Edited by: Dr. José Luis del Cura Rodríguez - Servicio de Radiodiagnóstico, Hospital Universitario Donostia, Donostia-San Sebastián, España
More infoInterventional procedures have become a routine part of breast imaging unit’s activity. Most interventional procedures in breast imaging are done under ultrasound guidance.
The list of ultrasound-guided interventional procedures performed by breast imaging units is long. This chapter will review the different techniques, placing emphasis on the most cost-effective indications.
La radiología de la mama ha incorporado el intervencionismo como una faceta más de su actividad asistencial. La mayoría de las lesiones se van a caracterizar mediante la punción percutánea guiada con ecografía.
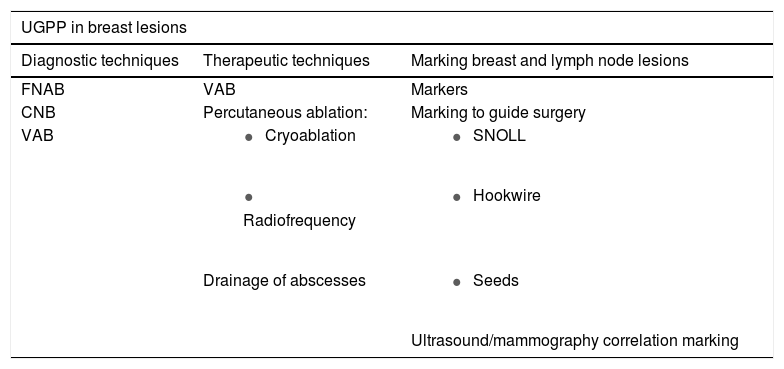
La cartera de servicios del intervencionismo guiado con ecografía en la mama es amplia. Se puede dividir en tres grupos según su utilidad: las técnicas diagnósticas, las terapéuticas y las dirigidas a localizar las lesiones de mama y axila. En este capítulo se van a revisar haciendo hincapié en sus indicaciones más costo-efectivas.
Breast radiology has incorporated interventional radiology as one more facet of its healthcare activity. Most breast interventional techniques are ultrasound-guided.
There is a wide array of ultrasound-guided interventional services in the breast. These services can be divided into three groups according to their use (Table 1). This chapter will review the different techniques with an emphasis on the most cost-effective indications.
Array of ultrasound-guided percutaneous puncture services in breast lesions.
| UGPP in breast lesions | ||
|---|---|---|
| Diagnostic techniques | Therapeutic techniques | Marking breast and lymph node lesions |
| FNAB | VAB | Markers |
| CNB | Percutaneous ablation: | Marking to guide surgery |
| VAB |
|
|
|
| |
| Drainage of abscesses |
| |
| Ultrasound/mammography correlation marking | ||
CNB: core-needle biopsy; FNAB: fine-needle aspiration biopsy; UGPP: ultrasound-guided percutaneous puncture; VAB: vacuum-assisted biopsy.
These diagnostic techniques enable characterisation of most breast lesions and locoregional lymph nodes with suspected metastasis. Hence, they make up the bulk of interventional procedures on a breast radiology unit. Fine-needle aspiration biopsy (FNAB) yields a cytology diagnosis and core-needle biopsy (CNB) yields a histology diagnosis. Specimens for microbiological study can be obtained using either technique.1
FNAB needle calibre ranges from 25 G to 21 G. For cytology diagnosis, 25-G calibre needles are usually used, since they are able to obtain a high-quality aspirate with little blood contamination. Needles with a calibre of 21 G are reserved for draining symptomatic cysts, small abscesses and breast and peri-implant collections. Fluid aspirated from simple cysts is typically discarded and not sent for cytological study. If a cyst is complicated — that is, if it has a somewhat thickened wall, colour Doppler signal or contents with a solid appearance, or if the aspirate is bloody — cytological study is usually ordered, since high-grade papillary lesions and carcinomas may present as complicated cysts.2 CNB is the method of choice for diagnosing lesions suspected of malignancy that are visible on ultrasound, as it is capable of characterising most of them.1 CNB is performed using needles with a calibre ranging from 18 G to 11 G and is always done with local anaesthesia. The usual calibre is 14 G and in general at least three cores are obtained.3
FNAB is a quick technique (it does not require local anaesthesia) with very few bleeding complications. It is highly cost-effective in diagnosing fibroadenomas (FAs) and other lesions with a benign appearance in young patients. In some cases, the FA aspirate is insufficient for diagnosis. CNB does not carry this limitation. The choice of FNAB versus CNB in the diagnosis of FA is at the discretion of the radiologist.
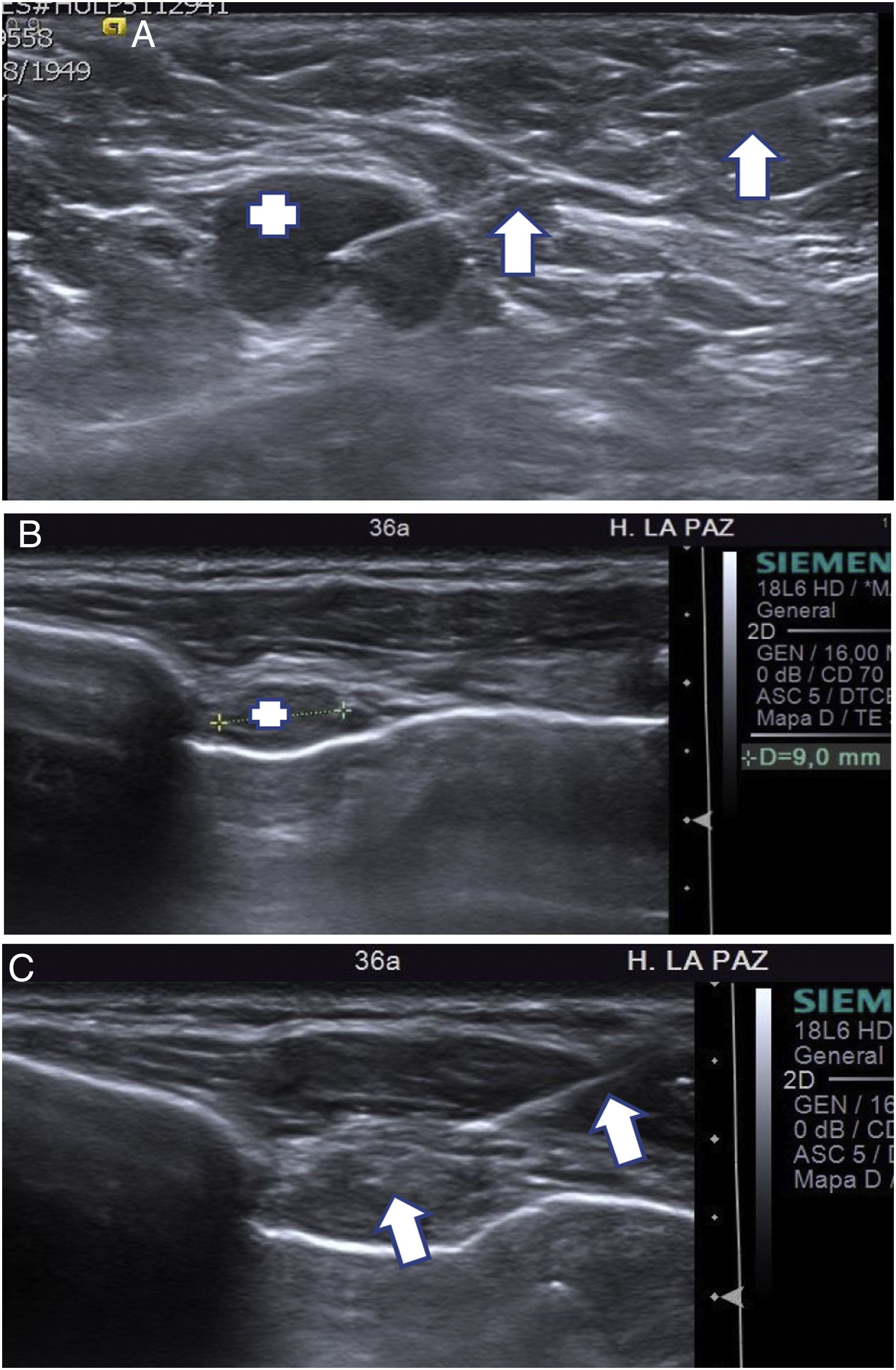
CNB is somewhat more sensitive than FNAB in diagnosing axillary lymph node metastases when staging breast cancer (BC).4 However, axillary FNAB remains the most commonly used technique, since it is quicker than CNB (enabling examination time to be optimised), and although it is less sensitive than CNB, the difference is not significant (Fig. 1A).5 The two techniques combined may increase effectiveness in the diagnosis of axillary metastases. If the result of the initial FNAB is inconsistent (i.e. lymph node metastasis is suspected but the FNAB result is benign) or the specimen is not sufficient, it may be followed by CNB. FNAB enables level-II and level-III lymph nodes (those in an interpectoral or subclavicular location) as well as cervical and internal breast lymph nodes to be characterised relatively easily (Fig. 1B and C). In general, these are small lymph nodes in which CNB is a more complicated technique to perform.
