Sarcoidosis is a multisystemic granulomatous disease of unknown etiology. It mainly affects the thoracic lymph nodes and the lungs. The staging of sarcoidosis, which classifies patients according to their probability of spontaneous remission, is based on the plain chest film findings. Plain chest films are not as sensitive as high resolution computed tomography (HRCT) at detecting involvement of the lymph nodes, lungs, or bronchi. The high resolution CT findings can be typical, practically pathognomic, or atypical. High resolution CT provides information about the activity of the disease and detects incipient signs of fibrosis and other complications. To reach the diagnosis, it is necessary to correlate the clinical and radiological findings (and often the histological findings).
Cardiac involvement can cause sudden death. The diagnosis of cardiac involvement is difficult; it is based on various imaging tests, such as magnetic resonance imaging, which is more specific, and positron emission tomography. Diagnostic confirmation by endomyocardial biopsy is obtained in few patients.
La sarcoidosis es una enfermedad granulomatosa multisistémica de causa desconocida, que afecta principalmente a los ganglios linfáticos torácicos y a los pulmones. La estadificación, que agrupa a los pacientes con similar probabilidad de remisión espontánea, se basa en los hallazgos de la radiografía de tórax, que tiene menor sensibilidad que la tomografía computarizada de alta resolución (TCAR) para detectar la afectación ganglionar, pulmonar y bronquial. Los hallazgos en TCAR pueden ser típicos, prácticamente patognomónicos, o atípicos. La TCAR aporta información sobre la actividad de la enfermedad y detecta incipientes signos de fibrosis y otras complicaciones. Para realizar el diagnóstico es precisa la correlación clínica, radiológica, y en muchos casos anatomopatológica.
La afectación cardiaca puede provocar muerte súbita; el diagnóstico, difícil, se basa en varias pruebas de imagen, como la resonancia magnética, que tiene mayor especificidad, y la tomografía por emisión de positrones; la confirmación mediante biopsia endomiocárdica se obtiene en pocos pacientes.
Sarcoidosis (from Greek “sark oid”, meaning flesh-like) is also called Besnier-Boeck's disease after the Norwegian dermatologist Caesar Boeck, who in 1899 described the skin lesions of the disease and coined the term “benign sarcoid” because of its histologic similarity to sarcoma. Later, he described the involvement of the lungs, bone, lymph nodes, spleen, nasal mucosa and conjunctiva. In 1915, Kusnitski and Bittorf described the chest radiographic features.1
Sarcoidosis is a systemic granulomatous disease whose cause is still unknown.2 However, infectious factors such as viruses, mycobacteria (mycobacterial DNA has been detected by the polymerase chain reaction [PCR]) and fungi, as well as environmental agents (after 9/11, increased rates of sarcoidosis were described in fire-fighters who took part in the rescue work at the World Trade Center) or genetic factors may induce the abnormal immune response seen in the disease. It remains however unclear why this response is triggered.1 Thoracic and pulmonary lymph nodes are the most common sites, and even though eyes, skin, salivary glands, liver, spleen, heart, bone and the central nervous system can also be involved, pulmonary sarcoidosis accounts for the highest rates of morbidity and mortality. Although the prognosis of the disease is usually favorable, its course cannot be predicted and in 10–30% of patients is chronic or progressive.
Sarcoidosis is one of the most common diffuse infiltrative lung diseases (DILD). In a series of 500 consecutive patients with suspected DILD, sarcoidosis was the most common disease (19% of patients).3 Its incidence is heterogeneous among different populations,4 and it seems to be rarely reported in Ecuador and more common in Northern European countries (60 cases/100000 population) and among African Americans. There is a higher disease rate for women in their 3rd and 4th decades.
Approximately 30% of patients are asymptomatic, while the most common clinical manifestations in symptomatic patients are erythema nodosum and occasionally respiratory symptoms, including dry irritating cough, chest pain and dyspnea, with dissociation between clinical and radiological findings (with more radiological than clinical involvement). Some patients develop Löfgren's syndrome, which may cause fever, erythema nodosum, arthralgia and bilateral hilar lymphadenopathy. Patients >70 years usually have systemic symptoms and salivary gland involvement.5 Elevated angiotensin-converting enzyme (ACE), hypercalciuria, decreased diffusion capacity and restrictive pattern on pulmonary function tests as well as increased CD4/CD8 ratio in the bronchoalveolar lavage (BAL) fluid may also appear.
The characteristic histologic lesion of sarcoidosis is a well-formed, non-necrotizing granuloma with histiocytes, epithelioid cells and multinucleated giant cells surrounded by a rim of lymphocytes.6 Sarcoid granulomas may develop fibrotic changes and occasionally focal coagulative necrosis.1 In the early stages of the pulmonary disease, before the formation of granulomas, there is lymphocyte accumulation in the alveolar septa (lymphocyte alveolitis). Subsequently, granulomas form in the perilymphatic interstitium (with a peribronchovascular, centrilobular and subpleural distribution) and, less frequently, in the interlobular septa.7 Microscopic granulomas may coalesce to form macroscopic nodules.
Lymph node, pulmonary and bronchial involvementChest radiographic findings and stagingPA and lateral chest radiographic (chest X-ray) examinations are essential since sarcoidosis most commonly targets the thorax and thoracic sarcoidosis is seen in over 90% of patients at some stage of the disease.8 Nonetheless, patients with histologically proven lung parenchymal disease may have normal chest X-ray. In asymptomatic patients, radiographic findings are often the first manifestation of the disease.
Five decades ago, Scadding9 described four stages of sarcoidosis based on X-ray findings. Stage 0 was later added for those cases with normal chest X-ray. Patients are classified according to the likelihood of spontaneous remission, which occurs in 55–90% of patients with stage I and in 0% of patients with stage IV disease.
Stage 0: absence of chest X-ray abnormalities.
Stage I: bilateral hilar lymphadenopathy that may be accompanied by right paratracheal and aortopulmonary window adenopathy.8 The differential diagnosis should include fungal and mycobacterial infections, malignancies such as lymphoma, bronchogenic carcinoma, and extrathoracic carcinoma. However, in the absence of specific symptoms, the most common cause of bilateral hilar adenopathy is sarcoidosis.10 Calcification of long-standing lymph nodes is common,11 sometimes in an “eggshell pattern” similar to that of silicosis.
Stage II: bilateral hilar lymphadenopathy and parenchymal infiltration with a bilateral symmetric micronodular or reticulonodular pattern with predominant perihilar distribution, in middle and upper lung fields. Atypical findings are large nodules, masses, consolidations, atelectasis secondary to bronchial obstruction, cavities, pleural involvement and pneumothorax. The differential diagnosis includes pneumoconiosis and, if the X-ray shows masses and consolidations, tuberculosis and lymphoma should also be ruled out.
Stage III: parenchymal infiltration without hilar adenopathy. The differential diagnosis should include pneumoconiosis, lymphangitis carcinomatosis and, if the X-ray shows masses and consolidations, infections, cryptogenic organizing pneumonia, vasculitis, adenocarcinoma and lymphoma should also be ruled out.
Stage IV: fibrosis with evidence of reticular pattern with traction bronchiectasis, masses causing architectural distortion or honeycomb cysts, predominantly in the upper fields. The differential diagnosis includes complicated pneumoconiosis and other causes of fibrosis such as chronic extrinsic allergic alveolitis and idiopathic pulmonary fibrosis (IPF).
