Breast implants are associated with well-known common complications that have been widely studied, such as rupture and capsular contraction. However, the increasingly growing number of patients with breast implants has led to the increased likelihood of coming across less common complications; these include seromas or late infection; adenopathies in the internal mammary chain; granulomas in the capsule of the implant, which in some cases can extend beyond the fibrous capsule; desmoid tumours associated with the implants; and breast implant-associated large cell anaplastic lymphoma.
This article aims to review the main uncommon complications associated with breast implants and to describe and illustrate their findings in different imaging techniques. Proper management of these complications is important; this is especially true of late seroma and the diagnosis of breast implant-associated large cell anaplastic lymphoma for their repercussions.
Los implantes mamarios se asocian a complicaciones frecuentes ampliamente conocidas y estudiadas como la rotura y la contractura capsular. Sin embargo, debido al número cada vez mayor de pacientes portadoras de implantes mamarios, podemos encontrarnos con patología más infrecuente como la presencia de seroma o infección tardía, adenopatías en la cadena mamaria interna, granulomas en la cápsula del implante –que en algunos casos pueden extenderse más allá de la cápsula fibrosa–, tumores desmoides asociados a los implantes y el linfoma anaplásico de células grandes asociado a implantes mamarios. El objetivo de este artículo es revisar las principales complicaciones infrecuentes asociadas a los implantes mamarios y sus hallazgos radiológicos en las diferentes técnicas. Es importante un correcto manejo de esta patología, principalmente del seroma tardío, para diagnosticar precozmente el linfoma anaplásico de células grandes por su mayor transcendencia.
Breast implants are among the most commonly used medical devices, either for aesthetic reasons or for breast reconstruction following breast cancer surgery. The prostheses consist of a silicone covering or coverings filled with saline solution, silicone gel or both. The surface of the implant may be smooth or textured, and the shape may be rounded or anatomical. Depending on their site of placement, they may be retroglandular or retropectoral.1–4
The complications associated with breast implants may be classified as early, when they occur in the post-operative period, or late, when they occur months or years following the operation. The main early complications include infection and periprosthetic collections, and the main late complications include rupture and capsular contracture.1
Recently, more uncommon late adverse effects have been reported, some of greater significance, such as breast implant-associated anaplastic large cell lymphoma (BIA-ALCL), breast fibromatosis associated with implants, formation of granulomas secondary to silicone in the fibrous capsule, and lymphadenopathy in patients with implants, which in certain cases may represent a diagnostic dilemma.
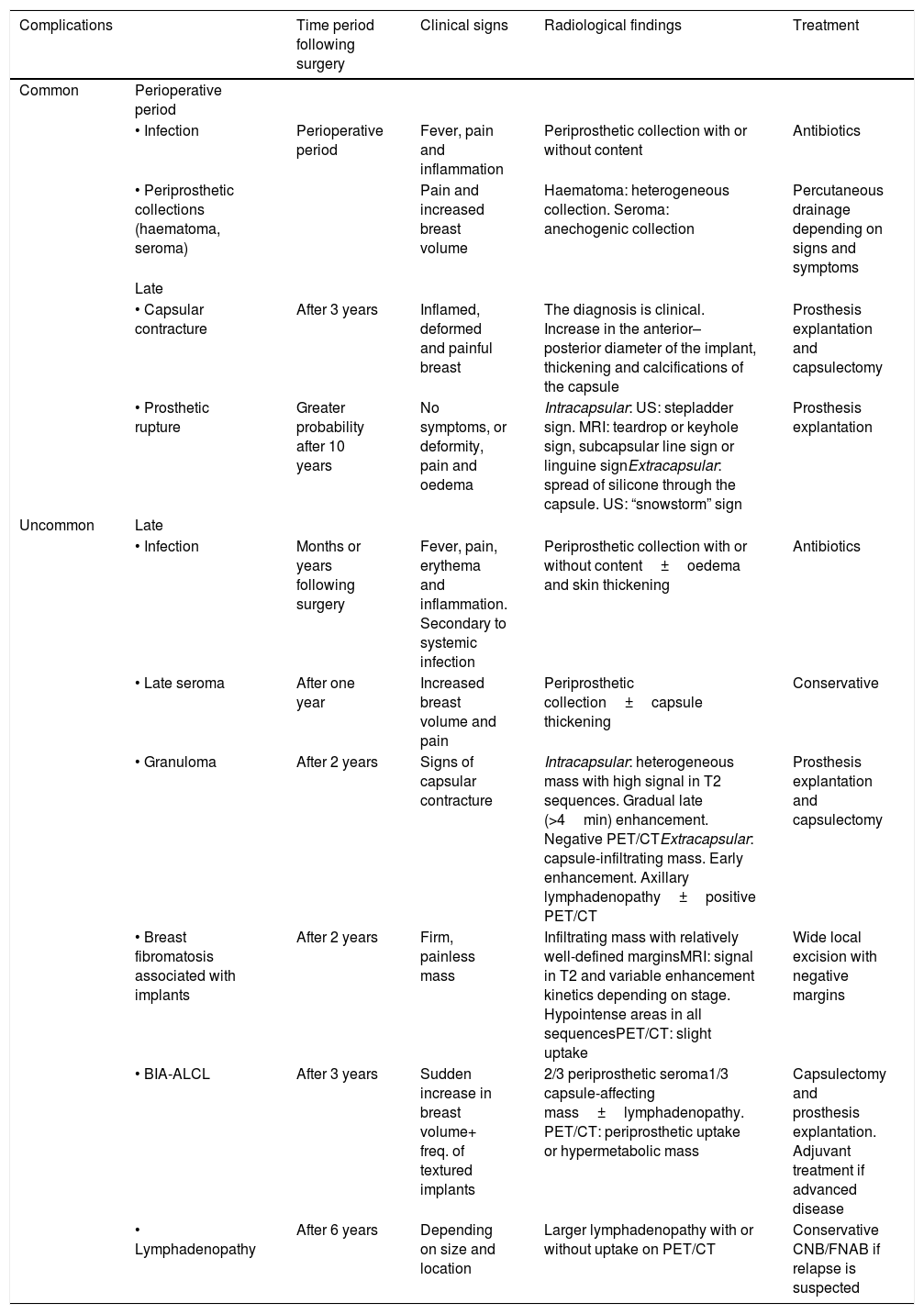
Common complications associated with breast implants have been widely studied and are known to all radiologists. This article conducts a review of uncommon pathology associated with breast implants, with a focus on radiological findings and on the clinical and radiological management of said pathology for a proper differential diagnosis (Table 1). Predictably, the prevalence of these complications will increase as a result of increased use of breast implants; therefore, knowledge and suitable management thereof are important.
Summary of complications associated with breast implants.
| Complications | Time period following surgery | Clinical signs | Radiological findings | Treatment | |
|---|---|---|---|---|---|
| Common | Perioperative period | ||||
| • Infection | Perioperative period | Fever, pain and inflammation | Periprosthetic collection with or without content | Antibiotics | |
| • Periprosthetic collections (haematoma, seroma) | Pain and increased breast volume | Haematoma: heterogeneous collection. Seroma: anechogenic collection | Percutaneous drainage depending on signs and symptoms | ||
| Late | |||||
| • Capsular contracture | After 3 years | Inflamed, deformed and painful breast | The diagnosis is clinical. Increase in the anterior–posterior diameter of the implant, thickening and calcifications of the capsule | Prosthesis explantation and capsulectomy | |
| • Prosthetic rupture | Greater probability after 10 years | No symptoms, or deformity, pain and oedema | Intracapsular: US: stepladder sign. MRI: teardrop or keyhole sign, subcapsular line sign or linguine signExtracapsular: spread of silicone through the capsule. US: “snowstorm” sign | Prosthesis explantation | |
| Uncommon | Late | ||||
| • Infection | Months or years following surgery | Fever, pain, erythema and inflammation. Secondary to systemic infection | Periprosthetic collection with or without content±oedema and skin thickening | Antibiotics | |
| • Late seroma | After one year | Increased breast volume and pain | Periprosthetic collection±capsule thickening | Conservative | |
| • Granuloma | After 2 years | Signs of capsular contracture | Intracapsular: heterogeneous mass with high signal in T2 sequences. Gradual late (>4min) enhancement. Negative PET/CTExtracapsular: capsule-infiltrating mass. Early enhancement. Axillary lymphadenopathy±positive PET/CT | Prosthesis explantation and capsulectomy | |
| • Breast fibromatosis associated with implants | After 2 years | Firm, painless mass | Infiltrating mass with relatively well-defined marginsMRI: signal in T2 and variable enhancement kinetics depending on stage. Hypointense areas in all sequencesPET/CT: slight uptake | Wide local excision with negative margins | |
| • BIA-ALCL | After 3 years | Sudden increase in breast volume+ freq. of textured implants | 2/3 periprosthetic seroma1/3 capsule-affecting mass±lymphadenopathy. PET/CT: periprosthetic uptake or hypermetabolic mass | Capsulectomy and prosthesis explantation. Adjuvant treatment if advanced disease | |
| • Lymphadenopathy | After 6 years | Depending on size and location | Larger lymphadenopathy with or without uptake on PET/CT | Conservative CNB/FNAB if relapse is suspected |
CNB: core needle biopsy; FNAB: fine needle aspiration biopsy; MRI: magnetic resonance imaging; PET/CT: positron emission tomography/computed tomography; US: ultrasound.
