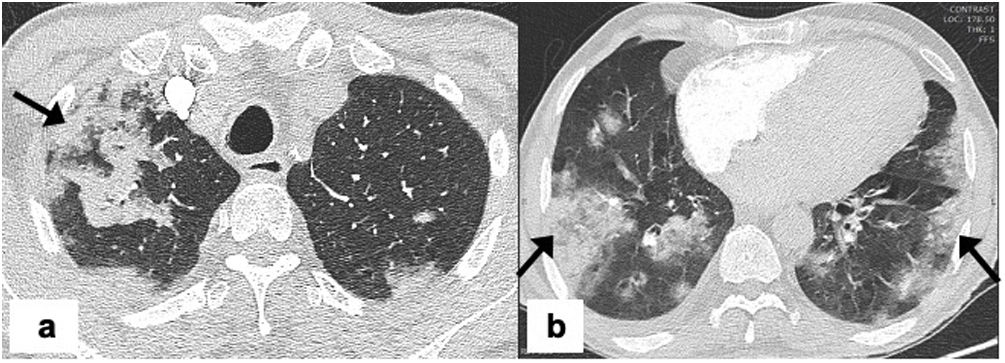
Mucormycosis is also known as ‘black fungus’ in the lay literature and is caused by inhalation of fungal spores that are present in the air, soil, compost, piles, decaying organic matter, fruits, or vegetables. Paranasal sinuses and lungs are the most affected sites.45 Diabetes mellitus is the most common risk factor, followed by immunosuppression, hematological malignancy, stem cell and organ transplantation.46 Chest CT findings are non-specific including the presence of nodules, consolidation, or ground-glass opacities (GGO).47 The reverse halo sign, lymphadenopathy, and/or pleural effusion may also be seen. The areas of consolidation may show cavitation on follow-up imaging.48 Rarely, mucormycosis may be associated with invasion of the pulmonary artery, superior vena cava, or other neck vessels48,49 (Figs. 4 and 5). The bird’s nest sign refers to the appearance created by a reverse halo sign with associated irregular and intersecting areas of stranding or irregular lines within the area of ground-glass opacity.50,51 When the bird’s nest sign is observed in the context of COVID-19 infection it should raise the suspicion for COVID-19 associated mucormycosis (CAM) infection. In practice, differentiating CAM from CAPA is difficult due to overlapping imaging features. The presence of bird’s nest sign, more than 10 lung nodules, pleural effusion, and concurrent sinus infections favors mucormycosis over invasive pulmonary aspergillosis.52 Bird’s nest sign is not specific for CAM and has been associated with cryptogenic organizing pneumonia, bacterial pneumonia, sarcoidosis, Wegeners granulomatosis, paracoccidioidomycosis, pulmonary infarction.53
Pulmonary mucormycosis in a 45-years-old man with a past medical history of diabetes mellitus, two weeks back was diagnosed and treated for COVID-19 elsewhere with symptomatic treatment and oral steroids. Patients now presents with persistent fever and dyspnea. Chest CT on admission, (a) axial image showing bilateral peripheral subpleural multifocal ground glass opacities (dashed black arrow) consistent with underlying COVID-19 pneumonia, (b) axial image showing large central cavitation in the left upper lobe associated with irregular intersecting lines within an area of consolidation and marginal ground glassing consistent with birds-nest sign (white arrow).
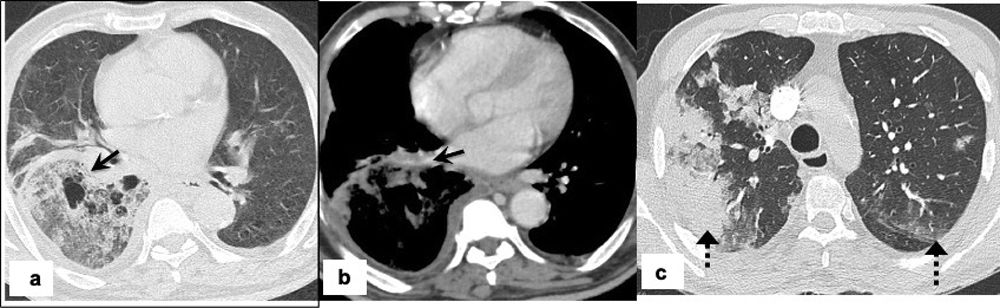
Pulmonary mucormycosis in 64-years-old man with a past medical history of diabetes mellitus, presents with fever, cough and breathlessness for the last 4 days. Real-time PCR was positive for COVID-19. The patient had a severe COVID-19 pneumonia requiring ICU admission and non-invasive ventilation. Admission chest CT, (a) axial image showing a large air-space consolidation with ground-glass attenuation in the right lower lobe with areas of cavitation’s (arrow), (b) axial post-contrast image shows vessel wall irregularity and luminal narrowing of the right inferior pulmonary vein suggesting vascular invasion (arrow) and (c) axial image showing peripheral subpleural consolidation on right upper lobe and patchy ground glass opacities in left upper lobes (dashed arrow) consistent with underlying COVID-19 pneumonia.