(A) Ultrasound-guided axillary FNAB. A 68-year-old patient with probable carcinoma in the left breast. The axillary ultrasound on the left identified a level-I lymph node (+) with a metastatic appearance. The FNAB needle can be seen with the tip inside the lymph node (arrows). The cytology results confirmed the suspected lymph node metastasis. (B, C) Ultrasound-guided lymph node FNAB in the inner breast region. A 36-year-old patient with treated carcinoma in the left breast, in follow-up. A follow-up positron emission tomography/computed tomography (PET/CT) scan detected a small lymph node in the left inner breast region suspected of metastatic involvement. (B) Targeted ultrasound showed that the small lymph node measured 9 mm (+). (C) The FNAB needle can be seen inside the lymph node (arrow). The cytology study confirmed metastatic involvement.
Vacuum-assisted biopsy (VAB) is a technique that yields thicker cores than CNB. Needle calibre ranges from 10 G to 7 G. VAB is a more costly and complex procedure than CNB. On the one hand, the cost of VAB needles ranges from around €250–€280 versus €20–€25 for CNB. On the other hand, VAB requires more examination time than CNB, as its needles are more difficult to handle and bleeding complications are more common than with CNB.
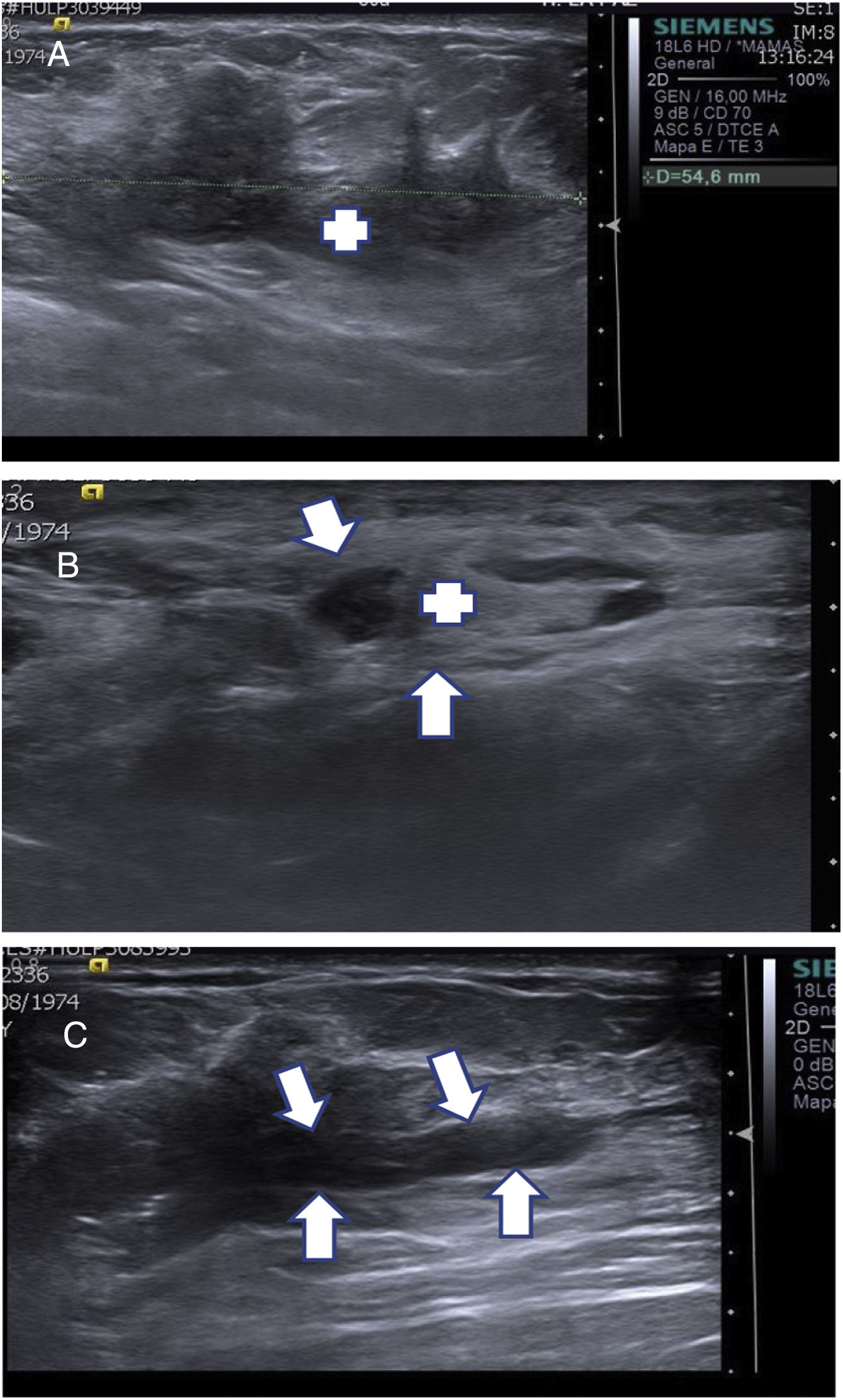
Although CNB is the method of choice for diagnosing lesions suspected of malignancy that are visible on ultrasound, it occasionally yields results that are inconsistent with radiological suspicion or inconclusive. In these cases, VAB is indicated to arrive at a diagnosis. The alternative to VAB is surgical biopsy, which entails much more morbidity for the patient and higher costs for the hospital6,7 (Fig. 2A–C).
(A–C) Use of diagnostic vacuum-assisted biopsy (VAB). A 39-year-old patient with a palpable mass in the right breast (RB). (A) Ultrasound of the RB confirmed that the palpable mass corresponded to a solid lesion with irregular borders consistent with breast cancer (+). (B) Axillary ultrasound identified a nonspecific lymph node (+) with a cortical thickness of 4 mm (arrows). CNB was performed on the palpable lesion with a result of intraductal carcinoma, and axillary FNAB was performed on the nonspecific lymph node with a result of metastatic involvement. (C) As CNB was inconsistent, VAB was performed on the lesion to characterise the carcinoma. A post-VAB ultrasound showed an anechoic cylindrical area inside the carcinoma corresponding to the haematoma and the tissue extracted by VAB (arrows). The result was consistent with oestrogen receptor (OR) 100%, progesterone receptor (PR) 95%, Ki 10%, HER2 — infiltrating ductal carcinoma.
Microcalcifications suspected of malignancy can sometimes be detected by targeted ultrasound. This is very useful in patients with microcalcifications that cannot undergo conventional stereotactic biopsy (as they are in a location that is very posterior or very superficial or in small breasts). Although microcalcifications visible with targeted ultrasound can be biopsied by CNB, with 11-G needles, VAB is associated with better performance since, in the diagnosis of intraductal carcinoma, VAB has higher precision than CNB1,7,8 (Fig. 3A–D).