Infections in the recent post-operative period are among the most common complications, with an incidence of approximately 1.7%. However, late infections (months or years following surgery) are an uncommon complication with an incidence of approximately 0.8% and are usually secondary to a systemic infection.1,5–7
The incidence of infection is even greater in patients with post-mastectomy reconstruction, mainly with immediate reconstruction.5,8
It usually presents with fever, pain, erythema and inflammation. The most commonly found pathogens are those located on the skin surface, such as Staphylococcus aureus.1,5,8
If there is no improvement with antibiotic treatment, explantation is recommended. Explantation is also recommended in cases of fungal infection or if there are signs of systemic infection.1
Hypoechoic collections that may have echogenic content are found on ultrasound. Ultrasound serves as a guide for percutaneous fluid aspiration, which allows for cytology testing, immunohistochemistry testing and culture for a proper differential diagnosis.
Radiological findings on magnetic resonance imaging (MRI) are the presence of a periprosthetic collection, breast oedema and skin thickening1,5,7–9 (Fig. 1).
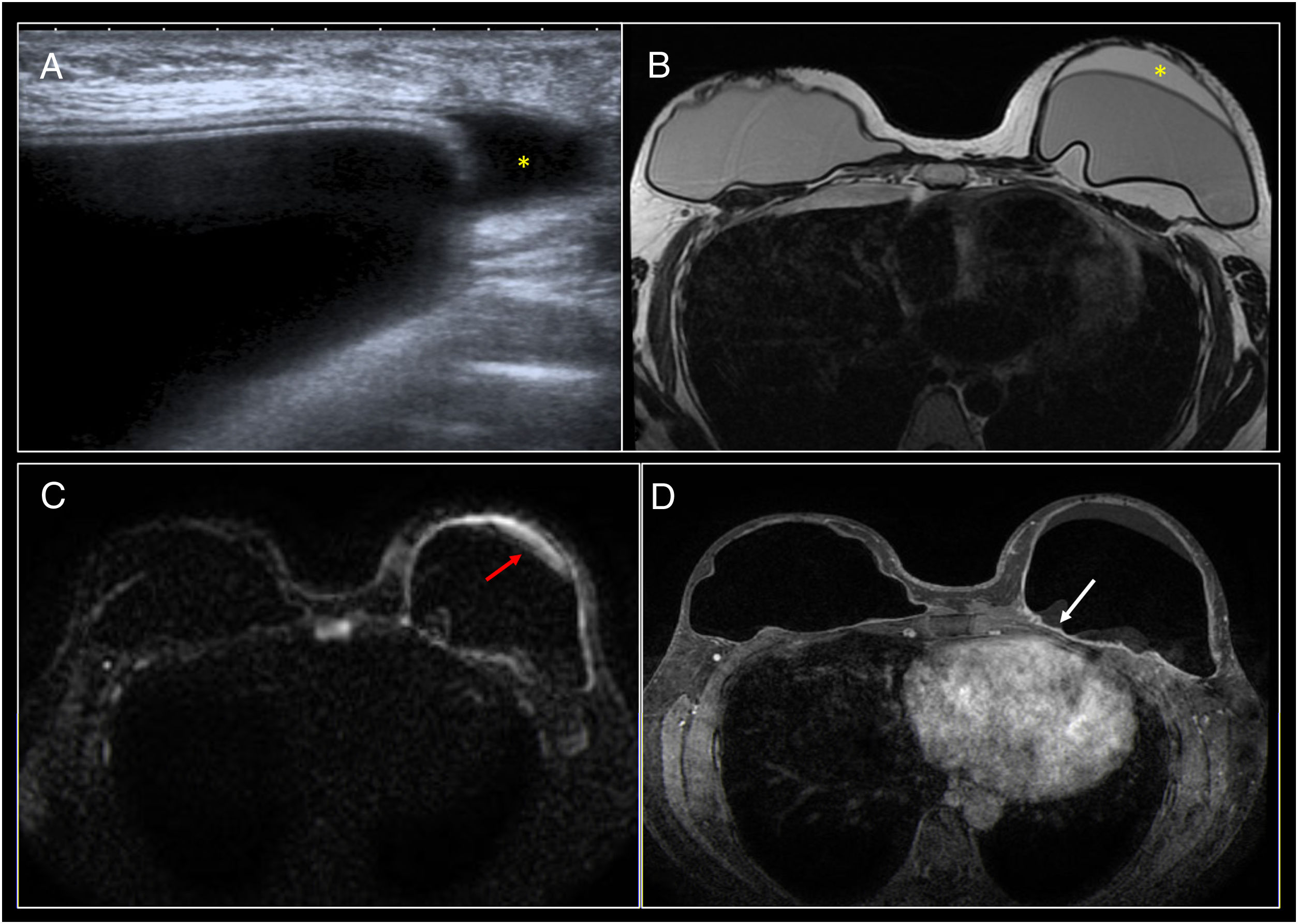
Late infection. (A) A heterogeneous periprosthetic collection (*) with the presence of septa (arrow) was identified on ultrasound. (B) Axial STIR. (C) Diffusion. (D) Sagittal T2. (E) Axial T1 following administration of intravenous contrast. Moderate periprosthetic seroma (*) with intact implant. It presented restricted diffusion and diffuse enhancement of the fibrous capsule (arrow). (F) Cell block (200× H&E staining) consisting of polymorphonuclear leukocytes in a proteinaceous fluid.
Normally, 75% of lymphatic drainage of the breast occurs through the homolateral axillary lymph nodes, and the other 25% occurs through the internal mammary chain lymph nodes, the contralateral breast and the inferior phrenic lymph nodes. This drainage may become abnormal following lymphadenectomy or sentinel lymph node biopsy.10
In patients with breast reconstruction with prostheses, enlarged lymph nodes secondary to a non-specific inflammatory reaction or a reaction to a foreign body due to migration of silicone to the lymph nodes may be found in the axillary region or in the internal mammary lymph node chains. It usually occurs six to ten years following implantation.4,10,11
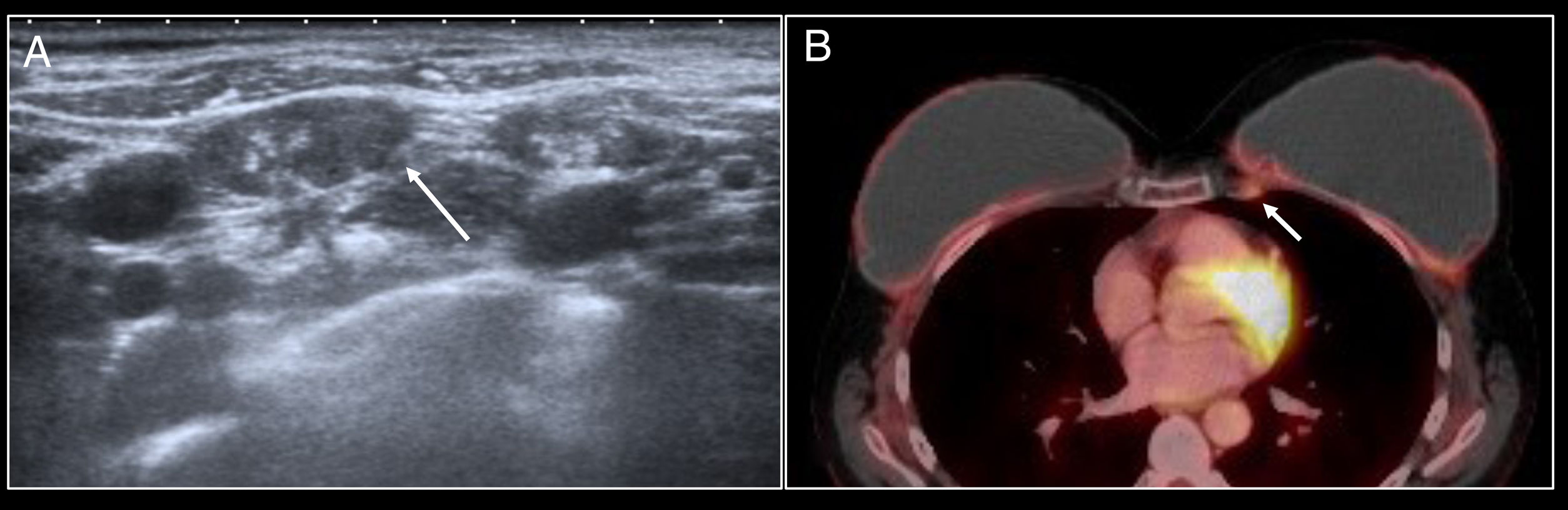
These lymphadenopathies may present intense fluorodeoxyglucose uptake on positron emission tomography/computed tomography (PET/CT) and therefore represent a diagnostic dilemma in patients with a history of cancer4,10 (Fig. 2).