Staging has prognostic and therapeutic implications and is still done using the same criteria, based on imaging findings.10
High resolution computed tomography findingsHRCT, either sequential or volumetric with IV contrast, can detect lesions that are not visible on chest X-ray, proving that lung and lymph node involvement is more common that the involvement seen on radiographic images. Chest radiographs only detect 50–60% of lymph nodes and 30–40% of parenchymal abnormalities found with HRCT. Only in a few cases, patients with sarcoidosis confirmed by transbronchial biopsy (TBB) have normal HRCT scans.10
HRCT scans of most patients with pulmonary disease show involvement of multiple lymph node chains, usually the right paratracheal and bilateral symmetric hilar chains accompanied by prevascular, left paratracheal, aortopulmonary window, paraaortic and subcarinal lymph nodes. Isolated unilateral enlargement and involvement of the internal mammary and posterior mediastinal nodes (paravertebral and retrocrural) is less common.12
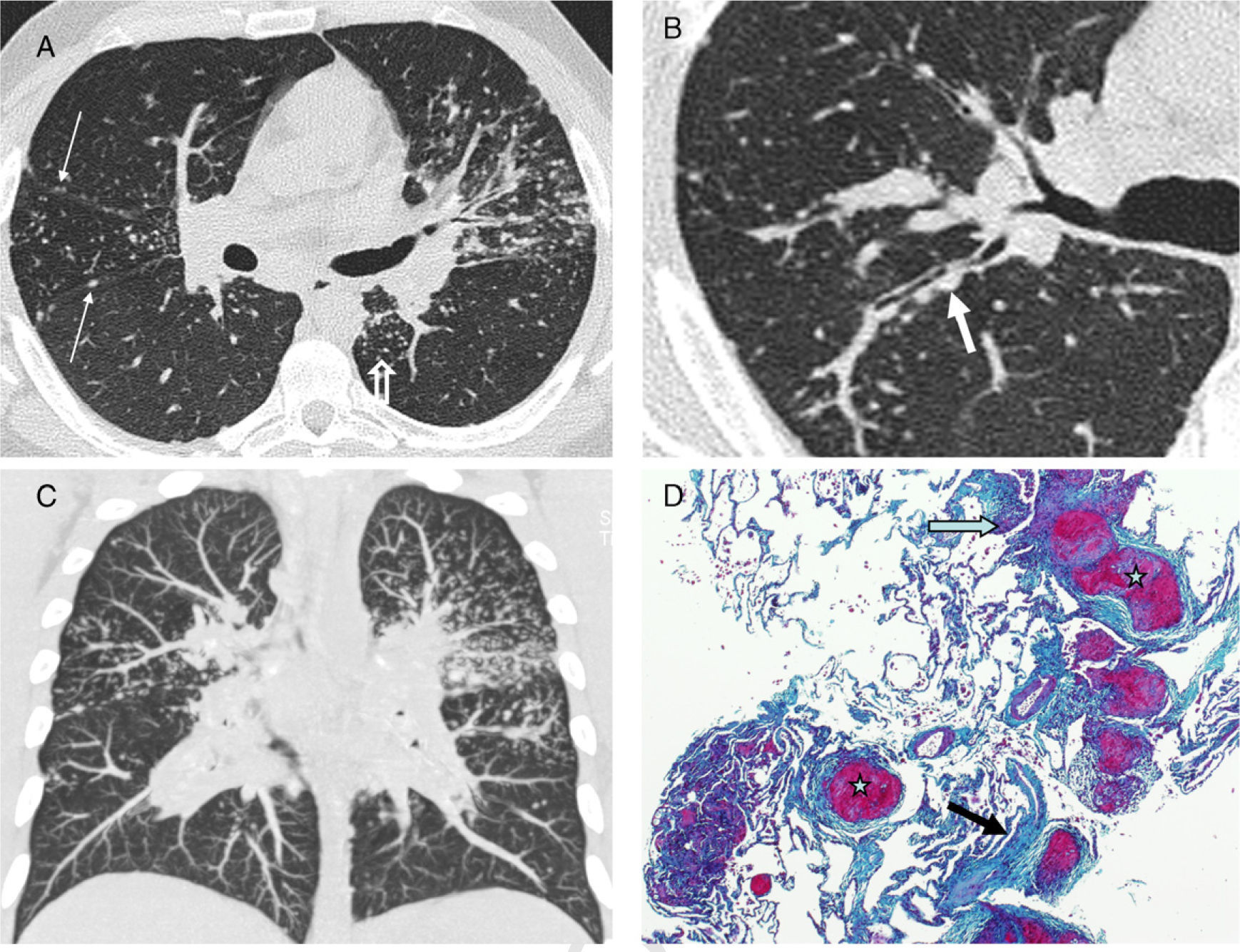
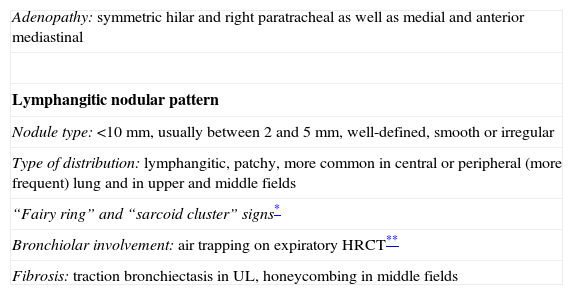
Perilymphatic distribution of pulmonary granulomas is readily detected on HRCT.13 The most common findings and those that represent the characteristic appearance of the disease are well-defined, small nodules <1cm (most frequently between 2 and 5mm), smooth or irregular, with lymphangitic distribution (lymphangitic nodular pattern): peribronchovascular, centrilobular, subpleural and, to a lesser extent, in the interlobular septa14,15 (Fig. 1). Although the lesions can be seen in the central lung, usually in a peribronchovascular and centrilobular distribution, they are more commonly seen in the peripheral lung, usually in a centrilobular and subpleural distribution and along the fissures. Moreover, the involvement is commonly symmetric and patchy, and the lung zones most often involved are the upper and middle fields16 (Table 1).
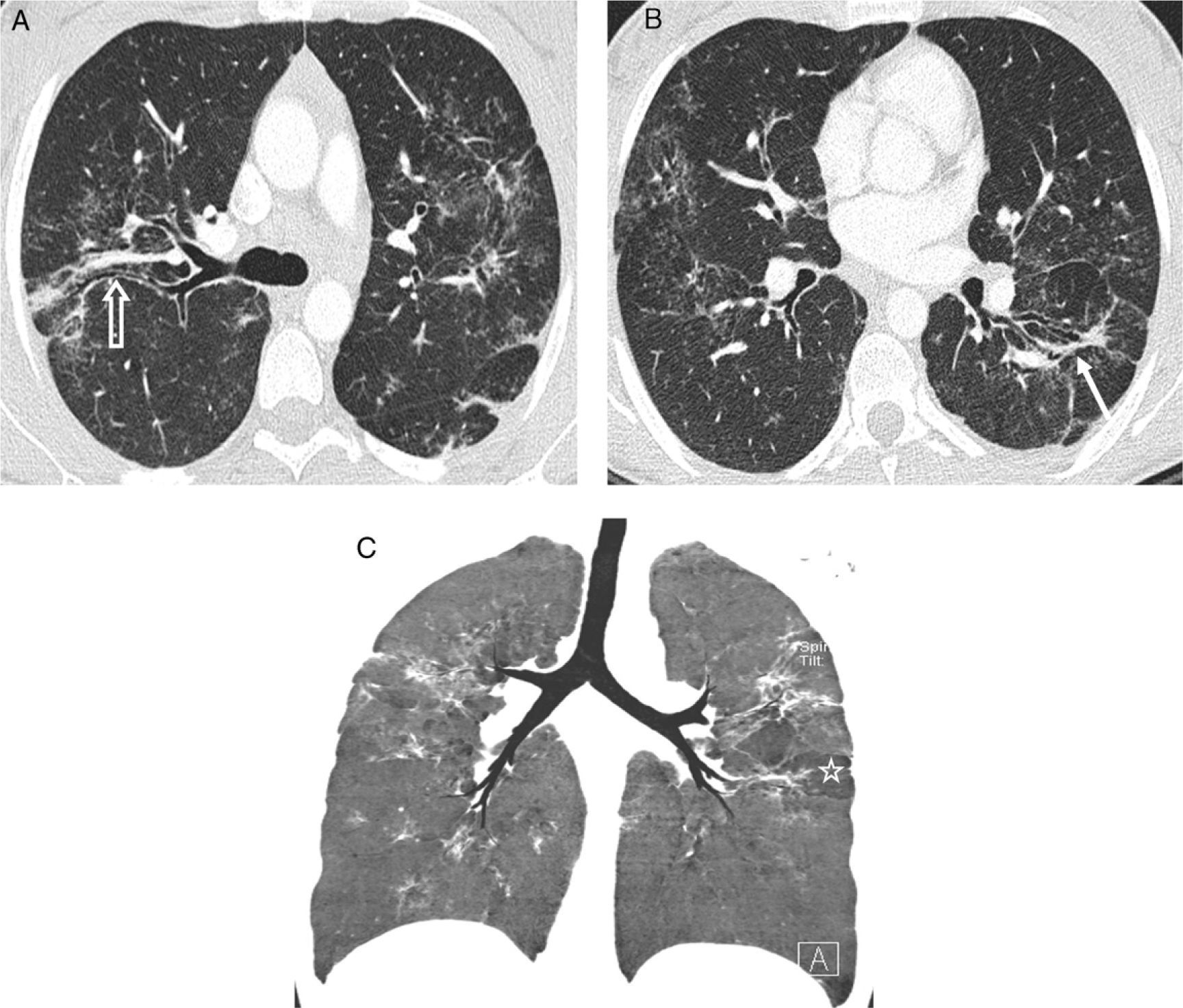
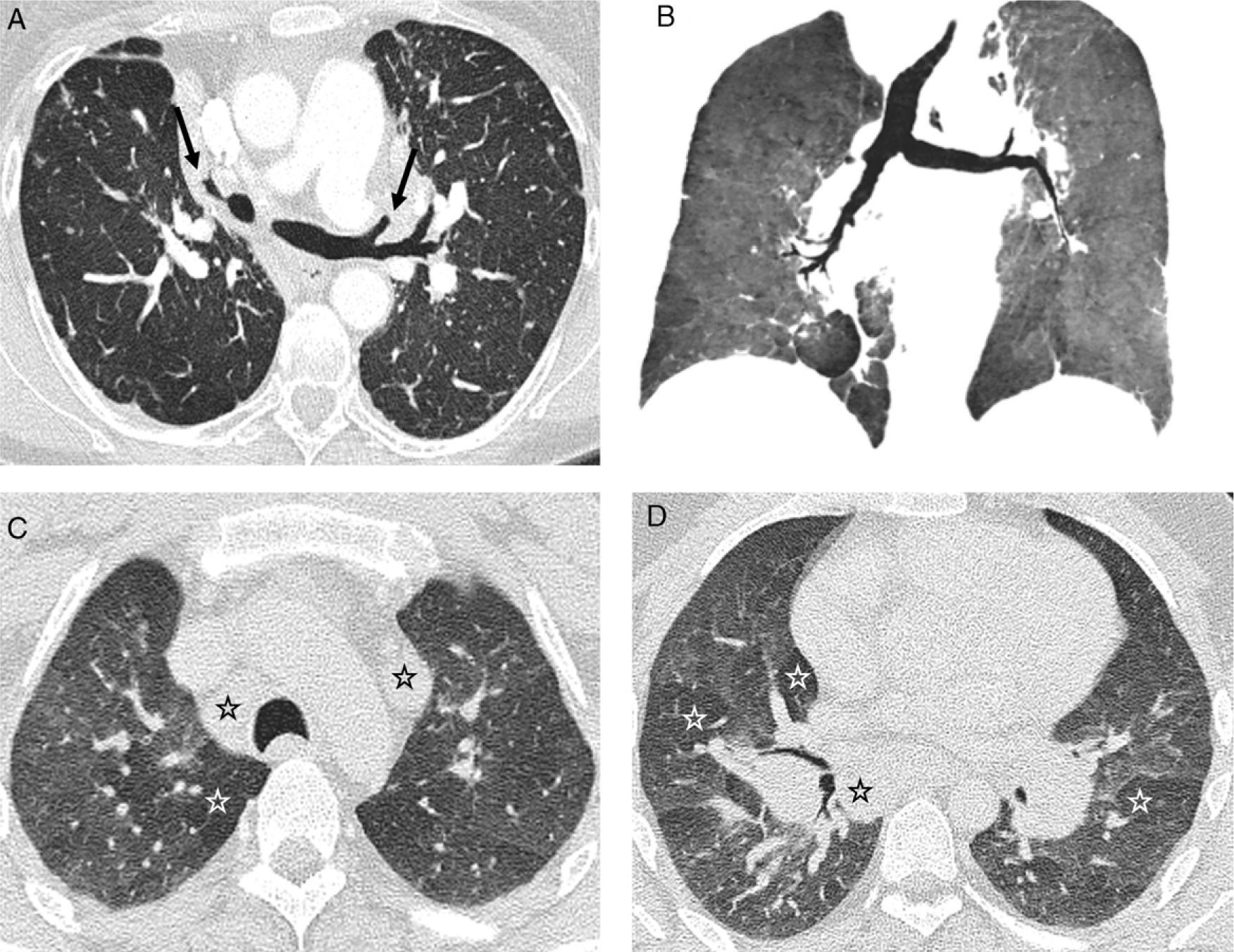
Typical pattern of sarcoidosis. (A and B) Axial HRCT: patchy lymphangitic nodular pattern with peribronchovascular (thick arrow), centrilobular (open arrow) and subpleural (thin arrows) small nodules. (C) Coronal MIP image: predominant perihilar distribution in the middle field. (D) Photomicrograph of transbronchial biopsy specimen: well-formed granulomas (stars) in a lymphangitic distribution (interlobular septum–thick arrow–and bronchovascular bundle–open arrow) (Masson's trichrome).