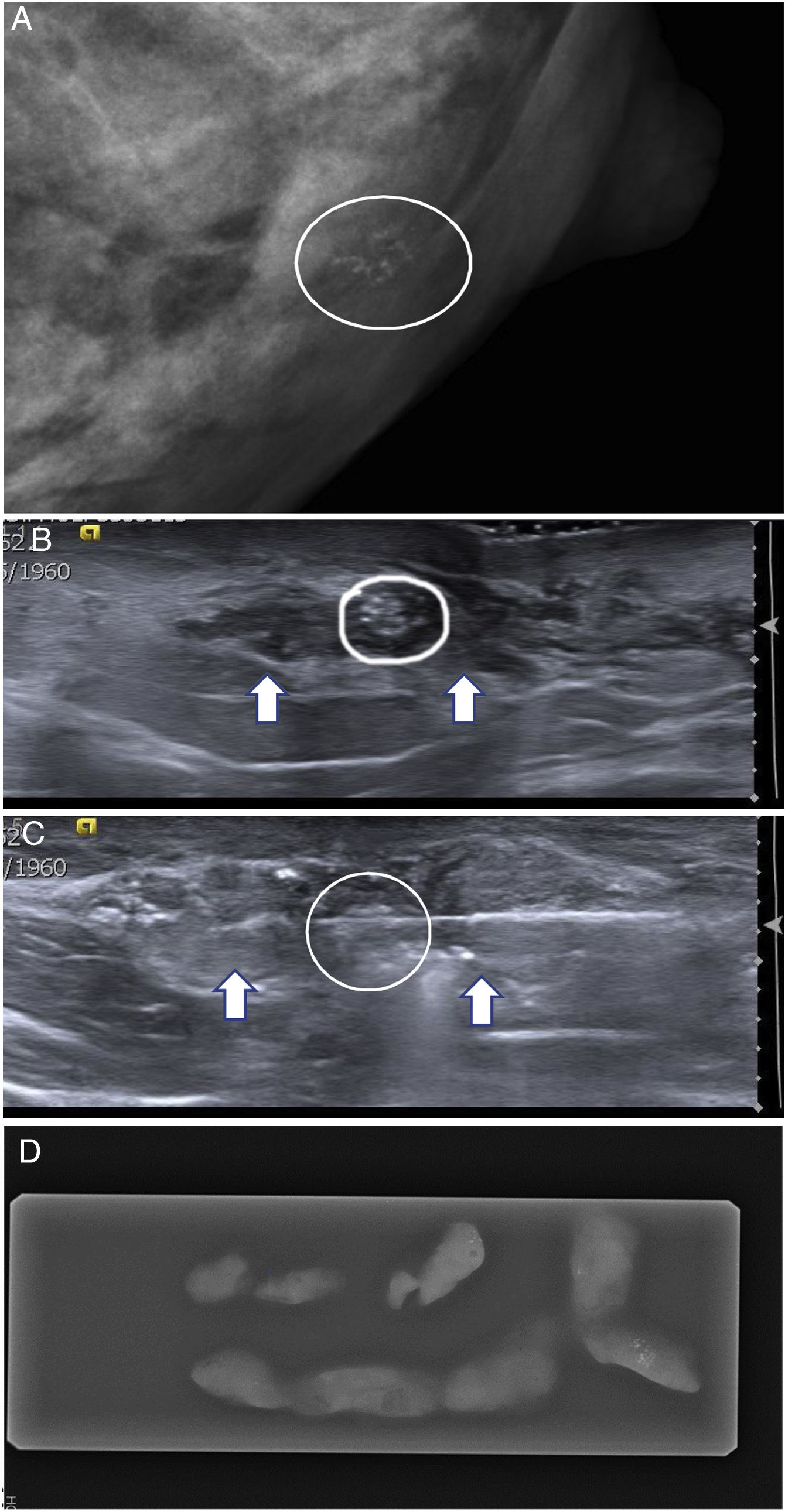
(A–D) Use of ultrasound-guided vacuum-assisted biopsy (VAB) of microcalcifications that were difficult to biopsy with conventional stereotactic biopsy. A 54-year-old patient. (A) Screening mammography detected an accumulation of retroareolar microcalcifications (circle) in the left breast, very close to the nipple, with a BI-RADS 4A nonspecific appearance. (B) On targeted ultrasound they corresponded to a small retroareolar echogenic nodule (circle), adjacent to the nipple (+). Their detection on ultrasound was assisted by said accumulation’s location in an area with a hypoechogenic appearance (arrows). (C) Ultrasound-guided VAB was performed using a needle with a calibre of 10 G. The VAB needle was visualised at the time of aspiration (arrows). (D) An X-ray of the cylinders confirmed the presence of the accumulation (circle). The histology result confirmed its benignity.
Another indication in which VAB could be useful that is being studied at present is the possibility of screening patients with BC who have achieved complete disease response after primary systemic therapy (PST). If these patients could be screened with VAB of the carcinoma bed, subsequent surgery could be avoided. Preliminary results have estimated a rate of false negatives of around 10%, corresponding to a negative predictive value for VAB of close to 90%.9
Therapeutic techniquesVacuum-assisted biopsy with excisional intentHistological B3 benign lesionsVAB with excisional intent is the most cost-effective way to manage patients with histological B3 lesions, which make up a heterogeneous group of lesions of uncertain potential for malignancy (with an increased risk of association with malignancy) diagnosed with CNB.7,10,11
B3a lesions are benign, but they sometimes develop foci of atypical ductal hyperplasia (ADH) or of BC. This means that there may be a sampling problem with CNB and that lesions may be underestimated histologically. The most common ones are benign papillomas (BPs) without atypia and complex sclerosing lesions (CSLs) without atypia (Fig. 4A, B). Rates of underestimation of carcinoma with CNB are 2%–28% in CSLs and 9%–3% in BPs.12–14 Most BPs and CSLs are small (less than 2 cm) and visible on ultrasound. In these cases, excision with VAB constitutes the most suitable management.7
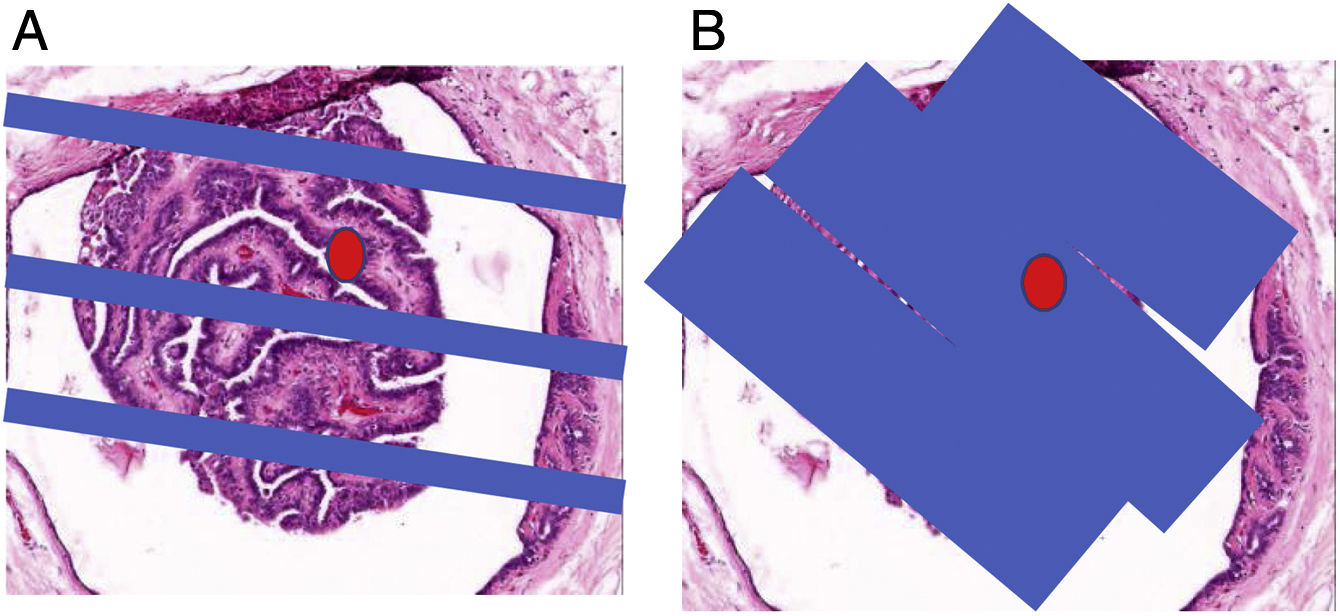
(A, B) Use of excision with ultrasound-guided VAB of histological B3 lesions (papillomas and complex sclerosing lesion [CSL]). Histological slice × 100 (haematoxylin–eosin) of a small papilloma. The red dot corresponds to a focus of papillary carcinoma. (A) The blue bands represent the CNB cylinders. As can be seen, the area of the carcinoma was not diagnosed using CNB. In our experience, up to 5% of benign papillomas (BPs) diagnosed using CNB show areas of papillary carcinoma when their excision is completed with VAB. In this regard, CSLs are similar, although in our experience underestimation occurs at a lower rate with CNB, 2.7%. (B) The blue bands represent the VAB cylinders that spanned nearly the entire BP; hence, we refer to “excision”, even though we know that unexcised lesion areas may remain. Our experience is that through excision with VAB their histological characterisation can be completed and the focus of papillary carcinoma can be diagnosed in 99.99% of papillomas (two false negatives with VAB in 11 years in 366 papillomas excised with VAB) and in 100% of CSLs (no false negatives in eight years in 52 CSLs excised with VAB).
B3b lesions are considered premalignant and carry a higher risk of developing BC than B3a lesions. They include atypical intraductal epithelial proliferation (ADH, squamous epithelial atypia, columnar cell hyperplasia with atypia and sclerosing adenosis with atypia) and lobular neoplasms (atypical lobular hyperplasia and lobular carcinoma in situ). In this group, traditional management has consisted of surgical excision. While a personalised approach should be taken in each case, VAB is an alternative to surgery in cases in which the entire lesion can be excised. If VAB were to confirm that the lesion is not associated with BC, the patient could be managed with follow-up.7
Next, protocols for management by means of excision with VAB of the most common B3 lesions, BPs and CSLs, will be reviewed.