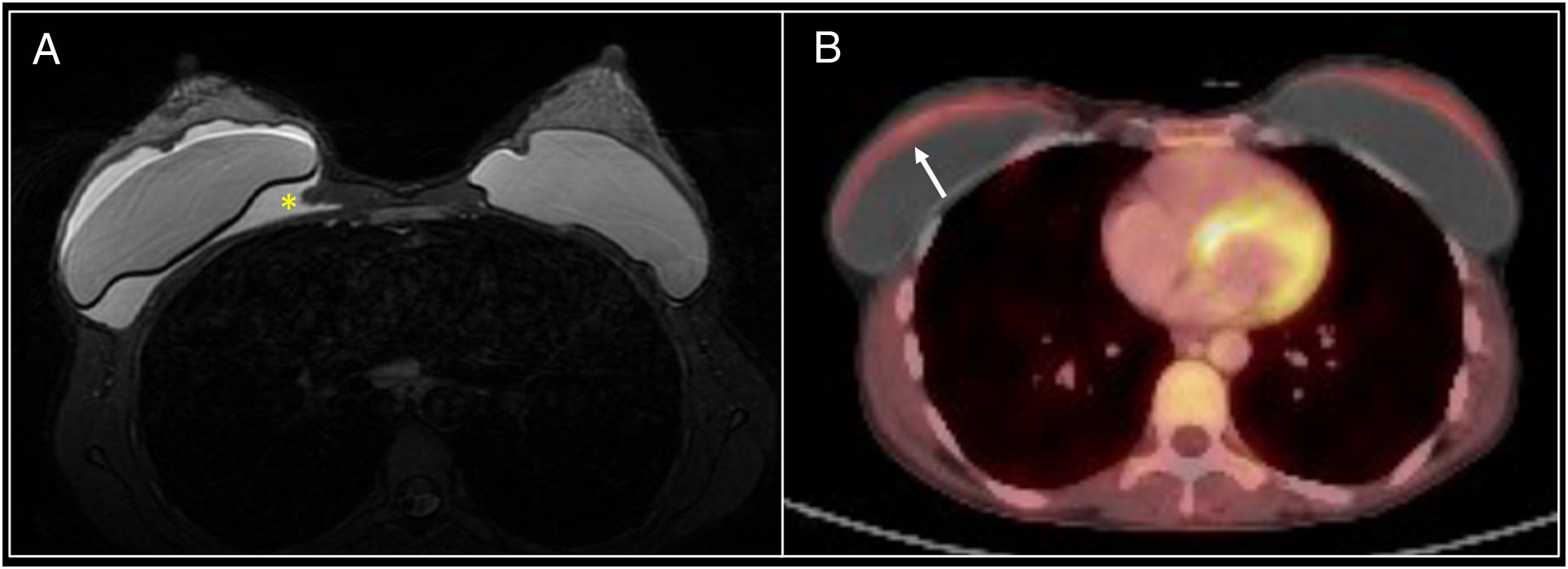
Lymphadenopathy in the internal mammary lymph node chain. A patient with a history of left mastectomy due to breast cancer and right prophylactic mastectomy with reconstruction with bilateral prostheses. The follow-up PET/CT scan identified the appearance of lymphadenopathy in the left internal mammary lymph node chain with uptake arousing suspicion of relapse (arrow). Fine needle aspiration biopsy was performed but did not identify any signs of relapse.
Some studies have reported percentages of lymphadenopathy in the internal mammary lymph node chains close to 30% in patients with breast surgery and reconstruction with prostheses, some with uptake on PET/CT, although with very low positive predictive values for malignancy; therefore, in the absence of other data arousing suspicion of relapse, it might be managed conservatively by means of radiological follow-up.10
Late seromaLate seroma is a rare complication, defined as any periprosthetic collection that occurs after the year following surgery. Its exact incidence is unknown, although approximate figures of 0.5%–1.84% are reported, and it is most commonly associated with textured and polyurethane implants.12–15
Its pathophysiology is unknown. It may include mechanical factors such as trauma and synovial metaplasia secondary to microtrauma, and non-mechanical factors such as clinical and subclinical infection, inflammation and lymphoproliferative processes.12,16 However, in most cases, no cause is found; these are deemed idiopathic.14
On ultrasound and MRI it presents as a periprosthetic collection that may be associated with fibrous capsule thickening (Fig. 3).
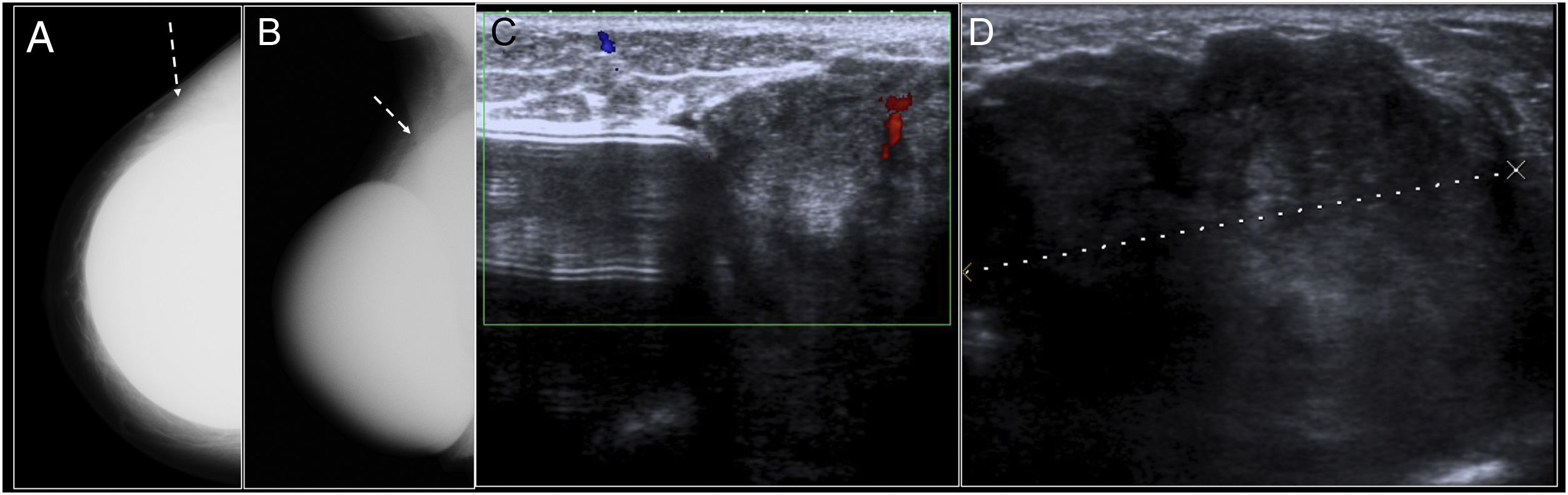
Late seroma. (A) Craniocaudal mammography of the right breast showing a modest increase in density adjacent to the inner aspect of the prosthesis, with no abnormality on its contour (arrow). (B) On ultrasound it corresponded to a small amount of anechogenic fluid (*). (C) STIR axial MRI. (D) Sagittal T1 following administration of intravenous contrast. Small periprosthetic seroma (*) with implant integrity. It was associated with diffuse modest enhancement of the fibrous capsule (arrow). Evacuating fine needle aspiration biopsy of the periprosthetic fluid was performed and no cause could be identified (idiopathic seroma).
It is necessary to rule out infection and malignancy by means of ultrasound-guided aspiration of the periprosthetic fluid, which should be sent for culture and cytology in which cell composition, cell morphology and percentage of CD30+ cells are assessed.15–17
Treatment is conservative, and even if cultures are negative, the possibility of subclinical infection should be considered. If a seroma is resistant to treatment, explantation of the prostheses and capsulectomy are recommended.12,15,16
Silicone-induced granulomasOne recently reported complication is formation of masses on the periprosthetic fibrous capsule in patients with intact implants as a result of an inflammatory response to silicone.
All types of implants, even the most modern, have demonstrated filtration of silicone particles that, when they come into contact with the fibrous capsule, induce a type 2 inflammatory response with increased IgE and IgG levels1 and chronic activation of T lymphocytes with consequent granuloma formation.18,19 This immune response will be mild when there is a predominance of giant cells and more aggressive when there is a greater lymphocyte component.18
The intracapsular space has limited vascularisation; therefore, in early phases, when the fibrous capsule is intact, the granuloma is found to be located in the intracapsular space and this is a limited condition with a good prognosis.