Typical HRTC findings of sarcoidosis.
| Adenopathy: symmetric hilar and right paratracheal as well as medial and anterior mediastinal |
| Lymphangitic nodular pattern |
| Nodule type: <10mm, usually between 2 and 5mm, well-defined, smooth or irregular |
| Type of distribution: lymphangitic, patchy, more common in central or peripheral (more frequent) lung and in upper and middle fields |
| “Fairy ring” and “sarcoid cluster” signs* |
| Bronchiolar involvement: air trapping on expiratory HRCT** |
| Fibrosis: traction bronchiectasis in UL, honeycombing in middle fields |
Other nodular patterns have been described in patients with sarcoidosis including the centrilobular distribution, due to isolated or predominant involvement of the centrilobular interstitium,17 and the random or miliary pattern18; both atypical and uncommon findings of the disease.
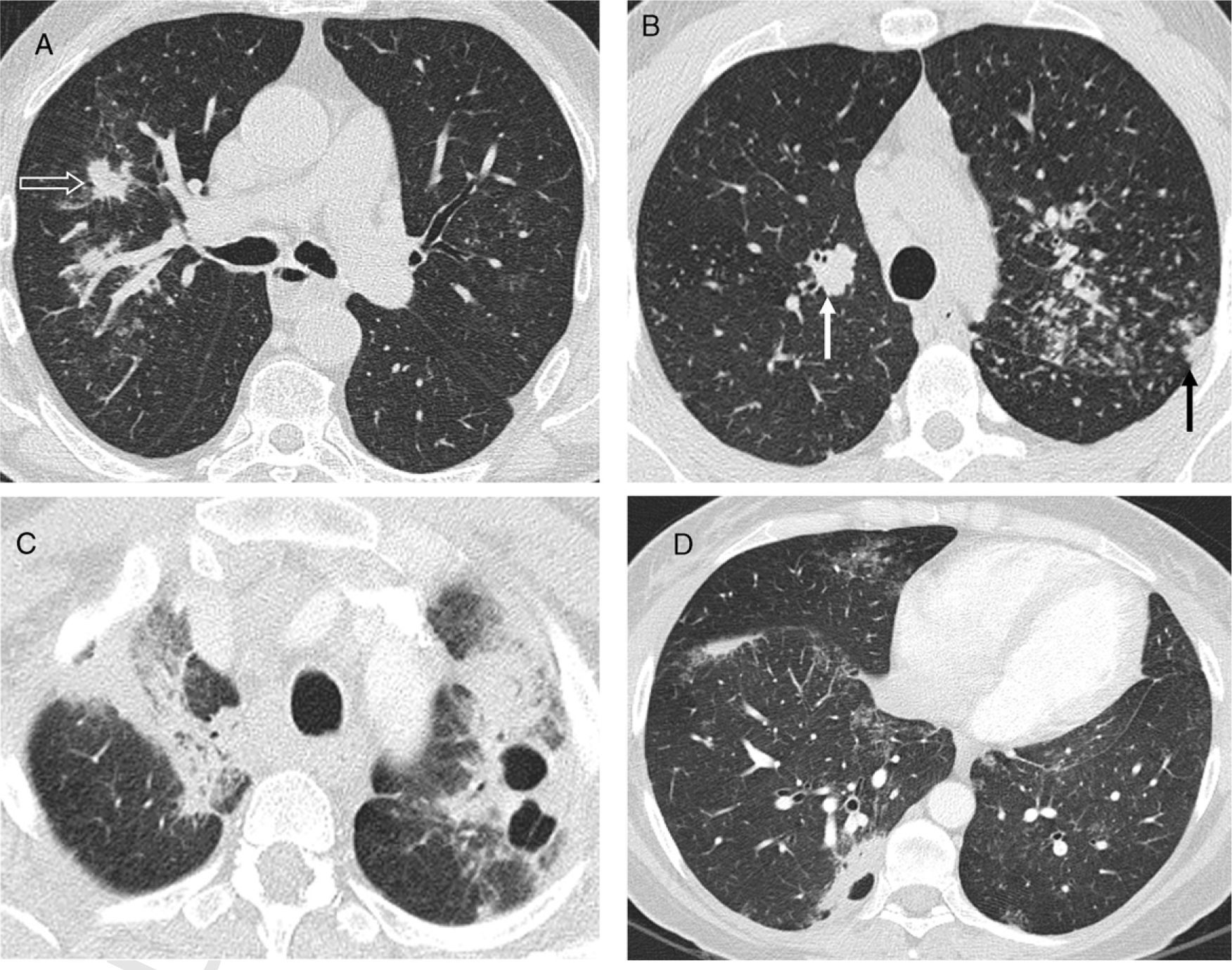
Other atypical pulmonary lesions, secondary to coalescent granulomas,19 are large nodules, masses, ground-glass opacities and consolidations12 (Table 2). Large nodules, 1–3cm in diameter, and masses >3cm may cavitate and very seldom calcify.20 These nodules may appear as a single finding or associated with other lesions (Fig. 2). Ground-glass appearance always accompanies other pulmonary abnormalities and sometimes superimposes the nodular pattern. The presence of consolidations (“alveolar sarcoidosis”), usually peribronchovascular and with aerial bronchogram, is usually associated with a nodular pattern (Fig. 3). Both the ground-glass attenuation and consolidations are due to interstitial accumulation of granulomas, with or without microscopic fibrosis. This accumulation leads to partial (ground-glass) or total (consolidation) collapse of the alveolar spaces.
Atypical HRCT findings of sarcoidosis.
| Adenopathy: unilateral, internal mammary, posterior mediastinal |
| Pulmonary patterns: centrilobular nodular, miliary, large nodules and masses, ground-glass, consolidations, paving, interlobular septal and calcified micronodules |
| Pulmonary fibrosis: similar to IPF |
| Bronchial, pleural, cardiac, mediastinal and vascular involvement |
IPF: idiopathic pulmonary fibrosis.
Atypical pattern of sarcoidosis. (A) Axial HRCT: large spiculated nodules in RUL (open arrow). (B) Axial HRCT axial: polylobulated peribronchovascular large nodules in RUL (white arrow) and subpleural in LUL (black arrow) as well as centrilobular and subpleural small nodules. (C and D) Axial HRCT: cavitating masses in a different patient.
Atypical pattern of sarcoidosis. (A) Axial HRCT: ground-glass areas with “black bronchus” sign and superimposed small nodules; some thickened interlobular septa (arrow). (B) Sagittal MIP image in the same patient shows centrilobular and supleural small nodules predominantly in the posterior part of the lower lobes; nodules in minor fissure (arrow). (C and D) Axial HRCT in a patient with “alveolar” sarcoidosis: peribronchovascular consolidations with aerial bronchogram, and multiple centrilobular and subpleural small nodules, some of them clustered together. Ground-glass opacity superimposing many of the small nodule areas.