Percutaneous management with vacuum-assisted biopsy of patients with papillomasA. Indications
- 1.
Lesions with ultrasound findings characteristic of small benign papillomas, BI-RADS 3
Excision with VAB, without prior CNB, can be initially planned. It must be borne in mind that most patients present with discharge from a single orifice and that this symptom, together with ultrasound findings, renders a diagnosis of BP highly likely (Fig. 5A–F).14
- 2.
BI-RADS 4 benign papillomas previously characterised with core-needle biopsy
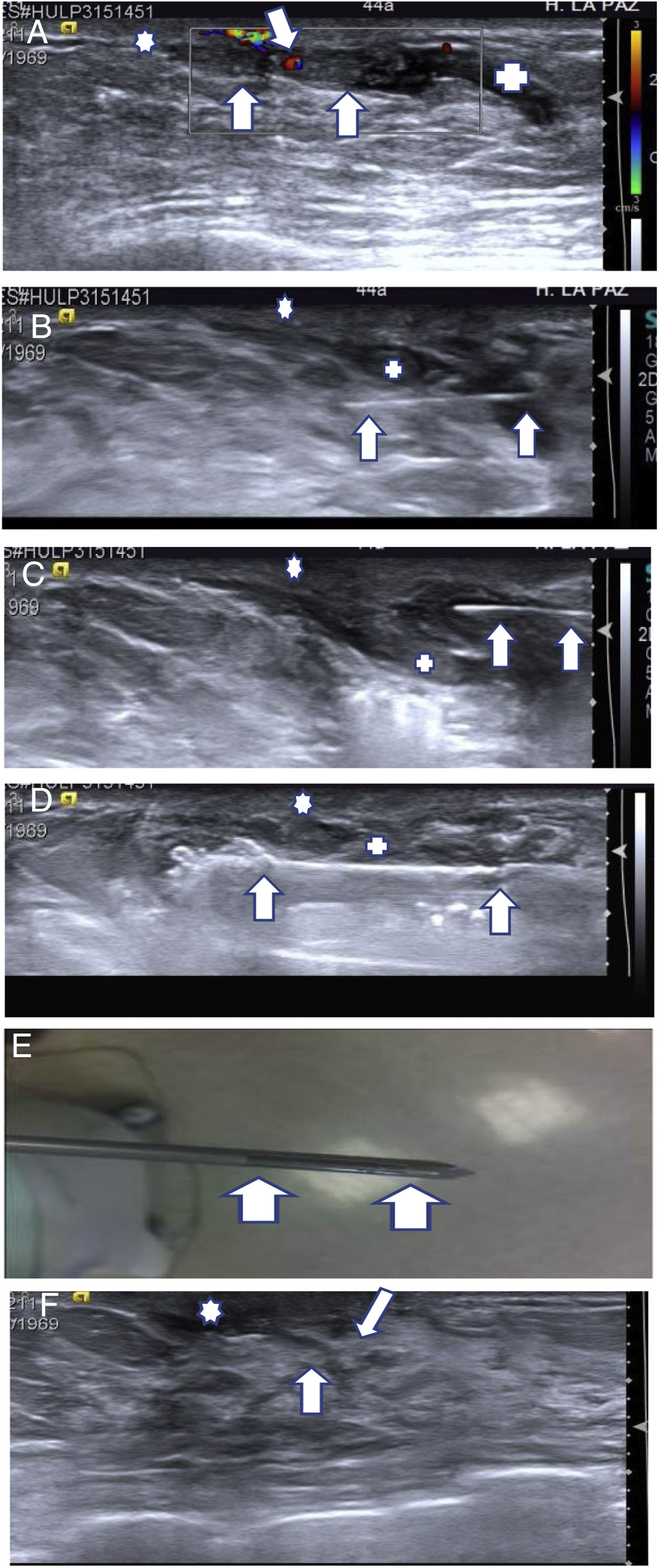
(A–F) Excision using ultrasound-guided VAB of a probable benign papilloma (BP) associated with discharge and ultrasound-follow-up. (A) Ultrasound imaging characteristic of BP. A 44-year-old patient seen for discharge from a single orifice from the right breast. Mammography showed no significant findings. Targeted ultrasound showed a dilated secretory duct (+) with a solid intraductal lesion with colour Doppler signal (arrows), measuring 1.5 mm × 7 mm, in the retroareolar region, close to the nipple (*), consistent with a BP (BI-RADS 3). In these cases we perform excision with VAB given that both the ultrasound and clinical findings (discharge) are consistent with a BP with near-complete certainty. (B–E) VAB excision technique. (B) First, we insert the thin (25-G) needle (arrows) under the lesion (+) to anaesthetise the entire trajectory that the VAB needle will follow, from the anterior part of the lesion to the skin. (C) We then insert the needle (arrows) over the lesion (+) to anaesthetise the superior region and in this case separate it a bit from the skin; however, as can be seen in the following figure, the anaesthesia quickly diffuses and the lesion is scarcely separated from the skin. In this case, it would be a good idea to use hyaluronic acid to separate the papilloma from the skin. (D) After waiting five minutes for the anaesthesia to take effect, we insert the VAB needle, placing the aspiration window under the papilloma. The window can be seen before aspirating through the small notch at the tip of the needle (arrows). When we have confirmed that the needle window is under the papilloma, we aspirate until we can no longer see it. We can then turn the window and aspirate both sides of the papilloma, spanning 180°, to minimise the risk of leaving any residual lesion. (E) Aspiration window of a 10-G VAB needle (arrows). The size and shape of the window (22 mm in length × 3 mm in thickness) are well-suited to those of most BPs. In larger papillomas, we use needles with a calibre of 7 G. (F) One-year follow-up ultrasound. Small scar (arrows) in the excision region, without signs of relapse. The patient reported that she no longer had discharge after VAB.
These BPs present on ultrasound as non-specific solid nodules or nodules with findings characteristic of papillary lesions, but do not allow papillary carcinoma to be ruled out owing to their size and shape. If the BP is of a size suited to percutaneous management — less than around 2–3 cm (most are) — then excision with VAB can be planned.14
Excision of papillomas achieves two objectives: first, it completes their histological characterisation, and second, it eliminates the symptom of discharge (which is present with approximately two-thirds of papillomas). If a result of papillary carcinoma is obtained after VAB, it is a good idea to place a marker in the biopsy bed to guide subsequent surgery.14
B. Follow-up and salvage vacuum-assisted biopsy
For the first two years after VAB, follow-up is performed with ultrasound every six months as relapses occur in this period (at a rate of around 15%).14 Although magnetic resonance imaging (MRI) is the most sensitive imaging method for the follow-up and diagnosis of papillary lesions, it has less availability than ultrasound. Most authors use ultrasound as a follow-up technique.14–16 Relapses can be treated with another (“salvage”) VAB such that percutaneous management can be continued and surgery avoided (Fig. 6A, B).14
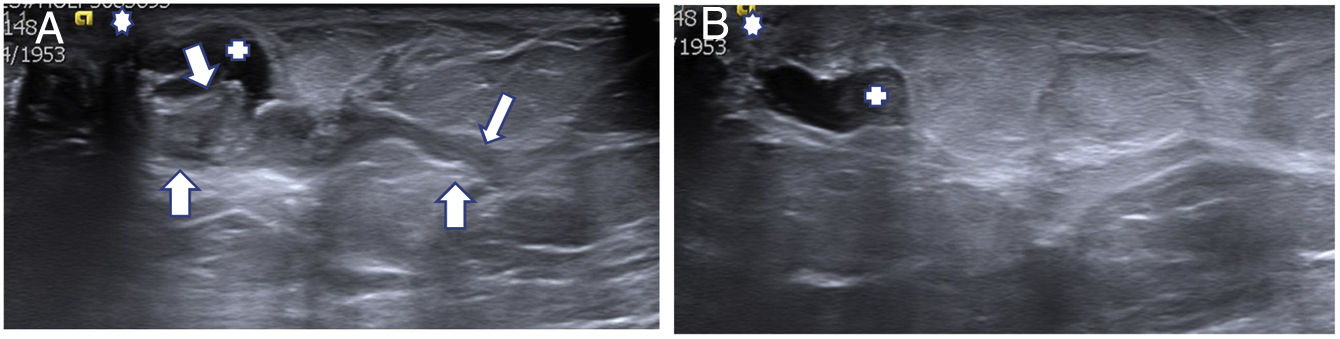
(A, B) Relapse of a benign papilloma (BP) excised with vacuum-assisted biopsy (VAB). A 60-year-old patient with discharge from a single orifice from the left breast. (A) Targeted ultrasound showed a probable BP (29 mm × 4 mm) larger than in the previous example (arrows). Secretory duct (+). Nipple (*). Excision was performed with VAB and a diagnosis of BP was confirmed. The discharge stopped after the procedure. (B) The patient’s 22-month follow-up ultrasound detected a probable relapse measuring 4 mm (+) that was excised with further VAB (which we termed “salvage”) and diagnosed as BP. The patient remained asymptomatic, without discharge. No relapses were detected in subsequent follow-up.