Extracapsular granuloma occurs when the mass invades the fibrous capsule and spreads to the adjacent tissue. Exposure to the intracapsular content may cause a more striking – even systemic – immune reaction, with the possibility of finding regional lymphadenopathy with silicone infiltration.18,20
Clinically, granulomas present with signs of capsular contracture such as stiffness and pain in the affected breast. As associated findings, patients may present arthralgia, pruritus and asthenia, commonly reported symptoms in the autoimmune syndrome induced by adjuvants such as silicone.18–22 In addition, there may be periods of spontaneous remission or remission with anti-inflammatory agents/corticosteroids.18
Granulomas may be associated with a haematoma or intracapsular seroma, enhancement of the fibrous capsule to different degrees or capsular contracture.
Prevalences of intracapsular granulomas of 27% have been reported; of these, 12% were associated with a seroma and 3% showed signs of extracapsular involvement.20
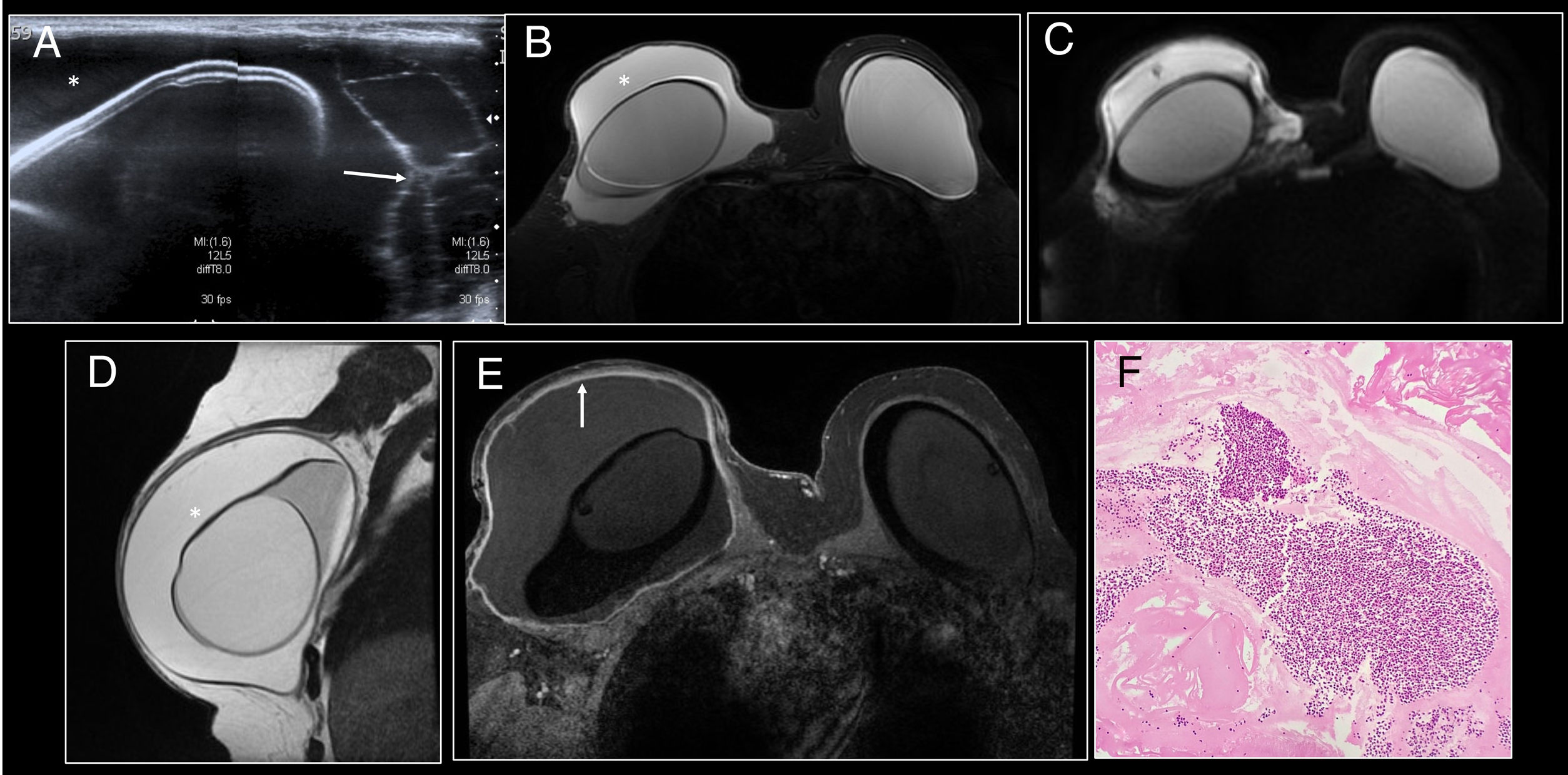
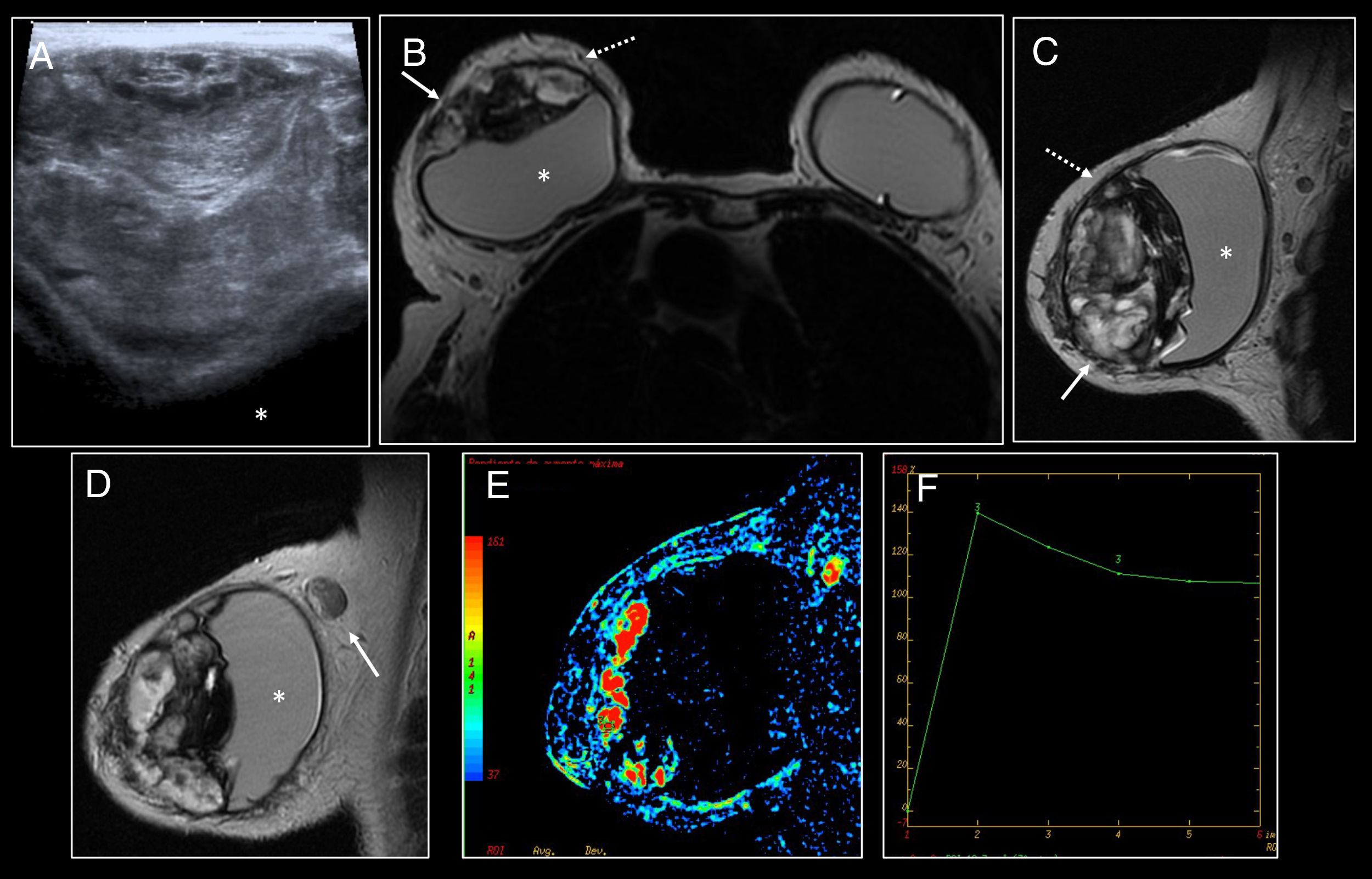
Histologically, they are formed by extracellular or intracellular silicone, histiocytes, chronic granulomatous inflammatory infiltrate with multi-nucleated giant cells and mixed lymphocytic infiltrate with no atypia20 (Fig. 4).