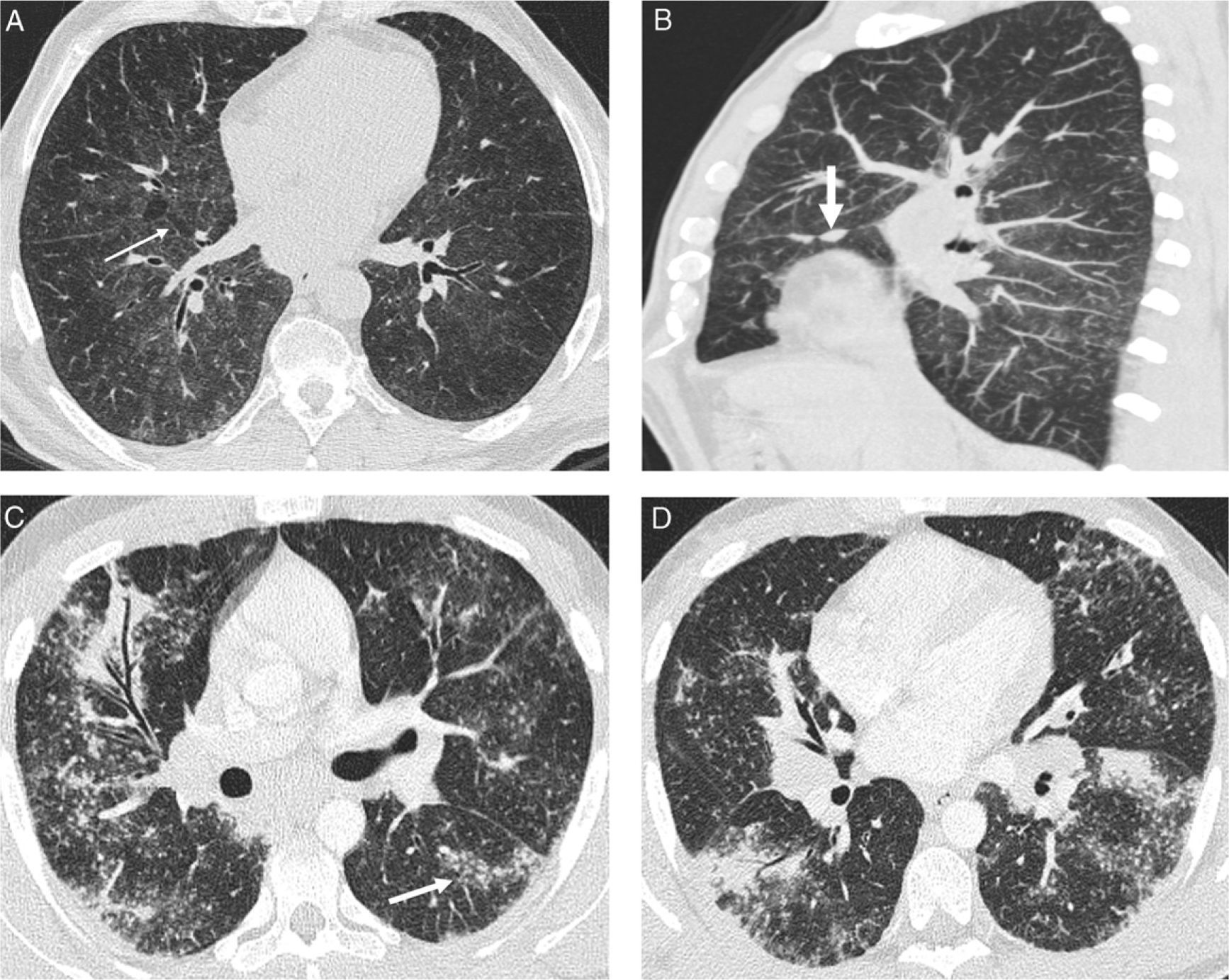
Other less common findings include the “paving” pattern,21 the septal pattern as dominant finding (Fig. 4), the calcified micronodular pattern simulating pulmonary alveolar microlithiasis, the halo sign22 and reversed halo sign (small nodules in the central ground-glass area and surrounding the outer areas of consolidation).23
Atypical pattern of sarcoidosis. (A) Coronal HRCT: smooth thickened interlobular septa (arrow), predominantly in upper fields, and multiple subpleural and centrilobular small nodules. (B) Coronal MIP images detect better the lymphangitic nodular pattern and calcified mediastinal adenopathy some of them in an eggshell pattern (arrow).
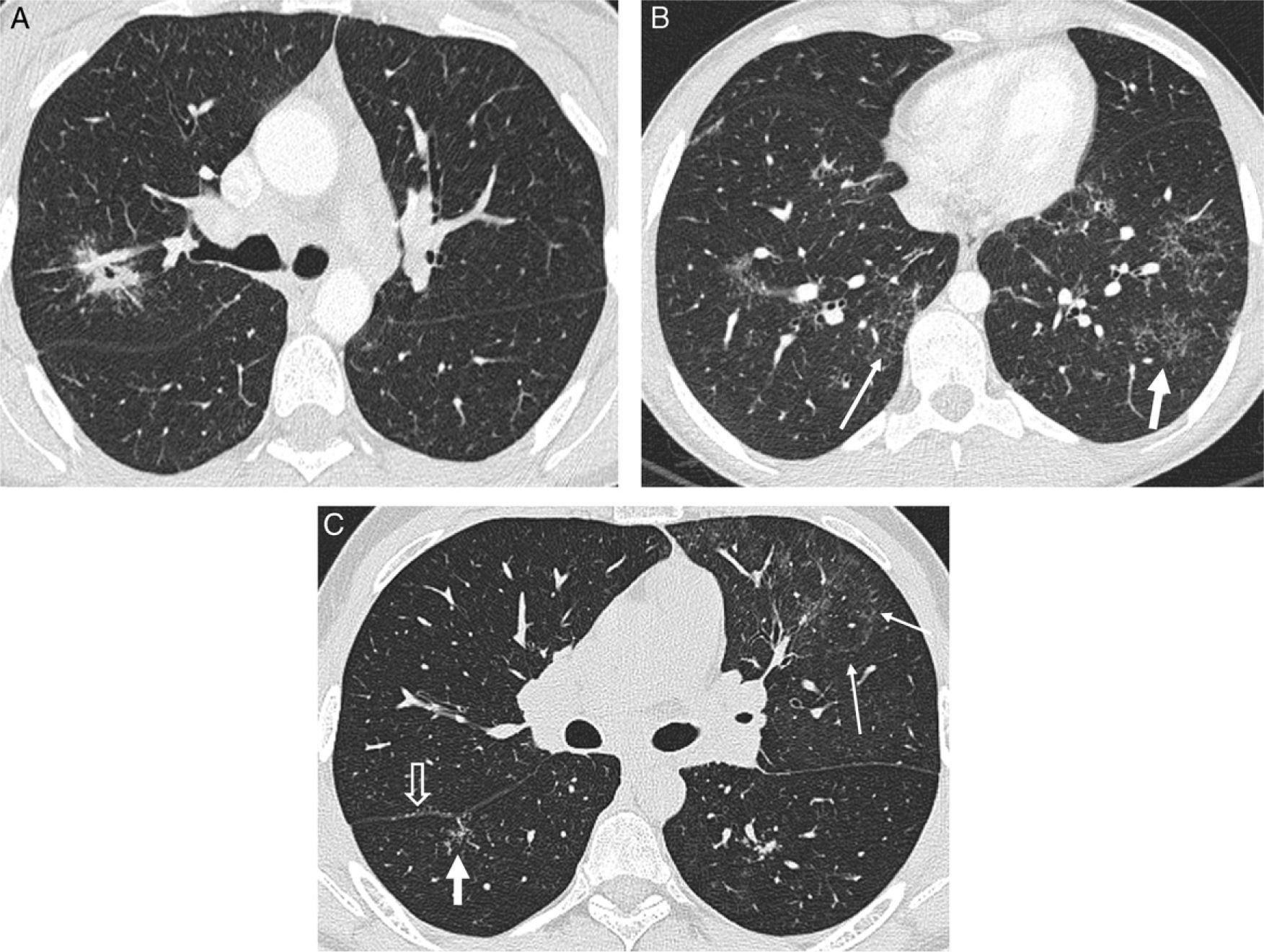
In some cases, the “sarcoid galaxy” sign appears as a result of the particular distribution of granulomas: a large nodule that arises from the coalescence of granulomas surrounded by tiny satellite lesions.24 This finding may also be seen in tuberculosis and lung carcinoma. Other typical signs of sarcoidosis are the “fairy ring”, where tiny nodules organize in a circumferential fashion,25 and the recently described “sarcoid cluster” sign, where multiple tiny centrilobular nodules, very close to each other but noncoalescing, cluster together in the peripheral regions of the lung.26 When the clusters are located in the subpleural peripheral regions, the clusters contain tiny subpleural nodules (Fig. 5). Studies on the correlation between imaging and histopathologic findings reveal that the small nodules correspond to noncaseating, noncoalescing granulomas with a predominance of CD4+ lymphocytes, without fibrosis and with lymphangitic distribution. The “sarcoid cluster” sign has also been found in one patient with tuberculosis.27
Signs of sarcoidosis. (A) Axial HRCT: “sarcoid galaxy” sign, where the central cavitating nodule is surrounded by satellite tiny nodules. (B) “Sarcoid cluster” sign: multiple clusters of tiny centrilobular (thick white arrow) nodules and centrilobular and subpleural (thin white arrow) tiny nodules. (C) “Sarcoid cluster” (thick arrow) and “fairy ring” sign (thin arrows). Subpleural small nodules in the right major fissure (open arrow).