Some authors have defended conservative management of BPs diagnosed with CNB in asymptomatic patients (without discharge) as a result of not finding cases of carcinoma in follow-up.17–19 In our experience, up to 5% of BPs diagnosed with CNB that are excised with VAB present foci of carcinoma. Therefore, we recommended that they be excised with VAB.14
Percutaneous management with vacuum-assisted biopsy of patients with complex sclerosing lesionsA. Indications
- 1.
Complex sclerosing lesion that presents as architectural distortion visible on mammography or tomosynthesis
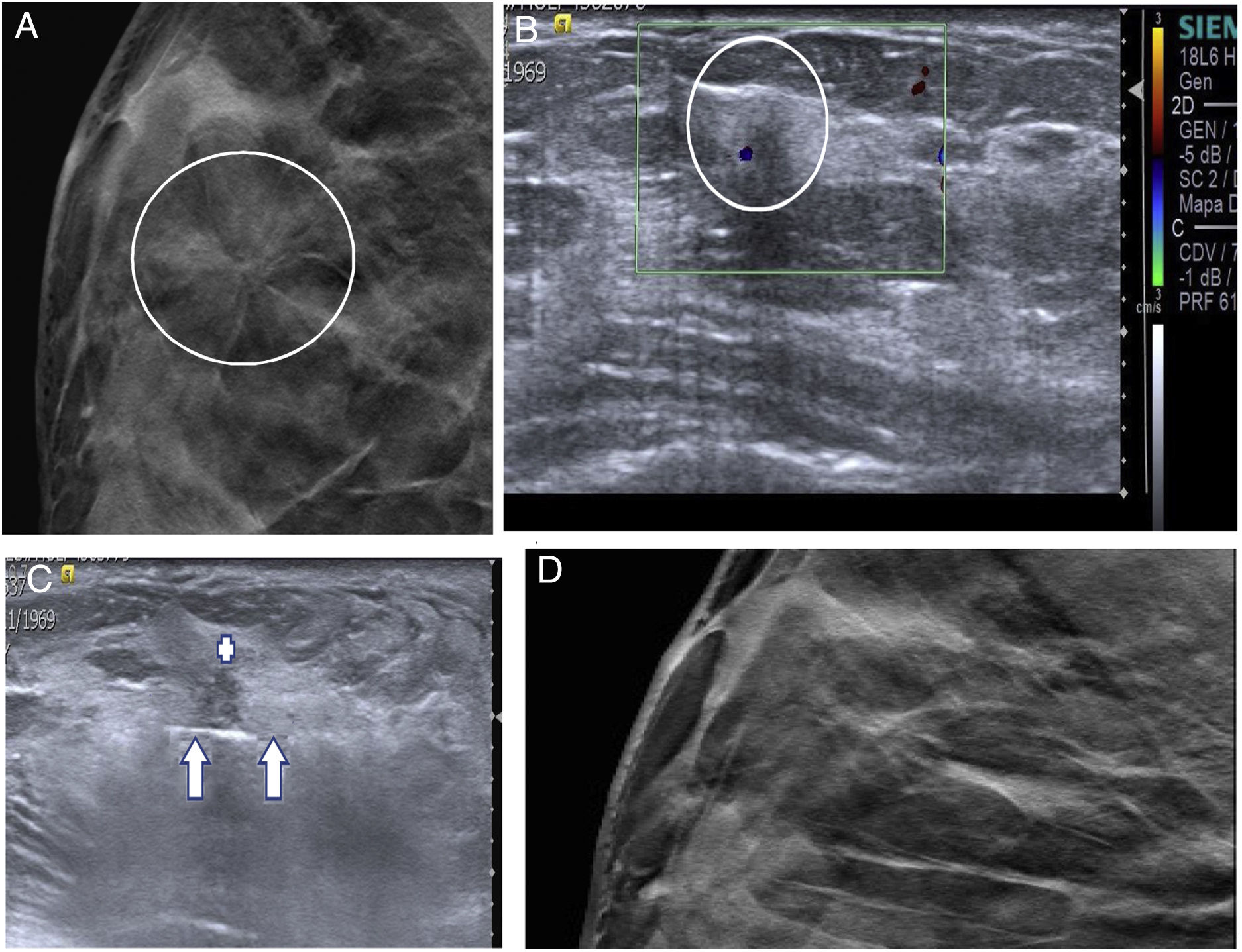
Architectural distortion is an uncommon BI-RADS 4B finding. Most are non-palpable and are subtle findings on mammography (sometimes they are only visible on tomosynthesis). Approximately 30%–40% are BC and the other 60%–70% are benign lesions (mostly CSLs). They are usually detected by means of targeted ultrasound; hence, they can be characterised with CNB20 (Fig. 7A–D).
- 2.
Complex sclerosing lesion that presents as a BI-RADS 4 nodule visible on ultrasound
(A–D) Excision with ultrasound-guided vacuum-assisted biopsy (VAB) of a complex sclerosing lesion (CSL). A 47-year-old patient. Screening mammography detected architectural distortion (AD) in the outer quadrants of the right breast. (A) Complementary tomosynthesis was performed which enabled us to localise it in the upper outer quadrant (circle). (B) Subsequently, targeted ultrasound detected a solid lesion measuring 4 mm with colour Doppler signal that corresponded to the AD (circle). CNB was done and CSL without atypia was diagnosed. (C) Excision was performed with ultrasound-guided VAB. The VAB needle (arrows) is seen under the CSL (+), prior to its aspiration. The VAB result confirmed the diagnosis of CSL without atypia. (D) One-year follow-up tomosynthesis in which the distortion is no longer visible. Ultrasound showed no significant findings.
Around 15%–20% of CSLs present as small, non-specific nodules that cannot be palpated but are visible on ultrasound.21
In both cases, once the CSL has been characterised with CNB, excision with VAB can be planned with the goal of confirming the diagnosis and avoiding histological underestimation.11
B. Follow-up
These patients are usually followed up with mammography/tomosynthesis and ultrasound on a yearly basis. Some authors have defended CSL follow-up after diagnosis with CNB, as rates of underestimation of carcinoma are very low (in our experience, 2.7%).21,22
FibroadenomasExcision of FAs by means of VAB as an alternative to surgery is indicated for FAs measuring less than 2 cm. When they are larger than that, percutaneous excision with VAB can be considered, although the odds of incomplete resection are higher — as high as 67.5% in lesions around 4–5 cm.23
Breast carcinomaPercutaneous excision of BC by means of VAB is not indicated in the clinical guidelines.23 Published studies have found rates of residual carcinoma after VAB ranging from 62.7% to 100%. In cases of BC measuring less than 10 mm, rates of complete resection of 65% can be achieved.24
Percutaneous ablationSeveral percutaneous techniques destroy breast lesions without excising them: cryoablation, radiofrequency, microwave ablation, high-intensity focused ultrasound, laser therapy and irreversible electroporation. The only one that has been approved by the United States Food and Drug Administration (FDA) with a level of evidence of A (indicated in clinical practice) is cryoablation for the treatment of fibroadenomas. BC treatment by means of cryoablation or radiofrequency has not been approved by the FDA, but each has a level of evidence of B (relatively common use in clinical practice with quite a few published studies in the literature). All the other techniques have lower levels of evidence, and some of them are experimental; hence, they will not be discussed.23
CryoablationCryoablation is a percutaneous ablation method whose mechanism of action consists of generating extremely low temperatures around the needle, which cause cell destruction due to coagulative necrosis. The temperature should be at least as low as −20 °C to achieve ablation. There are two types of cryoablation devices: those that use liquid nitrogen and those that use argon gas. Liquid nitrogen devices require higher-calibre needles (10 G and 12 G) compared to argon gas devices (17–14 G). The most significant advantage of cryoablation versus all the other ablation techniques is that it is done on an outpatient basis with local anaesthesia.23 It takes 45−60 min. For it to be duly effective, it must be done in three phases (with a duration ranging from 5 to 10 min): an initial freezing phase, followed by a passive heating phase and finally another freezing phase. The tissue located at the centre of the lesion, adjacent to the cryoablation needle, undergoes direct coagulative necrosis due to the extreme temperatures generated — as low as −140 °C. The goal of the three-phase process is to destroy the cells on the periphery of the ice ball where the temperatures hovers around −20 °C. In the first phase, the cell undergoes a dehydration process, since the first thing that happens is that the extracellular space freezes; this results in a change in osmotic pressure that draws the water out of the cytoplasm. In the second phase, passive heating, the osmotic gradient is reversed and there is a massive influx of fluid into the cytoplasm, resulting in cell swelling and rupture. In the third phase, freezing, the ablated volume is enlarged, since the tissue that became necrotic in the previous phases handles the cold better.25
The procedure should be planned in advance. If the lesion to be treated is BC, needle(s) that exceed the contours of the lesion by at least 10 mm must be selected, since the minimum temperature required for tissue necrosis (–20 °C) is reached at around 5 mm within the boundaries of the ice ball. In fibroadenomas, the capsule need not be exceeded. Once the procedure has been planned, the needle(s) are inserted along the longest axis of the lesion. The growth of the ice ball can be monitored in real time and it can be ensured that a sufficient margin is reached (Fig. 8A–J).