Intracapsular granuloma. (A) Axial T2 MRI. (B) Axial T1 following administration of intravenous contrast. Hyperintense mass in T2-enhanced sequences (arrow) of intracapsular location posterior to the prosthesis in the left breast. Intact fibrous capsule. Following administration of intravenous contrast, thickening and enhancement of the posterior aspect of the fibrous capsule (dashed arrow) adjacent to the mass without clear enhancement of the mass was identified. (C) Diffuse modest uptake by the posterior aspect of the fibrous capsule (dashed arrow) with no uptake by the mass was identified on PET/CT. (D) 200× H&E staining of the fibrous capsule showing cystic spaces containing pale extracellular material consistent with silicone, surrounded by vacuolated histiocytes, lymphocytes and multi-nucleated giant cells.
Radiological findings of intracapsular granuloma:
- •
A heterogeneous intracapsular mass may be visualised on ultrasound; this may be associated with snowstorm artefact due to free silicone.20
- •
MRI is the best diagnostic technique for its assessment, since it also assesses implant integrity.
- •
The intracapsular mass shows a hypersignal in T2-enhanced sequences and a hyposignal in T1-enhanced sequences. It may exert a mass effect on the implant18–20,23 (Fig. 4).
- •
Late dynamic sequences (more than 4min) must be performed following administration of intravenous contrast in order to distinguish between intracapsular seroma/haematoma.18,23 It will present gradual enhancement with type I curves, sometimes with hypervascular nodular areas inside the mass.
- •
The black-drop sign may be identified; this consists of a focus of marked hyposignal at the interface between the implant covering and the granuloma in the dynamic study.18,20
- •
Radiological findings of extracapsular granuloma:
- •
A mass with peripheral vascularisation and areas with snowstorm artefact may be identified on ultrasound20,23 (Fig. 5).
Figure 5.Extracapsular granuloma. (A) Ultrasound showing a large heterogeneous mass in contact with the breast implant which was found to be displaced (*). (B–D) Axial and sagittal T2. Heterogeneous intracapsular mass leading to a mass effect on the retroglandular implant (*). Loss of the integrity of the fibrous capsule with incipient spread towards the adjacent parenchyma was identified (solid arrow). The normal capsule was visualised as a hypointense line surrounding the mass (dashed arrow). There was lymphadenopathy with signs of silicone infiltration (arrow in D). (E and F) Perfusion map and kinetic curve showing the mass with areas of high perfusion and kinetic curves with rapid initial uptake and late-phase washout (type 3).
(0.37MB). - •
A mass with infiltration of the fibrous capsule will be visualised on MRI. Following administration of intravenous contrast, due to the lack of barrier represented by the fibrous capsule, it may present enhancement in early phases. There may be axillary siliconomas20 (Fig. 5).
- •
Uptake by the mass as well as uptake by the axillary and mammary lymph nodes may be identified on PET/CT.20
The differential diagnosis of granulomas includes mainly seroma and late haematoma, for which administration of intravenous contrast will be necessary, as well as BIA-ALCL, which in a third of cases may present as a periprosthetic mass.18–20,23
Treatment consists of prosthesis explantation and capsulectomy.18,20
It is advisable to avoid percutaneous biopsies when an intracapsular granuloma is suspected due to the risk of rupture of the barrier that provides the fibrous capsule which could lead to a systemic reaction.19,23
Breast fibromatosisDesmoid tumours or fibromatoses comprise a rare type of benign stromal tumour that may be classified based on their location as extra-abdominal, abdominal or intra-abdominal.24–26 Breast fibromatosis is an extremely uncommon disease accounting for approximately 4% of extra-abdominal desmoid tumours and 0.2% of all breast tumours.27–30 Cases associated with breast implants are even rarer, with few published studies in the specialised literature.1,31,32
Breast fibromatosis may originate in the breast parenchyma, the aponeurosis of the pectoral muscle and probably in the periprosthetic capsule.1,27–29
They present infiltrative and aggressive local growth with a high percentage of relapse, but not a tendency to metastasise.27 Due to this growth, they may simulate malignancy, especially in patients with a history of prior breast surgery.27,28
Their pathogenesis is unknown. However, most desmoid tumours occur sporadically, being associated in up to 85% of these cases with a mutation in the beta-catenin gene.31 Cases associated with Gardner syndrome, trauma, surgery or augmentation mammoplasty have been reported.25,26,31 Increased tumour volume has been observed during pregnancy, suggesting a hormonal, mainly oestrogenic, influence.25,26,32
It was recently suggested that the presence of breast implants may be a risk factor, although a clear causal relationship has not yet been confirmed.29 More cases have been reported in association with silicone implants versus saline implants, although this may be due to the greater prevalence of silicone implants.27,28 Most cases have occurred two to three years after surgery, and in all reported cases the implants were found to be intact.27,28
Clinically, they usually present as a single, firm and painless palpable mass with rapid growth.1,28,32
Histologically, they are composed of small groups of spindle-shaped cells and fibroblasts separated by variable amounts of collagen.26,28 They usually present nuclear positivity for beta-catenin and negativity for oestrogen and progesterone receptors.28,30
Radiological findings are non-specific; therefore, a biopsy must be performed to diagnose them.
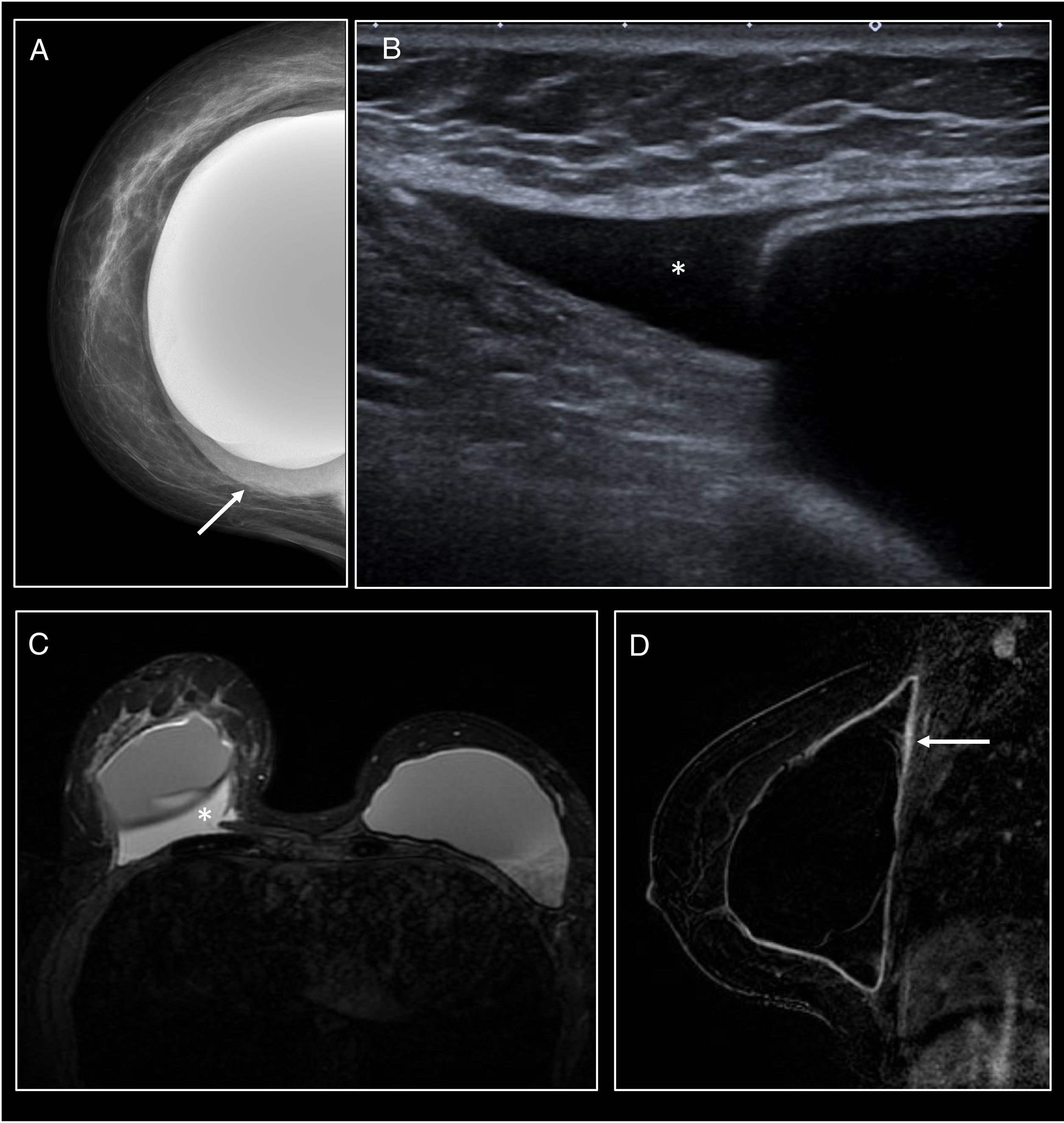
Breast fibromatosis presents an appearance provoking suspicion of malignancy on both mammography and ultrasound. However, cases associated with breast implants usually show a more benign appearance, with relatively well-defined margins despite their tendency towards local infiltration1,27,28 (Fig. 6).