P. jirovecii is associated with acquired immunodeficiency syndrome, organ transplant recipients, and patients undergoing immunosuppressive treatment with chemotherapy or steroids. The risk of Pneumocystis jirovecii pneumonia (PJP) increases significantly with CD4+ lymphocytopenia (<200 cell/μL).54,55 SARS-COV-2 infection in an already immunosuppressed patient, leads to further functional immunosuppression predisposing the patient to PJP.54 Due to overlapping radiological features of SARS-COV-2 with PJP, the presence of PJP co-infection with SARS-COV-2 may be difficult to diagnose on imaging. The radiologists should approach the CT scan with suspicion for PJP co-infection in an immunocompromised patient with CD4+ lymphocytopenia. Typical CT findings include patchy or geographic GGO with predominant perihilar or middle zone predilection.56 Less frequently smooth interlobar septal thickening in a crazy-paving pattern may be present. Upper zone predominance can be seen in patients on prophylactic therapy. Peripheral sparing is described in 40% of patients giving a batwing appearance57 (Fig. 6). Pleural effusions are uncommon with this infection, and the presence of effusion should prompt the suspicion of other pathology or infection.58 During the chronic phase, the areas of lungs affected by crazy paving patterns may be replaced with irregular lung cysts, increasing the risk of pneumothorax in such cases. Less common findings encountered include pulmonary consolidation and centrilobular nodules.58 In patients with CD4+ lymphocytopenia, and imaging features that are suspicious for co-infection with PJP, it is recommended to suggest testing with serum beta-D-glucan,59 or quantitative polymerase chain reaction (PCR) for PJP.60
Acute Pneumocystis jirovecii pneumonia in a 29-year-old man with HIV and low CD4+ count (<200 cells/μL). Patient presents to the emergency department with severe COVID-19 symptoms on day-6. Chest CT on day-10, (a) Axial image showing diffuse ground-glass opacities and confluent infiltrates in the bilateral lower lobes with septal line thickening indicating crazy paving (black arrows) reaching upto the peripheral lung fields keeping with COVID-19 pneumonia; (b) coronal image of the thorax shows diffuse ground-glass opacities with septal thickening involving bilateral lungs with relative peripheral sparing (dashed arrow) in upper and mid lung fields suggesting ‘bat wing’ appearance. There is considerable overlap of SARS-COV-2 infection with Pneumocystis jirovecii pneumonia making the diagnosis difficult.
These are commonly found in soil or bird droppings. SARS-COV-2 infection in patients with HIV accompanied by CD4+ lymphocytopenia (<200 cells/μL), or patients with stem cell transplants are susceptible to invasive cryptococcosis.61 Chest CT shows multiple pulmonary nodules of varying size (0.5cm–4cm), miliary pattern, or areas of consolidation (Fig. 7). Cavitation may occur within the nodules in immunocompromised patients.62 Pleural effusion or lymphadenopathy may also be seen. The clinical presentation with meningoencephalitis63 and Chest CT findings may be the first indication of invasive cryptococcosis co-infection.