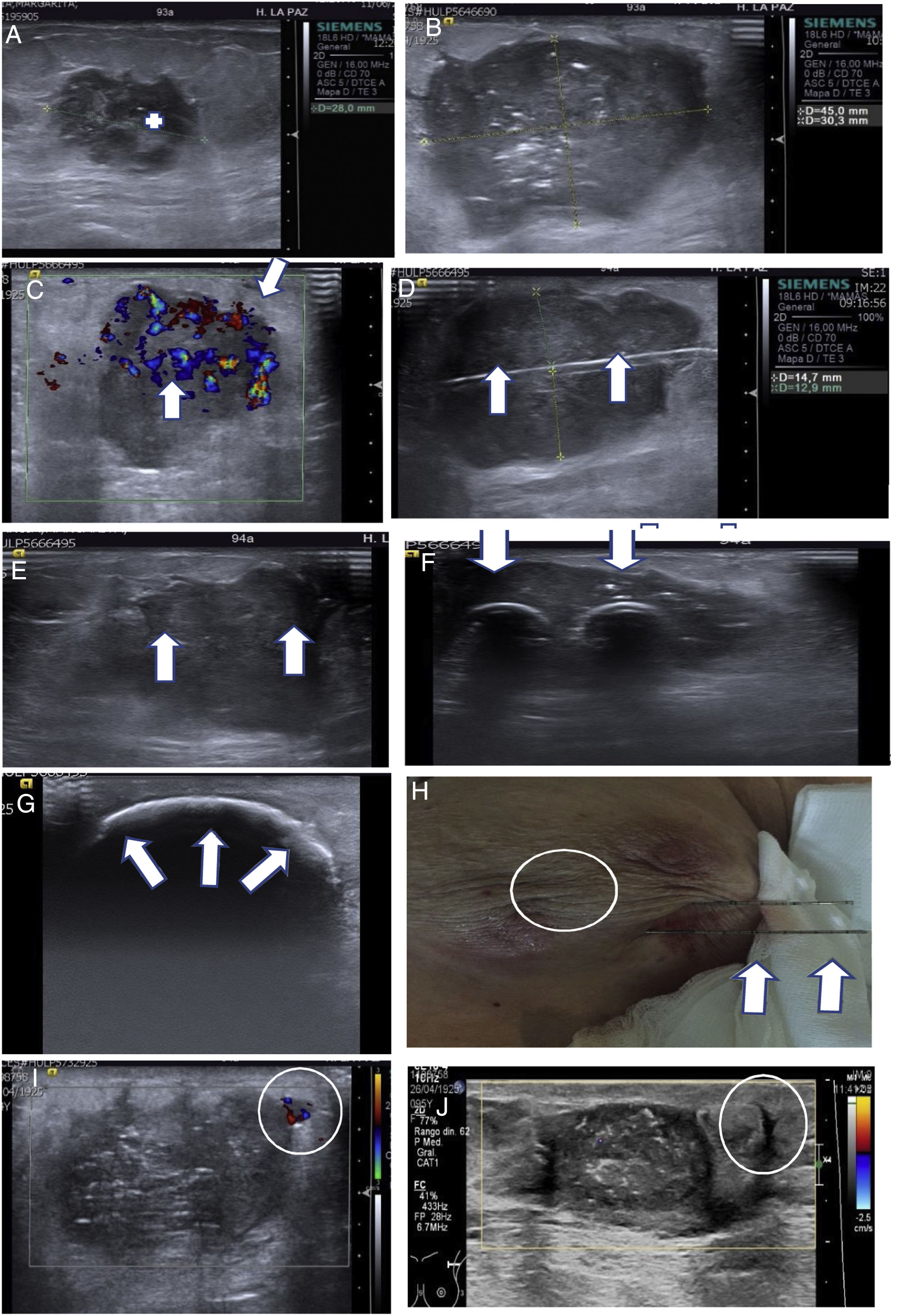
(A–J) Treatment of breast carcinoma (BC) using ultrasound-guided cryoablation. A 93-year-old patient with a palpable lesion in the left breast. (A) Ultrasound confirmed that the palpable lesion corresponded to a solid lesion with irregular borders, measuring 28 mm, consistent with BC (+). CNB was performed with a diagnosis of triple-negative (OR–, PR– and HER2–), androgen receptor 90%, Ki 70% infiltrating breast carcinoma. Due to comorbidity, she was not a candidate for surgery and was treated with hormone therapy (antiandrogen therapy). (B, C) A follow-up ultrasound after five months of antiandrogen treatment confirmed that the BC had enlarged to 45 mm. (C) Marked colour Doppler signal (arrows) indicating abundant vascularisation in the tumour can be seen. (D–H) Ultrasound-guided cryoablation. The use of several needles makes it possible to achieve ablation volumes that can treat large lesions. In this case, two IcePearl needles were used. (D) Ultrasound on the longitudinal plane of the BC showing one of the needles (arrows). (E) Ultrasound on transverse plane showing the two needles (arrows). (F) Ultrasound on transverse plane showing the initial advance of the ice ball (echogenic border) around the needles (arrows). (G) Ultrasound on transverse plane showing the formation of a large ice ball at the end of the freezing period spanning the entire lesion (arrows). (H) Small burn due to freezing on the skin (circle). When the ice ball comes into contact with the skin, it causes a mild burn. We usually place a bag of warm saline to minimise the effects of freezing. The two needles used can be seen parallel to each other (arrows). (I) A three-month follow-up ultrasound showed a peripheral nodule measuring 5 mm with colour Doppler signal corresponding to a small residual lesion (circle). A second cryoablation of the residual lesion was performed that we termed “salvage” cryoablation (as in “salvage” vacuum-assisted biopsies in B3 lesions). J) Follow-up ultrasound a year after the second cryoablation. It can be seen that the treated lesion is smaller and has no colour Doppler signal. The echogenic nodule (circle) corresponded to the residual lesion treated with the second cryoablation.
Breast cryoablation is not associated with significant complications. Mild or moderate haematomas and burns due to freezing may develop on the skin in adjacent lesions. Burns are usually mild (Fig. 8H).
It is a highly effective treatment option for FAs (up to 5 cm), with very little morbidity and very good aesthetic outcomes.23,26
It is also a treatment option in patients with BC and comorbidities that may complicate general anaesthesia or contraindicate surgery, especially elderly patients and patients with BC or other stage-4 tumours with good management of metastatic disease.27–29
At present, two phase-3 clinical trials are assessing cryoablation as a replacement for surgery in small tumours, low-grade tumours and tumours not associated with intraductal carcinoma.23 The preliminary results of one of them suggest that cryoablation will be offered to these patients as an alternative treatment to surgery.30
RadiofrequencyRadiofrequency is indicated in the treatment of BC in patients with a clinical context identical to those of cryoablation. It was the first ablation technique to be developed, in the 2000s.23 Its mechanism of action consists of generating an alternating current between the electrode and a grounding pad adhered to the patient, thus creating ion movement in the adjacent tissue at the active end of the electrode. The ion movement causes an increase in temperature due to friction. The temperature rising above 50 °C–60 °C causes liquefactive tissue necrosis. Although it has effectiveness similar to cryoablation, it carries the drawbacks of being very painful and requiring sedation or general anaesthesia. Skin burns may be serious, unlike burns due to freezing in cryoablation.31
Percutaneous drainage of abscessed mastitisInfectious mastitis presents as a painful area of the breast with erythematous changes in the skin that usually responds well to empirical antibiotic treatment. The type of bacteria most commonly responsible for the infection is Staphylococcus aureus. In some cases, mastitis becomes abscessed. Abscesses exceeding 3 cm are usually managed with surgical drainage. Radiologists can decrease the morbidity of surgical treatment (close to 50% of patients are young breastfeeding women) by means of ultrasound-guided percutaneous drainage.