HRCT is more accurate than chest radiography for the assessment of pulmonary fibrosis8 (Fig. 6), which causes central and peripheral bronchiectasis and significant lung distortion,28 linear opacities or irregular bands with traction bronchiectasis and volume loss (Fig. 7) or masses with traction bronchiectasis and volume loss. In either case, the upper and middle lung fields are more commonly affected typically resulting in a posterior displacement of the main bronchi and upper lobe bronchi. Sometimes a honeycomb pattern with peripheral distribution appears in the upper and middle fields. Only in a very small number of cases, the honeycomb pattern appears predominantly in the lower fields producing appearances similar to IPF29 (Fig. 8). Nodules can be seen associated with other patterns of fibrosis but not with honeycombing.30
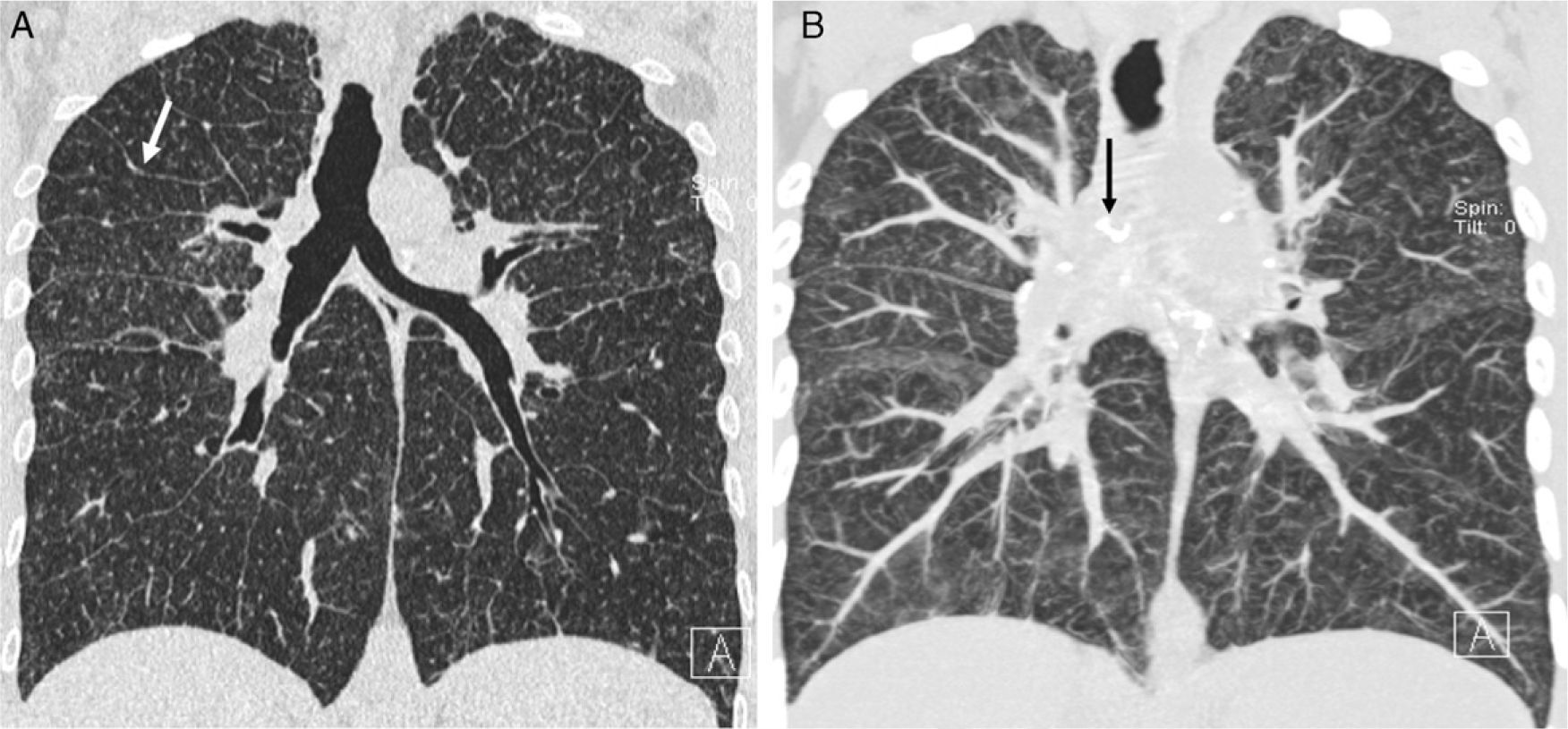
Stage II sarcoidosis. Discrepancy with HRCT findings. (A) Chest radiograph: lymph node and lung involvement. (B and C) Axial HRCT: signs of fibrosis (peripheral subpleural basal honeycomb cysts–black arrows–and traction bronchiectasis–white arrow) not visible on chest radiograph; and nodular pattern. (D) Sagittal MIP image: centrilobular (white arrows) and subpleural (black arrows) small nodules with a slight predominance in the lower half of the lung fields.
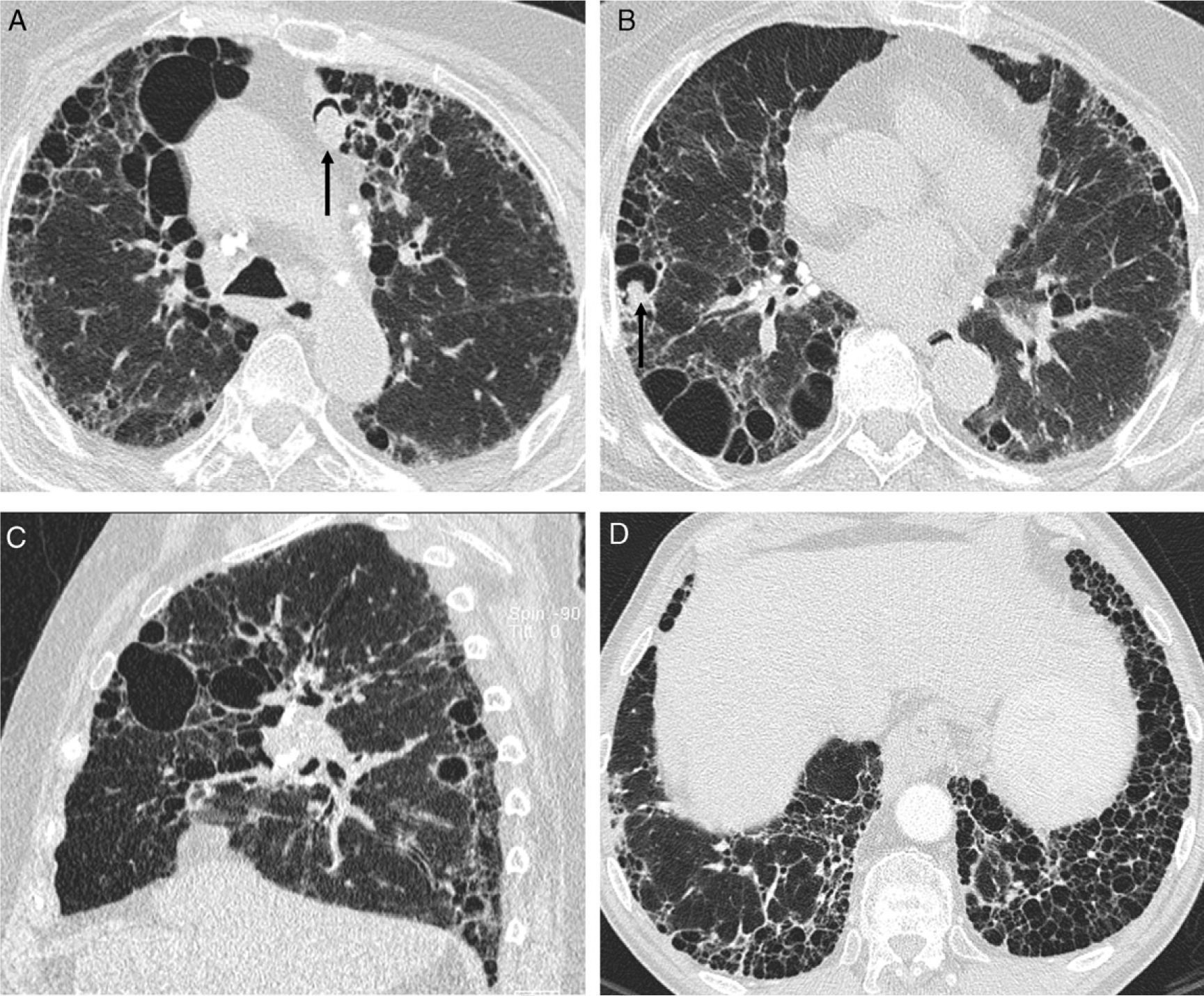
Stage IV sarcoidosis. (A and B) Axial HRCT: irregular dense bands (solid arrow) with traction bronchiectasis (open arrow) and distortion of lung architecture predominantly in the posterior segments of upper lobes and apical segments of inferior lobes. Multiple small nodules clustered together. (C) Coronal minIP image shows the predominant lesion distribution in middle fields, traction bronchiectasis and patchy areas of low attenuation secondary to small airway involvement (star).