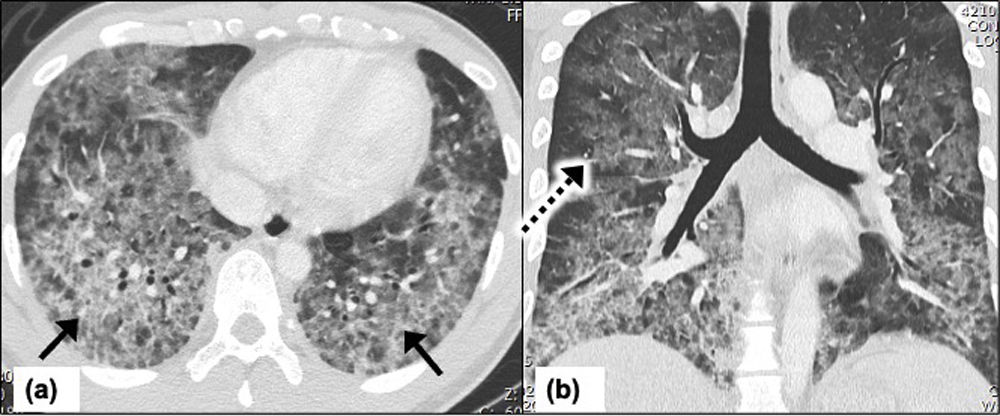
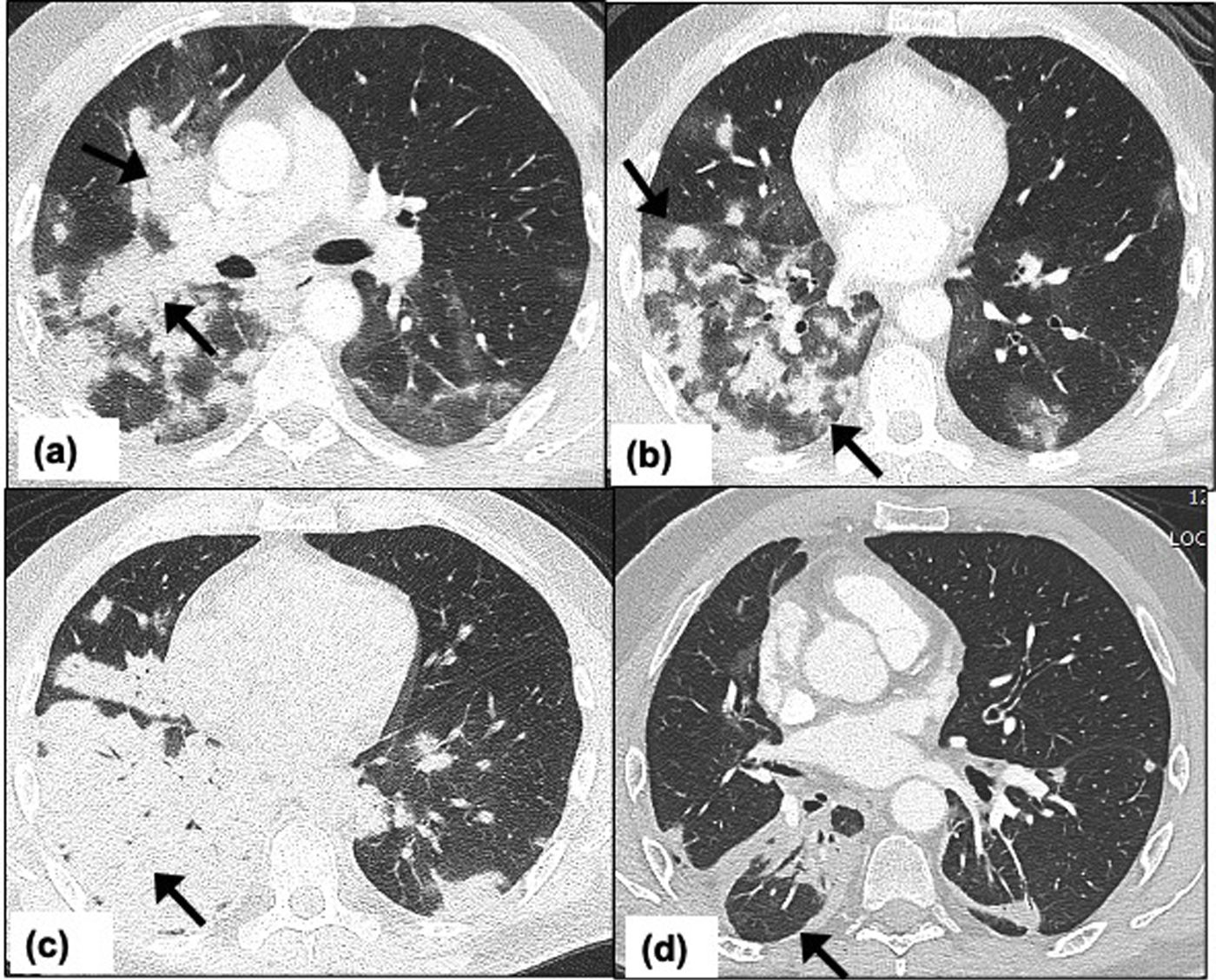
Pulmonary cryptococcosis in a 54-year-old man receiving immunosuppressive treatment for past medical history of inflammatory bowel disease. Patient presents to emergency department with moderate COVID-19 symptoms. Chest CT on day-10, (a) axial CT image at the level of carina shows confluent nodules forming a mass like peri-bronchial consolidation with surrounding ground-glass opacities (arrows), (b) axial CT image showing multiple nodules with surrounding ground-glass halo in bilateral lower lobes (arrows), (c) axial CT image taken 2 weeks later shows complete lobar consolidation in the right lower lobe suggesting significant interval progression (arrow), (d) axial CT image of the thorax 2 months after the symptom onset and shows significant interval improvement with residual peri-bronchial consolidation in right lower lobe (arrow).