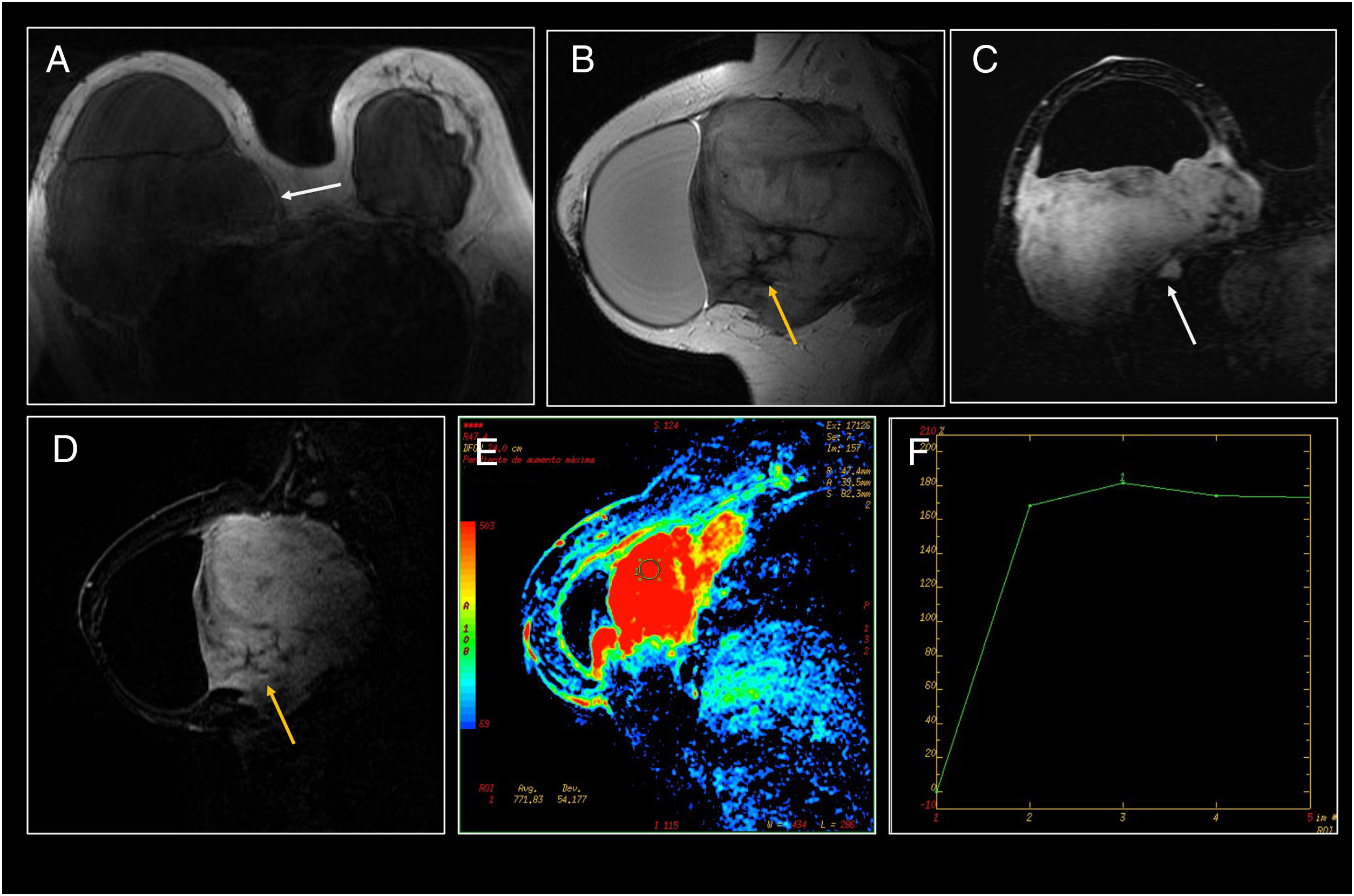
Breast fibromatosis. (A and B) Craniocaudal and mediolateral oblique mammography of the right breast. Mass with well-defined borders in the outer quadrants of the breast (arrow). The mass was found to be in close contact with the outer and posterior aspect of the prosthesis with effacement of the contour of the prosthesis and anterior displacement thereof. (C and D) On ultrasound it corresponded to a large heterogeneous mass with relatively well-defined borders in close contact with the prosthesis.
MRI is the imaging technique of choice to evaluate both tumour spread and the relationship of the tumour to adjacent structures.25,27,31,33,34 Masses are observed which may present well-defined margins (49%–54%) or irregular and infiltrative margins (46%–51%).31
Lesion signal and enhancement kinetics vary by amount of collagen and degree of cellularity.25,27,31 Three histological stages are reported depending on collagen content and cellularity which influence the signal in T2-enhanced sequences. In initial stages, they present a lesser amount of collagen and greater cellularity, which translates to high signal in T2, as well as lesser cellularity and a greater amount of collagen in end stages with low signal in T2-enhanced sequences.31 They often present gradual kinetics, although cases of plateau or washout kinetics in late phases have also been reported25,27,28,34 (Fig. 7).
Breast fibromatosis (same case as in Fig. 6). (A) Axial T1. A large isointense mass, located in close contact with and posterior to the right implant with anterior displacement of the prosthesis and a significant increase in breast volume. The split fat sign, consisting of a fine halo of fat surrounding the lesion, was identified (white arrow). (B) Sagittal T2 showing the hyperintense mass with small hypointense areas inside the lesion (orange arrow). (C and D) MRI following administration of intravenous contrast. The mass presented significant contrast uptake with the presence of small hypointense areas (orange arrow) corresponding to the presence of collagen fibres with limited cellularity. It presented local infiltration with spread through the intercostal space towards the thorax (white arrow). (E and F) Perfusion map and kinetic curve showing increased perfusion with a plateau (type 2) kinetic curve.
Fibromatoses usually show in 62%–91% hypointense areas inside the lesion in all sequences due to the presence of collagen fibres with limited cellularity27,31 (Fig. 7).
Other signs that may be visualised on different imaging techniques, although essentially on MRI, are the fascial tail sign, which consists of linear extensions across the fascial planes, and the split-fat sign, which consists of a fine halo of fat surrounding the lesion27,31,35 (Figs. 7 and 8).
Breast fibromatosis. (A and B) Ultrasound: well-defined hypoechogenic retropectoral mass in contact with the prosthesis extending through the intercostal space (dashed arrow). Fascial tail sign (solid arrow in (A)) consisting of linear extensions towards the lateral margins. (C) PET/CT: the mass was slightly hypermetabolic (solid arrow). (D) Pathology (200× H&E staining): fusiform cell bundles in a collagen-rich stroma with scattered lymphocytes and extravasated red blood cells.
PET/CT may aid in guiding the biopsy, evaluating the aggressiveness of the lesion and diagnosing disease recurrences and progression.36–38 Desmoid tumours are usually hypermetabolic, although there may be intralesional variability depending on the percentage of cells and the collagen content of the lesion37,38 (Fig. 8).