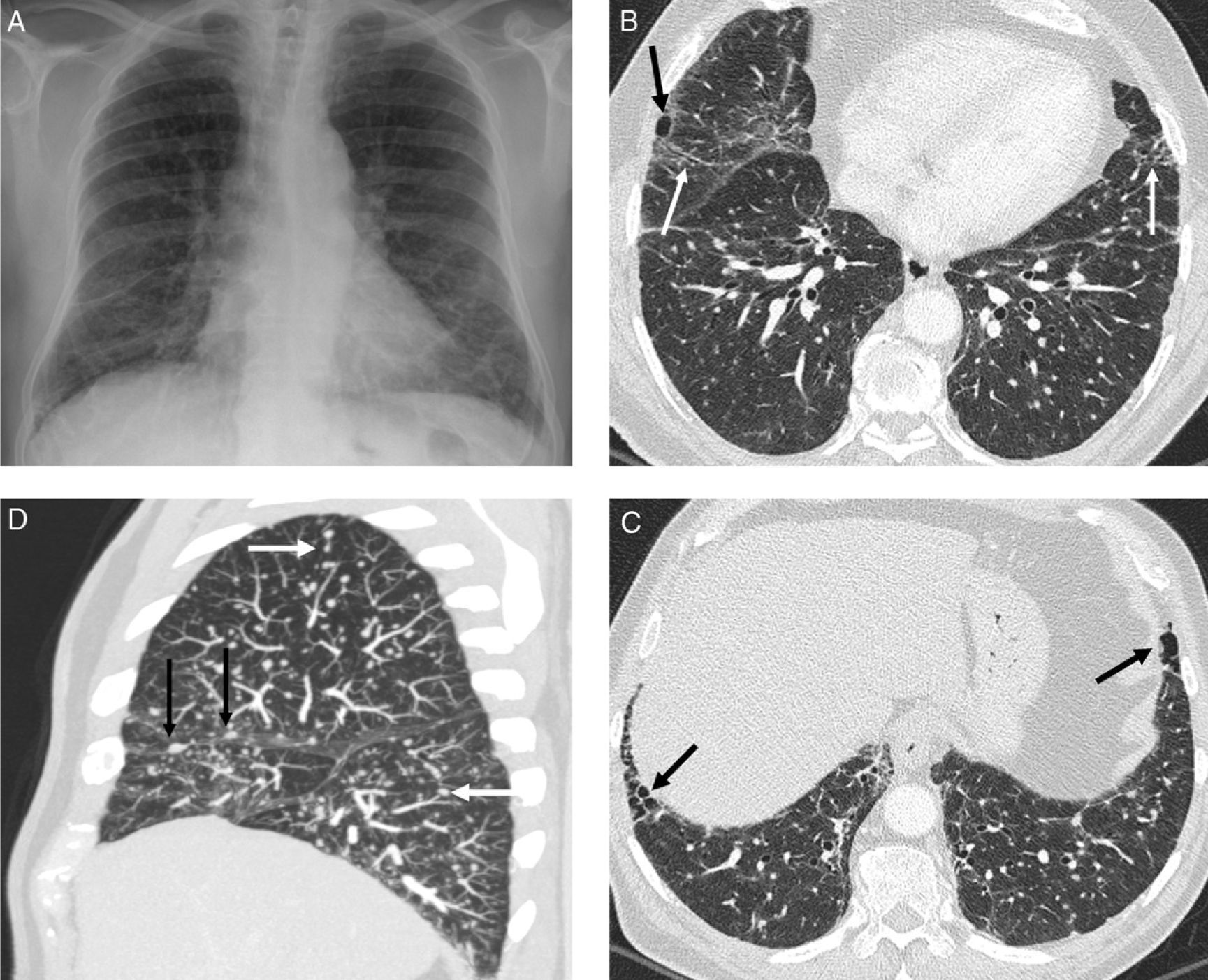
Stage IV sarcoidosis. (A and B) Axial HRCT: honeycomb cysts, some of them large in size, with predominant peripheral distribution. Mycetomas in two of the honeycomb cysts (arrows) and hilar and mediastinal calcified adenopathy. (C) Sagittal HRCT: honeycombing predominantly in the middle fields. (D) Axial HRCT of a patient with predominant basal honeycombing, similar to IPF (lymph node biopsy by mediastinoscopy).
HRTC may demonstrate signs of bronchial involvement31 such as bronchial wall thickening, stenosis and airway obstruction leading to distal atelectasis. These findings are secondary to intramural and endobronchial granulomas. Compression of the bronchi by adjacent lymph nodes may also occur. However, signs of bronchiolar involvement such as air trapping on expiratory HRCT are more common (Fig. 9). Air trapping is the second most common finding after the lymphangitic nodular pattern. It can appear at any stage of the disease and sometimes is the only finding in the lung fields. It is secondary to small airway obstruction caused by peribronchiolar or intraluminal granulomas.32,33 The morphology of air trapping on expiratory HRCT is lobular, with patchy areas of decreased attenuation in comparison with the rest of the parenchyma.34 Inspiratory HRCT shows air trapping areas with decreased vascularization caused by reflex vasoconstriction, and areas of decreased attenuation (vascularization and mosaic attenuation patterns). Maximum and minimum intensity projections (MIP and minIP) may also help in the visualization.35
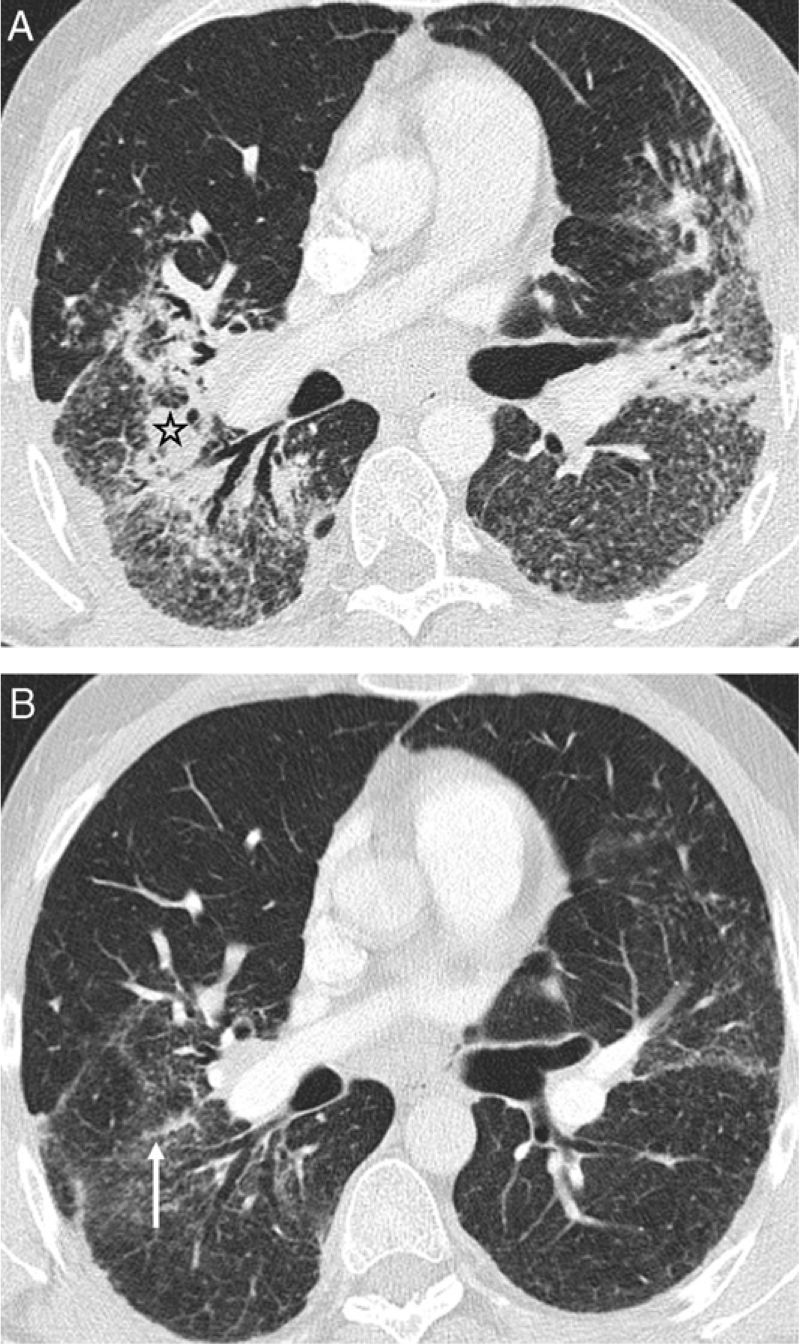
Airway involvement. (A) Axial HRCT and (B) coronal minIP image: complete bronchial obstruction of upper lobes (arrows), with distal atelectasis. Patchy areas of low attenuation secondary to small airway involvement. (C and D) Expiratory HRCT: patchy air trapping areas (white stars) as the predominant manifestation of small airway involvement in a patient with lymph node involvement (black stars) (chest radiographic stage I disease).