Candida also known as the ‘white fungus ‘is an organism that is part of normal human microbial flora in the oral cavity.64 Most patients with pulmonary candidiasis tend to have widespread systemic involvement. Invasive candidiasis was shown to occur more frequently in patients who received broad-spectrum antibiotics, parenteral nutrition, prolonged neutropenia and other immune impairment factor.65 Pulmonary involvement with candida pneumonia is non-specific and on imaging is commonly attributed to other opportunistic infections or COVID-19 pneumonia. Chest CT findings may include multiple solid nodules without a ground glass halo, GGO, miliary nodules, multifocal consolidation, or small pulmonary abscesses.66
Vigilance for opportunistic fungal infections in COVID-19The typical imaging patterns associated with fungal infections overlap with COVID-19 pneumonia and other viral, bacterial infections and even malignancy. In some patients, radiologic manifestations may be delayed or absent. The radiologist has a role in integrating the high-risk clinical features, flagging the chest CT findings such as the halo sign, bird’s nest sign, cavitations, bronchial or peri-bronchovascular involvement associated with fungal co-infections and recommending confirmatory laboratory, or histopathological sampling.67 Sputum analysis, syndromic molecular panels with quantitative polymerase chain reaction, culture for aspergillosis, mucormycosis or P. jirovecii of respiratory samples may be performed to help confirm the diagnosis. Depending on the positivity further confirmatory testing with blood biomarkers, using serum galactomannan, serum beta-D-glucan, cryptococcal antigen, blood quantitative PCR for mucormycosis, and aspergillosis would be necessary.52,68 Biomarkers for diagnosing invasive aspergillosis, like galactomannan and beta-D-glucan are negative in patients with mucormycosis, allowing the clinical team to refine the diagnosis with the presence of contributing history and/or radiological suspicion.
ConclusionCOVID-19 patients with immunocompromised status and underlying comorbidities are prone to develop fungal co-infections. In a background of COVID-19, the radiologists should approach the chest CT with a high index of suspicion, for CAPA when there is history of steroid and interleukin-6 therapy, for CAM in diabetes mellitus, for PJP in HIV patients with CD4+ lymphocytopenia, and for cryptococcosis when there is pre-existing malignancies or immunosuppression associated with clinical presentation of meningoencephalitis. Most fungal lung co-infections in a setting of COVID-19 are invasive with a propensity for rapid deterioration. It is important for radiologists to be familiar with the typical and atypical disease pattern of fungal co-infection on chest CT in COVID-19 pneumonia patients, so that further workup including serology and tissue sampling can be performed to confirm the diagnosis and advance patient care.
Authorship- 1.
Responsible for study integrity: MM, EP
- 2.
Study conception: MM, EP, JCM, OJT, JCP.
- 3.
Study design: MM, EP
- 4.
Data acquisition: MM, EP, JCM, OJT, JCP
- 5.
Data analysis and interpretation: N/A
- 6.
Statistical processing: N/A
- 7.
Literature search: MM, EP, JCM, OJT, JCP
- 8.
Drafting of the manuscript: MM, EP, JCM, OJT
- 9.
Critical review of the manuscript with intellectually significant contributions: MM, EP, JCM, OJT, JCP.
- 10.
Approval of the final version: MM, EP, JCM, OJT, JCP
Authors has not received any funding for this article.
Conflicts of InterestAuthors have no disclosure or no relevant relationships.