Management and treatment of these lesions is debated due to the few existing cases. Whenever possible, the treatment of choice will consist of wide local excision with negative margins in an attempt to decease their recurrence.1,26,28 Radiotherapy is usually the treatment pursued when the disease is unresectable or associated with surgery if negative margins cannot be achieved.26,28
Drug treatment is usually reserved for patients with recurrences and includes non-steroidal anti-inflammatory drugs, interferon, hormone therapy and cytotoxic agents with different degrees of success.32 Although desmoid tumours usually do not express oestrogen receptors, anti-oestrogenic agents (tamoxifen) have demonstrated disease management in some cases (from disease stabilisation to cases of complete remission).26 Tamoxifen's mechanism of action is not clear, but it appears to be independent of oestrogen receptor expression.26
Clinical and radiological follow-up are necessary due to the high rate of relapse, especially in the first three years.39
Anaplastic large cell lymphomaPrimary breast lymphoma (PBL) is a rare tumour accounting for 0.5% of cases of breast cancer and 2% of cases of extranodal lymphoma. PBL originates in the periductal and perilobar breast lymphoid tissue. Most PBLs derive from B cells; tumours with T cell phenotypes are rare (less than 6%).19
Anaplastic large cell lymphoma is a peripheral T cell lymphoma that accounts for 2%–3% of all non-Hodgkin lymphomas.19,40,41 It may affect a wide variety of tissues, including breast tissue. There are two variants of anaplastic large cell lymphoma: one is systemic, with lymphadenopathy and extranodal involvement; the other is cutaneous. In 2016, the World Health Organization (WHO) recognised BIA-ALCL as a discrete entity, diagnosed in the capsule or the periprosthetic fluid and rarely infiltrating the breast parenchyma.41,42 This is a CD30+, ALK-negative tumour with a better prognosis than systemic forms of anaplastic large cell lymphoma.19,40–45
The first case of BIA-ALCL was reported in 1997, and since then the number of cases has gradually increased, probably due to growing numbers of breast implants and greater knowledge of the disease.1,40,43 However, the true relative and absolute risk of lymphoma in women with implants is unknown.46
It is usually diagnosed three to seven years following surgery, with a mean of five years following mammoplasty.19 Cases have been reported in both mammoplasty due to aesthetic reasons, such as breast reconstruction, and in transgender patients. Most cases have been reported in textured implants.1,40,41,44,45
At present, there is insufficient evidence that different types of implant fillers (saline and silicone) are associated with greater risk.1,47 In addition, some reported cases have occurred in families with a high risk of breast cancer; therefore, there may be an increased risk in patients with a BRCA mutation.46
Their pathogenesis is unknown. The following have been reported as potential risk factors: capsular contracture, subclinical infection on the surface of the implant (biofilm), repeated capsular trauma, an immune response to silicone components, a genetic predisposition and an autoimmune mechanism.1,40,43,44
Notable among the main theories of the aetiopathogenesis of this activated T cell monoclonal neoplasm is the theory that it is due to chronic lymphocyte stimulation in predisposed individuals, secondary to bacterial contamination on the surface of the implant, or due to chronic inflammation caused by the silicone or polyurethane from which the implant is made.19,42,43,45,47 In addition, textured implants seem to cause a more marked local T cell-mediated response than implants with a smooth surface; hence, they may carry a greater risk of lymphoma.45
There are two main forms of presentation. The more common is as late-onset periprosthetic seroma, which usually corresponds to localised disease. The other is a mass in which the tumour grows on or through the capsule, with or without an associated seroma.40,43,45
Cases with lymphatic involvement with no breast mass, as well as cases in the non-explanted residual fibrous capsule near the prosthesis, have been reported.48 Other, less common presentations are pain, erythema, skin lesions, fever and systemic signs.40,42,43
Therefore, the development of a periprosthetic seroma of late onset is a suspicious sign that should be evaluated by ultrasound with fine needle aspiration biopsy (FNAB) of the fluid for microbiology, cytology and flow cytometry testing (including CD30 markers). When it presents as a mass or axillary lymphadenopathy, that mass or lymphadenopathy must be biopsied and the presence of CD30 markers must be assessed.45
Histological diagnosis consists of demonstration of T lymphocytes of atypical morphology (very large, aberrant cells) with strong CD30 expression and negativity for ALK.
Lymphomas presenting in an initial stage usually have an excellent prognosis only with explantation of the prostheses and the fibrous capsule.
When there is infiltration, management must be more aggressive, with removal of the mass with negative margins and of the lymphadenopathy. Adjuvant treatment should be considered, as higher relapse rates and mortality rates of approximately 40% in two years have been reported.40
Neither sentinel lymph node biopsy nor lymphadenectomy is indicated as neither has been shown to decrease recurrence rates40,43,45.
Involvement of the contralateral capsule has been shown in 4.6% of cases; therefore, bilateral explantation is recommended.40,45
Whenever BIA-ALCL is diagnosed, a suitable extension study must be conducted by PET/CT and bone marrow biopsy to rule out systemic forms of anaplastic large cell lymphoma.
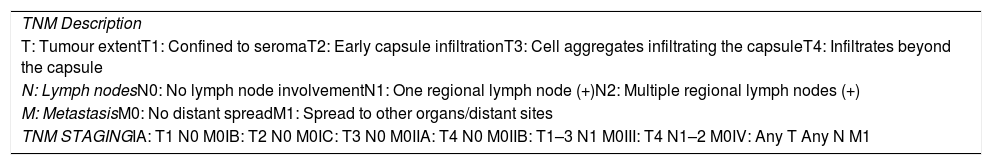
A specific TNM classification was recently proposed (Table 2).40,45,49
National Comprehensive Cancer Network BIA-ALCL TNM classification (2019).
| TNM Description |
| T: Tumour extentT1: Confined to seromaT2: Early capsule infiltrationT3: Cell aggregates infiltrating the capsuleT4: Infiltrates beyond the capsule |
| N: Lymph nodesN0: No lymph node involvementN1: One regional lymph node (+)N2: Multiple regional lymph nodes (+) |
| M: MetastasisM0: No distant spreadM1: Spread to other organs/distant sites |
| TNM STAGINGIA: T1 N0 M0IB: T2 N0 M0IC: T3 N0 M0IIA: T4 N0 M0IIB: T1–3 N1 M0III: T4 N1–2 M0IV: Any T Any N M1 |
BIA-ALCL: breast implant-associated anaplastic large cell lymphoma.
There are no specific radiological findings.
Mammography exhibits a sensitivity of 73% and a specificity of 50% for detecting abnormalities, some subtle such as capsule contour thickening or irregularity, but it does not distinguish between masses and seromas.40,50
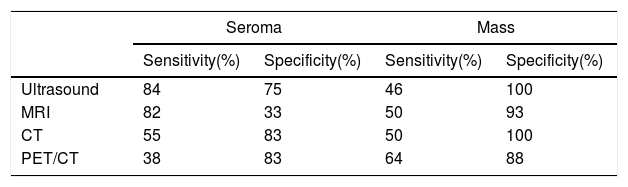
Ultrasound and MRI are the best imaging techniques for their diagnosis, since they are the most sensitive techniques for detecting periprosthetic fluid. The most sensitive technique in mass detection is PET/CT (Table 3).50
Sensitivity and specificity for each imaging modality in detecting a seroma or a mass in patients with BIA-ALCL.50
| Seroma | Mass | |||
|---|---|---|---|---|
| Sensitivity(%) | Specificity(%) | Sensitivity(%) | Specificity(%) | |
| Ultrasound | 84 | 75 | 46 | 100 |
| MRI | 82 | 33 | 50 | 93 |
| CT | 55 | 83 | 50 | 100 |
| PET/CT | 38 | 83 | 64 | 88 |
CT: computed tomography; MRI: magnetic resonance imaging; PET/CT: positron emission tomography/computed tomography.
Ultrasound is the first imaging test that must be performed in cases of clinical suspicion, in order to assess the presence of a seroma, a mass or lymphadenopathy.49 It is the most cost-effective technique, and furthermore serves as a guide for seroma drainage40,50,51 (Fig. 9).