HRCT is very useful in the assessment of the complications of sarcoidosis such as chronic respiratory failure secondary to fibrosis, pulmonary hypertension and hemoptysis.36 The presence of a mycetoma in a cavitating lesion is the main cause of hemoptysis in patients with sarcoidosis (Fig. 8). Other causes are bronchiectasis, necrotizing bronchial aspergillosis, semi-invasive pulmonary aspergillosis, erosion of a pulmonary artery due to an adjacent necrotic lesion, and endobronchial lesions.
Bronchomalacia, or bronchial collapse at the end of expiration, also has been described as a complication of the bronchial involvement of sarcoidosis37 and it is associated with greater air trapping.
High resolution computed tomography diagnosisThe distinctive nodular pattern with lymphangitic distribution of sarcoidosis on HRCT is virtually pathognomonic. Diagnostic accuracy increases when HRCT findings are integrated with clinical and chest radiographic data. A correct imaging diagnosis is almost always correct when based on these findings.38,39
Despite the discrepancy between chest radiographic and HRCT findings in a high number of patients due to the higher sensitivity of HRCT in the detection of adenopathy, lung abnormalities and fibrosis, the prognostic value of HRCT has not been sufficiently evaluated and staging is therefore still based on chest radiographic findings.1,10
There are no imaging criteria indicative of reversible or irreversible disease or of progression to fibrosis (which appears in 20% of patients). Nonetheless, HRCT may provide information about its potential reversibility38: nodules are reversible in most cases, but not always; distortion of the lung architecture, traction bronchiectasis and honeycombing are irreversible; ground-glass opacification is unpredictable since it may remain stable, progress or resolve, depending on whether it corresponds to active disease or macroscopic fibrosis (Fig. 10). A trial including 40 patients in a 7-year follow-up period40 reported that ground-glass opacities and consolidations most frequently progress to honeycomb cysts (5 and 3 patients, respectively), whereas abnormalities in nodular and large nodular pattern resolve or decrease in size. Fibrosis does not disappear and is associated with worse prognosis and increased morbidity and mortality.
Progression of the disease, before (A) and after (B) treatment: resolution of peribronchovascular consolidations and of many diffuse small nodules with randomly distributed predominantly in the lower lobes. Bronchiectasis and loss of volume persist in RLL. An irregular line (arrow), visible before treatment, has appeared in the site of the large nodule (star).
The studies regarding the correlation of HRCT with other markers of activity such as 67Ga scintigraphy, lymphocyte count and CD4/CD8 ratio in the BAL fluid and ACE assay differ. However, the study by Leung et al.41 reported good correlation between these tests and the extent of nodules and consolidation. Correlation between HRCT and pulmonary function tests differs among studies,8 but the presence of fibrosis is associated with abnormal tests, especially in patients with honeycomb cysts. Functional impairment is relatively minor in patients with linear opacities or irregular bands
HRCT scanning should not be performed indiscriminately in all patients with suspected or proven sarcoidosis.42 The joint statement of the ATS/ERS/WASOG (World Association of Sarcoidosis and other Granulomatous Disorders) issued in 19991 recommends lung CT scans in patients with atypical clinical and/or chest radiographic findings, when there is a normal chest radiograph but a clinical suspicion of the disease,43 and for the detection of complications of the disease such as bronchiectasis, aspergilloma, pulmonary fibrosis, traction emphysema, or superimposed infection or malignancy.
High resolution computed tomography in the differential diagnosis of sarcoidosisThe imaging differential diagnosis includes berylliosis (history of exposure to beryllium, with similar imaging and histologic findings), other pneumoconiosis (like silicosis and anthracosis), lymphangitis carcinomatosis and lymphoma.44,45 In the “alveolar” variant of the disease, the differential diagnosis includes vasculitis, infections, cryptogenic organizing pneumonia, adenocarcinoma and lymphoma.46 In patients with pulmonary fibrosis, the differential diagnosis includes other causes of fibrosis such as chronic extrinsic allergic alveolitis, IPF and lung fibrosis related to collagen diseases and to drug toxicity. The presence of a nodular pattern helps in the diagnosis of the disease.47
Tracheal involvementTracheal involvement is rare and is either secondary to compression from adjacent lymph nodes or to the presence of granulomas within the airway mucosa and submucosa resulting in luminal stenosis and wall thickening that can be smooth, irregular or nodular. Extensive tracheal involvement may cause bronchomalacia.47 Differential diagnosis includes tracheobronchopathy osteochondroplastica and relapsing polychondritis (which do not affect the posterior tracheal wall), amyloidosis and Wegener's granulomatosis.
Pleural involvementSarcoid granulomatous involvement of the pleura is rare. It causes pleural effusion that resolves in 2 or 3 months and may leave behind pachypleuritis.
Spontaneous pneumothorax is a rare complication that occurs in 2–5% of patients with long-standing sarcoidosis, especially in those with fibrosis. Pneumothorax can be recurrent, although it can also be the initial manifestation of the disease.48 Causes of pneumothorax include rupture of a subpleural bleb or necrosis of a subpleural granuloma. Bilateral and tension pneumothorax as well as hemopneumothorax have also been described.49
Vascular involvementSarcoid granulomatous involvement of the pulmonary arteries and veins is noted in 42–89% of lung biopsies. However, clinically significant pulmonary vascular involvement is uncommon.
Pulmonary hypertension occurs in 1–5% of patients and is associated with higher mortality rates.10,50 The mechanisms contributing to pulmonary hypertension include the fibrous destruction of the pulmonary vascular bed,51 extrinsic compression of the central pulmonary arteries by lymphadenopathy, granulomatous mediastinitis52 and intrinsic vasculopathy. Another cause of pulmonary hypertension is left ventricular dysfunction.
Cardiac involvementCardiac sarcoidosis can be primary or secondary. Secondary involvement corresponds to cor pulmonale secondary to pulmonary disease-related hypertension. Primary cardiac sarcoidosis is caused by myocardial infiltration with granulomas resulting in restrictive cardiomyopathy or heart failure.53 Its incidence in autopsies of patients with sarcoidosis ranges between 20 and 60%. Nonetheless, cardiac involvement is seldom recognized during the course of sarcoidosis since myocardial infiltration is usually silent and approximately only 10% of the patients have symptoms. Given the fact that cardiac sarcoidosis can lead to sudden death, early diagnosis and prompt treatment are essential for these patients. However, there is not scientific evidence supporting the screening for cardiac involvement in asymptomatic patients with proven sarcoidosis.54
The most common manifestations include atrioventricular conduction defects and branch blocks, followed by sustained and non-sustained ventricular tachycardia and atrial arrhythmia.54
Ventricular wall infiltration with granulomas may result in decreased myocardial contractility (systolic failure) or decreased ventricular relaxation (diastolic failure), with heart failure in both cases. Heart failure may also result from mitral dysfunction secondary to infiltration or rupture of papillary muscles. When heart failure occurs, the disease is generally in the late stages.
Since none of the diagnostic modalities available combines high sensitivity and high specificity, the diagnosis should be based on a combination of ECG, morphologic, functional and/or histologic findings. Magnetic resonance imaging (MRI) is the most sensitive and specific non-invasive technique for the diagnosis of cardiac sarcoidosis and the most useful imaging technique for early diagnosis.
The most common MRI findings are morphologic and functional abnormalities (areas of ventricular dilation, segmental contractility defects and areas of basal thinning of the left ventricle), valve insufficiency and pericardial effusion. In addition, MRI provides additional information during T2-weighted and delayed gadolinium enhancement sequences.55
Cardiac MRI helps identify the three stages in the disease process: the initial phase of edema that is followed by granulomatous infiltration, and last phase of postinflammatory scarring. These stages may superimpose and findings of the three stages may be found in the same patient.56
The administration of a contrast agent (0.1mmol/kg) is essential for the diagnosis of cardiac sarcoidosis. First step perfusion and “black myocardium” sequences are analyzed; this way, the normal hypointense myocardium contrasts with abnormal areas, characteristically hyperintense. Myocardial scarring appears as patchy areas of hypokinesis and myocardial thinning, with enhancement in a predominantly subpericardial distribution on “black myocardium” sequences. Cases of subendocardial enhancement in areas of hypokinesis and thinning have been described although, unlike ischemic heart disease, cardiac sarcoidosis shows no vascular distribution. Steroid treatment reduces the areas of edema and of delayed enhancement57 (Fig. 11).