If abscesses are less than 2−3 cm, they can be drained by FNAB. Microbiological study is required to confirm that the antibiotic treatment initiated is correct. A pigtail drainage catheter or an Abbocath catheter can be inserted into larger abscesses to drain them32 (Figs. 9 and 10A–D).
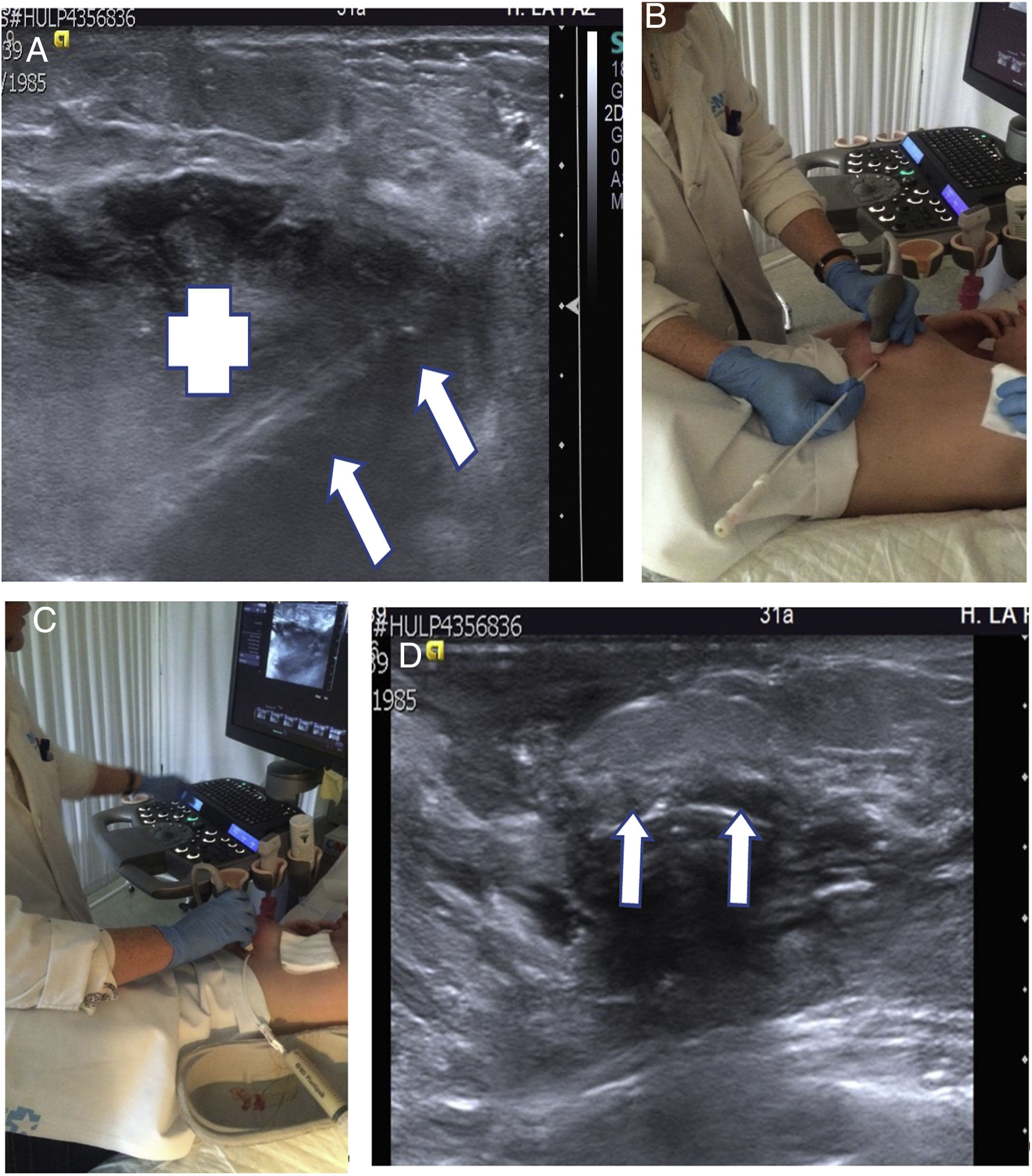
(A–D) Ultrasound-guided percutaneous drainage of abscessed mastitis. A 31-year-old breastfeeding patient visited the accident and emergency department due to a painful palpable mass with signs of skin inflammation. (A) Ultrasound slice on the longitudinal plane. A hypoechoic collection corresponding to an abscess can be seen (+). The drainage catheter is identified inside (arrows). (B, C) Percutaneous drainage technique. (B) The catheter is inserted with ultrasound guidance, perpendicular to the chest wall. (C) The catheter has been inserted into the abscess and the pus has been extracted (see syringe). (D) Longitudinal ultrasound slice showing the catheter in the abscess bed which has been completely drained (arrows).
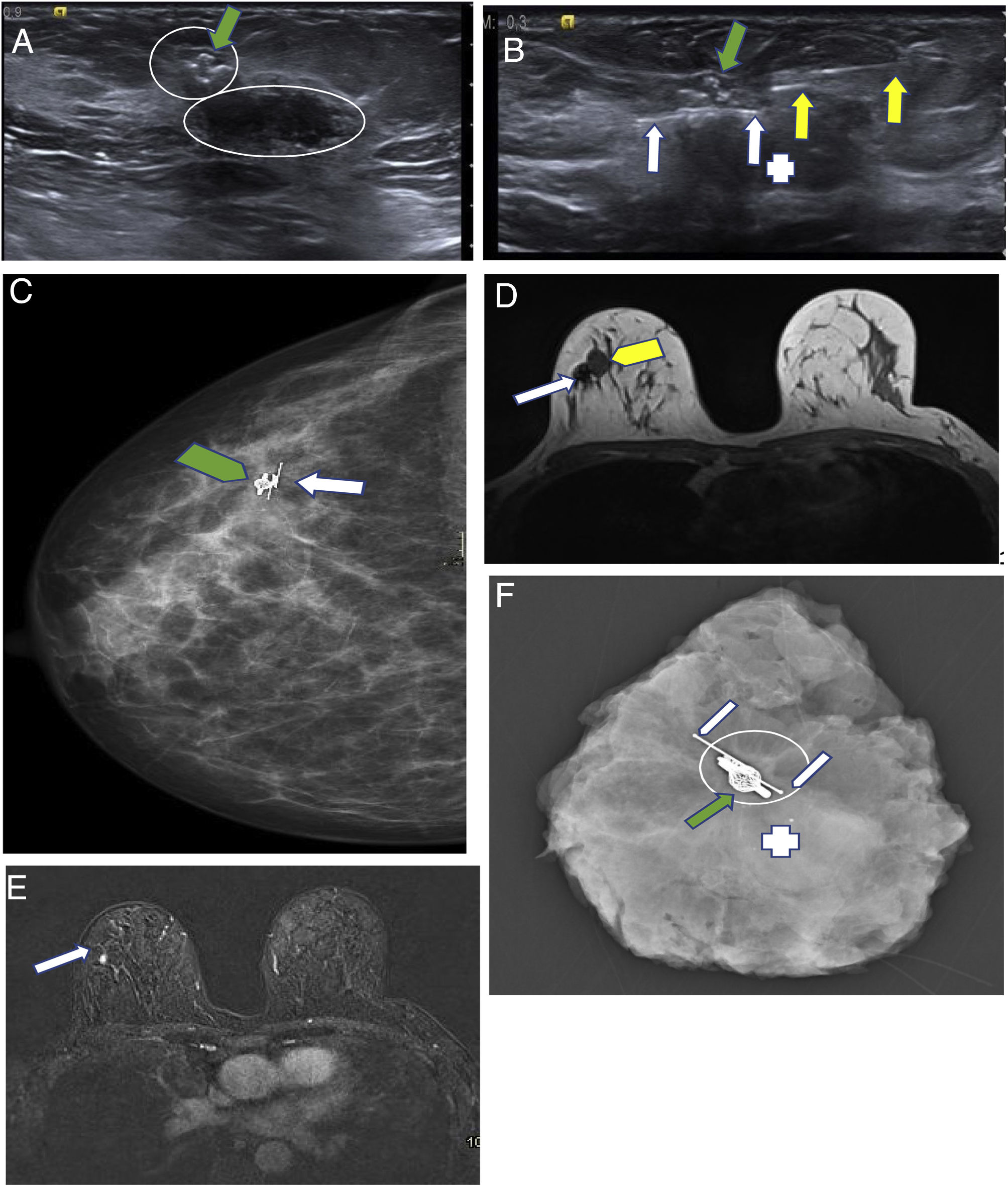
(A–F) Savi Scout reflector. The advantage of this seed over others is that it can be placed at the time of diagnosis, like a conventional marker, since it does not generate artefacts on magnetic resonance imaging (MRI). Thus, it spares expenses by acting as both marker and seed. A 49-year-old patient. Triple-negative breast carcinoma (BC). Ki 25% in upper outer quadrant of the right breast. It was treated with neoadjuvant chemotherapy. A marker was initially placed in the BC bed. (A) Ultrasound slice on the longitudinal plane of the lesion after six months of treatment. The metal marker that was initially placed is seen (green arrow). The residual lesion is visualised as the small hypoechogenic nodule that surrounds it (small circle). An adjacent fibroadenoma can be seen (large circle). (B) Ultrasound slice on the longitudinal plane during Savi Scout placement (white arrows). Insertion needle (yellow arrows). Marker (green arrow). Fibroadenoma (+). (C) Right mammography in a craniocaudal projection showing the marker (green arrow) and the Savi Scout (white arrow). (D–E) MRI to assess response to primary systemic therapy. It was performed after placing the Savi Scout. (D) Anatomical sequence on axial plane (T1). The small artefact created by the marker and the Savi Scout is seen (white arrow). Fibroadenoma (yellow arrow). (E) Dynamic subtraction sequence on axial plane at the same height as the prior image. The slight enhancement related to the residual BC (arrow) can be seen without the Savi Scout artefact or the marker interfering with the assessment thereof. The fibroadenoma shows no enhancement. (F) Mammography of the surgical specimen. The marker (green arrow) and the Savi Scout (white arrows) can be seen with the residual BC (circle) and the fibroadenoma (+).