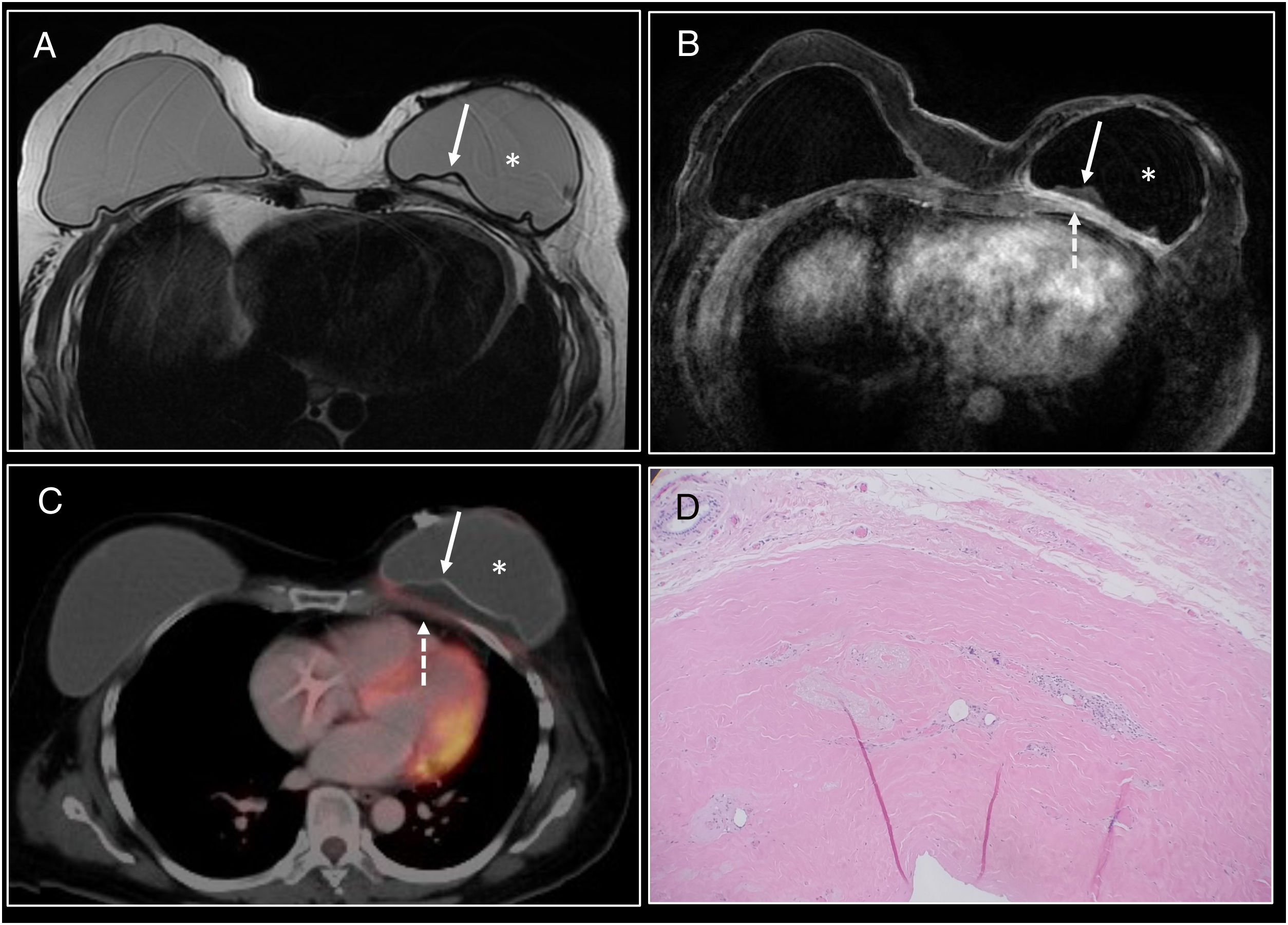
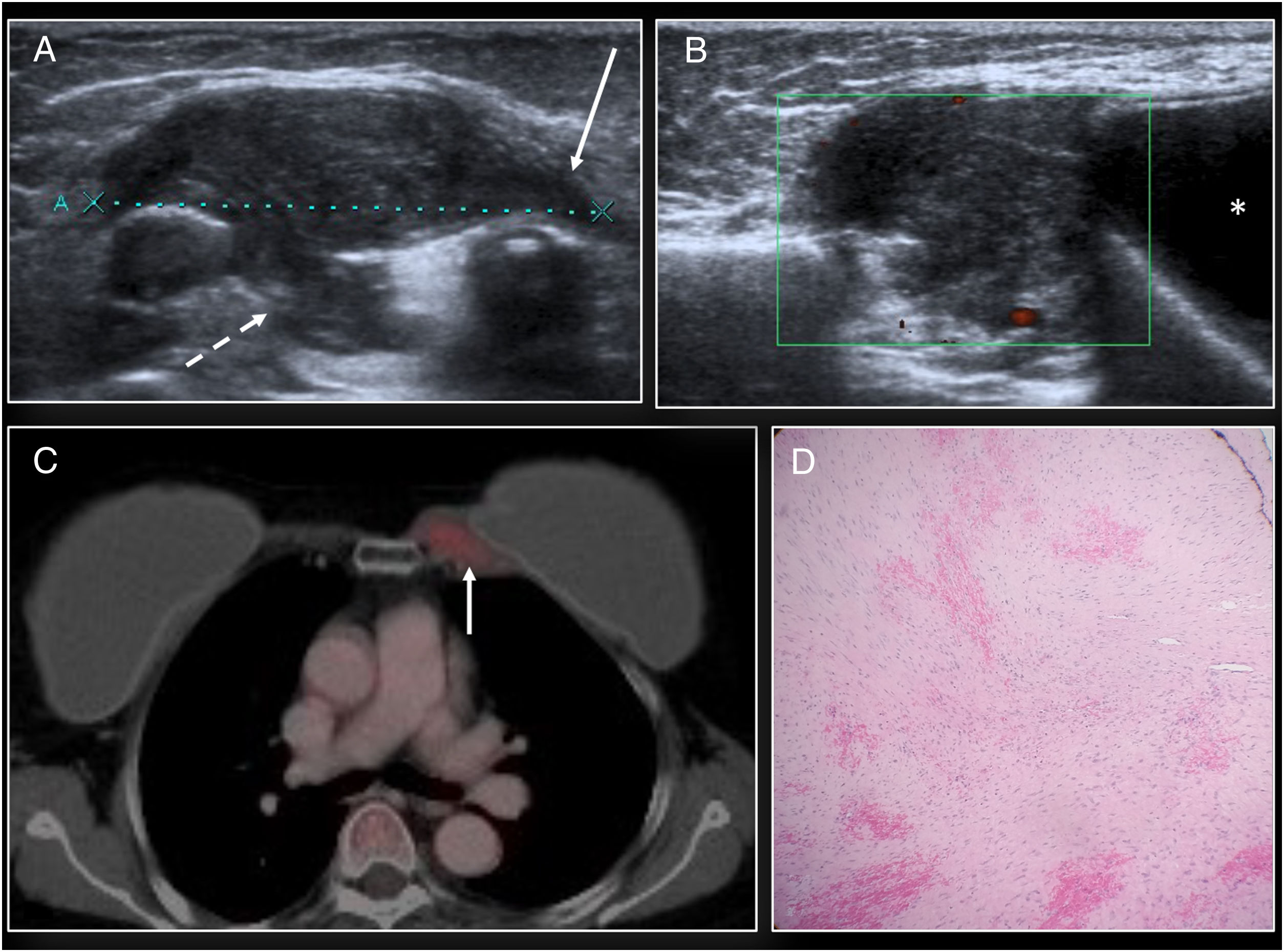
BIA-ALCL. (A) Ultrasound showing modest periprosthetic seroma in the left breast (*). (B) Axial T2 MRI. (C) Diffusion. (D) Axial T1 following administration of contrast. Modest periprosthetic seroma (*) with implant integrity. The seroma presented restricted diffusion (red arrow) and mild diffuse enhancement of the fibrous capsule (white arrow). Fine needle aspiration biopsy of the periprosthetic fluid was performed and confirmed the diagnosis.
Breast MRI is recommended when ultrasound findings are inconclusive.49 MRI assesses the presence of a seroma, a mass or lymphadenopathy. It also assesses implant integrity and associated findings such as capsule thickening and enhancement50 (Figs. 9 and 10).
BIA-ALCL. (A) Axial T2 MRI identifying a periprosthetic seroma with implant integrity. Evacuating fine needle aspiration biopsy was performed and confirmed BIA-ALCL. On PET/CT following seroma evacuation modest hyperuptake of the anterior aspect of the periprosthetic capsule (arrow) was identified.
Confirmed cases of BIA-ALCL are staged with PET/CT.49 PET/CT may identify diffuse or focal periprosthetic uptake, hypermetabolic masses and lymphadenopathy. However, no standardised uptake values (SUVs) have been established for the diagnosis of a seroma or a mass in BIA-ALCL40,43,50,52 (Fig. 10).
Prognosis and follow-upRelapse rates following complete surgery are approximately 6%–11% in the first year. Some series have reported local recurrence rates of approximately 36% and distant recurrence rates of 64%.40
If excision was complete with no residual disease, follow-up every three to six months for two years with subsequent follow-up depending on clinical findings is recommended. It is recommended that a CT or a PET/CT scan be done every six months for two years with subsequent follow-up depending on the patient's clinical picture.44,45,49
ConclusionBreast implants can be associated with a number of complications, some of which, such as rupture, are widely known to radiologists. However, recently, more uncommon complications have been reported, and some of them, such as BIA-ALCL, are more significant.
An increase in the prevalence of these more uncommon conditions can be predicted as a result of the growing use of breast implants. The clinician must be knowledgeable about them in order to be able to suitably diagnose and treat them. Of particular importance is proper management of late-onset periprosthetic seroma, the main sign of BIA-ALCL.
Authorship- 1.
Responsible for study integrity: NSR.
- 2.
Study conception: NSR and MJCF.
- 3.
Study design: NSR, MJCF, MMF, BLM and MDM.
- 4.
Data acquisition: NSR, MMF, MJCF, MDM and BLM.
- 5.
Data analysis and interpretation: NSR, MJCF, MMF, BLM and MDM.
- 6.
Statistical processing: N/A.
- 7.
Literature search: NSR, MDM and BLM.
- 8.
Drafting of the article: NSR.
- 9.
Critical review of the manuscript with intellectually significant contributions: NSR, MJCF, MMF, BLM and MDM.
- 10.
Approval of the final version: NSR, MMF, MJCF, BLM and MDM.
Please cite this article as: Sánchez Rubio N, Lannegrand Menéndez B, Duque Muñoz M, Montes Fernández M, Ciudad Fernández MJ. Complicaciones infrecuentes de las prótesis de mama. Radiología. 2020. https://doi.org/10.1016/j.rx.2020.01.008