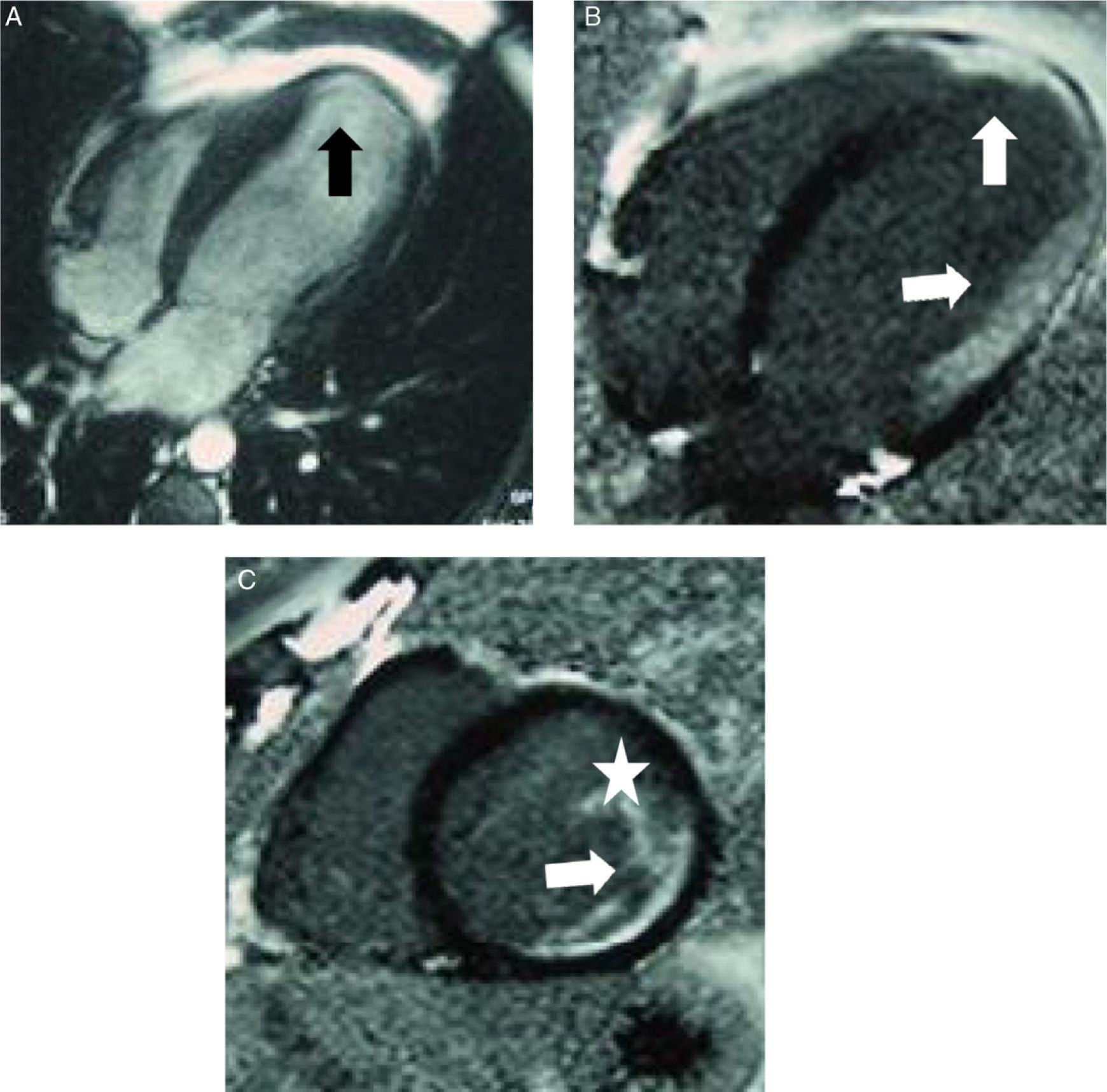
Cardiac sarcoidosis. Patients with heart rate alterations, cardiac enzymes and normal cardiac catheterism. The patient required cardiac transplantation and the explanted specimen confirmed the diagnosis. (A) Four-chamber gradient echo sequence: apical thinning of left ventricle, which appears slightly dilated (black arrow). (B and C) Four-chamber and short-axis, respectively, “black myocardium” sequences show and area of delayed transmural (arrow) and subendocardial enhancement in left ventricle that extends to the papillary muscle (arrow and white star).
Myocardial abnormalities are more common than pericardial, with a special predilection for the basal segments and free left ventricular wall and less commonly for the right ventricle and atria58,59 (Table 3).
Other diagnostic techniques include ECG, echocardiography, positron emission tomography (PET), gallium-67 and/or thallium-201 scintigraphy and endomyocardial biopsy.60
Histologic diagnosisThe aim of histologic examination in patients with suspected pulmonary sarcoidosis is to provide evidence of well-formed non-necrotizing granulomas with a perilymphatic distribution. This is achieved in most cases by transbrochial biopsy (TBB), which has a sensitivity of 80–90%. TBB may detect pulmonary disease in the absence of HRCT abnormalities. HRCT increases the diagnostic accuracy of TBB by helping in the selection of the sites with sarcoid activity and guiding the biopsy.61 Given the fact that endobronchial involvement is common in sarcoidosis, endobronchial biopsy can increase the diagnostic value of bronchoscopy when added to TBB.62
Surgical biopsy of mediastinal adenopathy, by mediastinoscopy or mediastinotomy, and lung biopsy, by open biopsy or videoassisted thoracoscopic, are indicated only in case of clinical and radiological suspicion of sarcoidosis when other types of biopsy are nondiagnostic.
Both TBB and lung biopsy should be performed at sites of disease activity, identified by HRCT. Some studies have reported that PET scanning help locate occult sites of active disease and perform the biopsy.64
In addition to detecting sarcoid granulomas, the pathologist should rule out bacterial, mycobacterial and fungal infections, berylliosis, granulomatosis caused by intravenous injection of talc, extrinsic allergic alveolitis, and drugs such as methotrexate, lymphoma and vasculitis. Sarcoidlike reactions in lymph nodes and lung parenchyma have also been described in patients with thoracic and extrathoracic malignancies.65 Granulomas with a sarcoidlike pattern are, by themselves and in the absence of an identifiable cause, nonspecific lesions and not diagnostic of sarcoidosis.66
In patients with suspected cardiac sarcoidosis, endomyocardial biopsy can demonstrate edema, non-necrotizing granulomas infiltrate and fibrosis. However, endomyocardial biopsy may frequently be negative in cardiac sarcoidosis. This is due to the patchy distribution of the cardiac lesions and to the fact that biopsy specimens are taken from the apical interventricular septum, at the side of the right ventricle.60 The sensitivity of endomyocardial biopsy is thus low (20–63%) which, together with the invasive nature of the technique, makes other diagnostic approaches more recommended. A positive biopsy is therefore not needed for diagnosis.1
Consensus diagnosis based on clinical imaging and pathologic findingsThe final diagnosis of pulmonary sarcoidosis is established when compatible clinical and radiological findings are supported by histologic evidence of noncaseating, or minimally caseating, granulomas in a lymphangitic distribution and once other diseases with similar findings are ruled out.67,68 In cases of typical presentation of Löfgren's syndrome, biopsy is not needed. A diagnosis of high probability without histologic confirmation is also accepted when compatible clinical and radiological findings are supported by a BAL CD4/CD8 ratio >3.5 (specificity 93–96%, and sensitivity 53–59%).63,69 In multidisciplinary teams, the diagnosis should be established by consensus.70
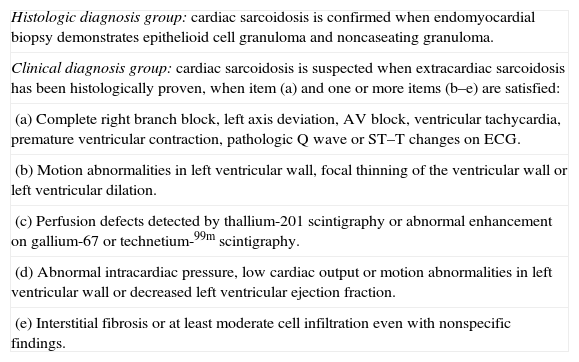
Although not widely accepted by the international scientific community, the Japanese Ministry of Health Guidelines are very useful in the diagnosis of cardiac sarcoidosis (Table 4).54 New research studies are thus needed to develop a diagnostic algorithm to assess the cardiac involvement of sarcoidosis.68
Criteria issued by the Japanese Ministry of Health for the diagnosis of cardiac sarcoidosis.
| Histologic diagnosis group: cardiac sarcoidosis is confirmed when endomyocardial biopsy demonstrates epithelioid cell granuloma and noncaseating granuloma. |
| Clinical diagnosis group: cardiac sarcoidosis is suspected when extracardiac sarcoidosis has been histologically proven, when item (a) and one or more items (b–e) are satisfied: |
| (a) Complete right branch block, left axis deviation, AV block, ventricular tachycardia, premature ventricular contraction, pathologic Q wave or ST–T changes on ECG. |
| (b) Motion abnormalities in left ventricular wall, focal thinning of the ventricular wall or left ventricular dilation. |
| (c) Perfusion defects detected by thallium-201 scintigraphy or abnormal enhancement on gallium-67 or technetium-99m scintigraphy. |
| (d) Abnormal intracardiac pressure, low cardiac output or motion abnormalities in left ventricular wall or decreased left ventricular ejection fraction. |
| (e) Interstitial fibrosis or at least moderate cell infiltration even with nonspecific findings. |
- 1.
Responsible for the integrity of the study: IHO.
- 2.
Conception of the study: IHO.
- 3.
Design: IHO.
- 4.
Data collection: IHO, LLG.
- 5.
Analysis and interpretation of data: IHO, LLG.
- 6.
Statistical analysis: N/A.
- 7.
Bibliographic search: IHO, LLG.
- 8.
Drafting of the manuscript: IHO, LLG.
- 9.
Critical review with intellectually relevant contributions: IHO, LLG.
- 10.
Approval of the final version of the manuscript: IHO, LLG.
The authors declare no conflicts of interests.
Please cite this article as: Herráez Ortega I, López González L. La sarcoidosis torácica. Radiología. 2011;53:434–48.