Around 70% of cases of BC managed with primary systemic therapy show a complete response on imaging. Despite this, the carcinoma bed must be surgically excised, since residual carcinoma is detected in the surgical specimen in up to 30% of these patients.33,34 For this reason, before treatment, a marker (or multiple markers, if the lesion is extensive) should be placed in order to delimit the carcinoma bed in cases of a complete response on imaging.
Patients with limited axillary involvement who show normalisation of their metastatic lymph node(s) after primary systemic treatment are usually managed conservatively. The sentinel lymph nodes are excised, and should a low tumour load or no tumour load be demonstrated intraoperatively, then the lymphadenectomy is not completed. In these patients it is necessary to mark the metastatic lymph node previously examined with FNAB or CNB to confirm that it is one of the excised sentinel lymph nodes.
There are many types of markers. All of them are visible with mammography. However, what is most important is that the marker is visible on ultrasound, since this greatly facilitates preoperative localisation (it is much quicker with ultrasound than with mammography). In addition, some areas of the breasts (close to the pectoral region and in the axillary tail of Spence) and lymph nodes can only be localised with ultrasound.
Marking to guide breast and axillary surgery- A.
Breast
For non-palpable BC to be surgically excised, its exact location must be marked to guide the surgeon. Most non-palpable cases of BC are visible on ultrasound. There are several ultrasound-guided preoperative localisation techniques: sentinel node and occult lesion localisation (SNOLL), hookwire localisation and seed localisation.1,35,36
SNOLL
This is a technique that requires cooperation with nuclear medicine and radiology. The day before surgery, the radioactive tracer (technetium-99m albumin colloid) is inserted into the BC bed using ultrasound-guided puncture. This radioactive tracer consists of a macrocolloid that remains stationed in the BC and another smaller colloid that enters the lymphatic circulation and reaches the sentinel lymph node. Thus, the BC and the sentinel lymph node can be localised in the operating theatre using a scintigraphic probe.1,35
Hookwire
On the day of surgery, ultrasound-guided puncture is used to insert a needle such that it crosses the BC bed, and through this a very thin metal guide that comes out the through the skin is released. The guide has an angled end (most are harpoon-shaped) such that it can be lodged in the lesion. Thus the surgeon advances the guide up to the distal end where the BC bed is located.1
Seeds
Seeds are small markers that are inserted into the lesion by percutaneous puncture and that can be detected by various methods depending on seed type.
The first ones to be developed were radioactive (iodine 125) seeds detected using a scintigraphic probe in a manner similar to SNOLL radioactive tracers. After that, magnetic seeds and radiofrequency reflectors were developed. The magnetic seed is a paramagnetic steel marker. Radiofrequency reflectors include the Savi Scout and the LOCalizer. Both magnetic seeds and radiofrequency reflectors require specific probes to localise them in the operating theatre.36
- B.
Axilla
Seeds are used to guide lymph node excision.36
The most cost-effective procedure in non-palpable BC surgery or sentinel lymph node surgery is SNOLL. It optimises operating time and radiologist time better than hookwires and is much more affordable than seeds. Nevertheless, each hospital will use the technique that it deems most appropriate. Magnetic seeds can be comfortably inserted, since the calibre of the insertion needle is 18 G. The LOCalizer has the drawback that the calibre of the insertion needle is 12 G and thus is more uncomfortable to use in the axilla. Both create MRI artefacts that limit imaging assessment, and therefore should not be placed before primary systemic treatment. The Savi Scout has two advantages: on the one hand, the calibre of the insertion needle is 16 G (such that it can be comfortably inserted into the breast and axilla); on the other hand, it can be placed before MRI, since it does not produce artefacts. This characteristic means that it can also be used as an initial marker, before primary systemic treatment. It is a marker that can also act as a seed, thus saving radiologist time, reducing patient morbidity (with one puncture versus two) and limiting expenses (with one device versus two) (Fig. 10A–F).36
Marking suspicious findings on mammography using targeted ultrasoundIn some cases, findings on mammography that must be biopsied have very subtle manifestations on ultrasound. If ultrasound-guided biopsy is to be performed, it must be ensured that these ultrasound findings correspond to the mammography findings. A 21-G needle can be inserted into the ultrasound findings, and then, with the needle inserted into the findings, one or two mammography projections can be taken to confirm whether they match.
Conflicts of interestThe author declares that he has no conflicts of interest.
Please cite this article as: Oliver Goldaracena JM. Intervencionismo ecográfico en imagen mamaria. Radiología. 2022;64:76–88.







![(A, B) Use of excision with ultrasound-guided VAB of histological B3 lesions (papillomas and complex sclerosing lesion [CSL]). Histological slice × 100 (haematoxylin–eosin) of a small papilloma. The red dot corresponds to a focus of papillary carcinoma. (A) The blue bands represent the CNB cylinders. As can be seen, the area of the carcinoma was not diagnosed using CNB. In our experience, up to 5% of benign papillomas (BPs) diagnosed using CNB show areas of papillary carcinoma when their excision is completed with VAB. In this regard, CSLs are similar, although in our experience underestimation occurs at a lower rate with CNB, 2.7%. (B) The blue bands represent the VAB cylinders that spanned nearly the entire BP; hence, we refer to “excision”, even though we know that unexcised lesion areas may remain. Our experience is that through excision with VAB their histological characterisation can be completed and the focus of papillary carcinoma can be diagnosed in 99.99% of papillomas (two false negatives with VAB in 11 years in 366 papillomas excised with VAB) and in 100% of CSLs (no false negatives in eight years in 52 CSLs excised with VAB). (A, B) Use of excision with ultrasound-guided VAB of histological B3 lesions (papillomas and complex sclerosing lesion [CSL]). Histological slice × 100 (haematoxylin–eosin) of a small papilloma. The red dot corresponds to a focus of papillary carcinoma. (A) The blue bands represent the CNB cylinders. As can be seen, the area of the carcinoma was not diagnosed using CNB. In our experience, up to 5% of benign papillomas (BPs) diagnosed using CNB show areas of papillary carcinoma when their excision is completed with VAB. In this regard, CSLs are similar, although in our experience underestimation occurs at a lower rate with CNB, 2.7%. (B) The blue bands represent the VAB cylinders that spanned nearly the entire BP; hence, we refer to “excision”, even though we know that unexcised lesion areas may remain. Our experience is that through excision with VAB their histological characterisation can be completed and the focus of papillary carcinoma can be diagnosed in 99.99% of papillomas (two false negatives with VAB in 11 years in 366 papillomas excised with VAB) and in 100% of CSLs (no false negatives in eight years in 52 CSLs excised with VAB).](https://static.elsevier.es/multimedia/21735107/0000006400000001/v2_202202200527/S2173510722000258/v2_202202200527/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)







