The main objectives of this study were to evaluate the sensitivity and specificity of duplex Doppler ultrasonography in the study of hemodialysis peripheral vascular access dysfunction and to analyze the resistance index and flow in the afferent artery.
Materials and methodsWe prospectively studied 178 patients with 178 peripheral vascular accesses that were dysfunctional in at least three consecutive hemodialysis sessions. Patients underwent duplex Doppler ultrasonography and clinical and laboratory follow-up for three months (provided angiography findings were negative). We calculated the sensitivity, specificity, predictive values, and coefficients of probability. We studied the morphology of the afferent artery, the arteriovenous anastomosis, and the efferent vein, and we measured the resistance index and the flow of the afferent artery, the diameter of the anastomosis, and the flow and peak systolic velocity in the efferent vein.
ResultsThe final sample consisted of 159 patients. The sensitivity, specificity, positive and negative predictive values, and positive and negative coefficients of probability were 0.98 (95% CI: 0.88–1.00), 0.74 (95% CI: 0.66–0.81), 0.96, 0.82, 3.7, and 0.03, respectively. The resistance index was less than 0.5 in 78.5% of the peripheral vascular accesses with normal function and greater than 0.5 in 86.1% of the dysfunctional peripheral vascular accesses. We found aneurysms in 19 of the native peripheral vascular accesses and pseudoaneurysms in 7 of the prosthetic grafts. Inverted flow was seen in 57 peripheral vascular accesses.
ConclusionDuplex Doppler ultrasonography is an efficacious method for detecting and characterizing stenosis and thrombosis in peripheral vascular accesses, and it provides information about the morphology and hemodynamics.
El objetivo principal del estudio es evaluar la sensibilidad y especificidad de la ecografía dúplex-Doppler para estudiar la disfunción de los accesos vasculares periféricos para hemodiálisis, y analizar el índice de resistencia y el flujo en la arteria aferente.
Material y métodosSe estudiaron prospectivamente 178 pacientes con 178 accesos vasculares periféricos disfuncionantes durante al menos 3 sesiones de hemodiálisis seguidas. Se realizaron ecografía dúplex-Doppler, angiografía y seguimiento clínico y analítico durante 3 meses (si la angiografía fue negativa). Se calcularon los valores de sensibilidad, especificidad, valores predictivos y cocientes de probabilidad. Se estudiaron morfológicamente la arteria aferente, la anastomosis arteriovenosa y la vena eferente, y se midieron el índice de resistencia y el flujo de la arteria aferente, el diámetro de la anastomosis, y el flujo y velocidad picosistólica en la vena eferente.
ResultadosLa muestra final la constituyeron 159 pacientes. Los valores de sensibilidad, especificidad, valor predictivo positivo y negativo y cociente de probabilidad positivo y negativo, fueron 0.98 (IC 95% 0,88–1), 0,74 (IC 95% 0,66–0,81), 0,96, 0,82, 3,7 y 0,03 respectivamente. El índice de resistencia fue <0,5 en el 78,5% de los accesos vasculares periféricos normofuncionantes y >0,5 en el 86,1% de los disfuncionantes. Se encontraron aneurismas en 19 de los accesos vasculares periféricos nativos y seudoaneurismas en 7 de los protésicos. El flujo invertido apareció en 57 accesos vasculares periféricos.
ConclusiónLa ecografía doppler dúplex es un método eficaz de detección y caracterización de estenosis y trombosis del accesos vasculares periféricos y aporta información morfológica y hemodinámica.
Chronic kidney disease (CKD) is an increasingly important health issue whose incidence and prevalence have skyrocketed during the last years.1 End-stage renal disease is defined as the situation in which native kidneys fail as the metabolic regulating organ of the internal environment leading to the necessary substitution of such kidneys through hemodialysis or kidney transplant. Hemodialysis that is still the main option when we talk about substitute therapy needs one hemodialysis fistula or peripheral vascular access (PVA).1 PVA needs at least 600–800ml/min of blood flow2–4 to allow the elimination of uremic toxins in a reasonable amount of time. This is why PVA (native or graft) is key for the survival of these patients. PVA lesions are common– primary patency is 79.5%/year and 48% every 4 years5– and they usually condition thrombosis and closure of PVA or flow reductions in cases of stenoses in venous or arterial branches. The PVA dysfunctions or thromboses are the top resource consumers in the population with CKD. It is necessary to proceed with the early detection of dysfunction in an attempt to fix the dose of hemodialysis and find treatable structural lesions to avoid thrombosis and increase survival.
Angiography is the agreed standard of reference for the monitoring of the PVA status but it is an expensive aggressive modality that needs to be avoided and used only for diagnostic purposes. Yet despite the following issues shown by the duplex-Doppler ultrasound (DDU): operator dependence, interference of bandages and lesions, and vessel calcifications that complicate the assessment of anastomotic stenoses,6 PVAs are more and more studied with DDU before the angiography given the information delivered by the DDU is not only information on the morphological fistula but also on the efferent artery and the inflow and outflow flows with the advantage that it is a non-invasive modality, does not use ionizing radiations or iodinated contrast media and is cheap and accessible. Even though there are several studies in which DDU is the same6–10 or even better7,11 than angiographies to locate and assess the degree of stenosis of the PVA there are not too many observational prospective studies to assess their performance when there is suspicion of PVA dysfunction and to determine the causes of the DDU error.6 The main goal of this study is to evaluate the sensibility and specificity of DDU in the dysfunctions of PVA. The secondary goal is to analyze the resistive index (RI) and the afferent arteriole flow of normal and dysfunctional PVAs and to show the main non-hemodynamic morphological findings associated.
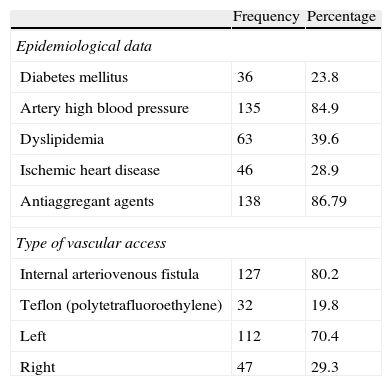
Materials and methodsPatientsFrom October 1, 2008 to December 31, 2011 the data of 178 PVAs with suspicion of dysfunction pertaining to 55 women (34.6%) and 104 men (65.4%) were prospectively collected. The average age was 24–92 years (media: 6611 years). The study was approved by the hospital ethical committee. In Table 1 the epidemiological data of our sample can be seen.
Epidemiological data and features of peripheral vascular access.
| Frequency | Percentage | |
| Epidemiological data | ||
| Diabetes mellitus | 36 | 23.8 |
| Artery high blood pressure | 135 | 84.9 |
| Dyslipidemia | 63 | 39.6 |
| Ischemic heart disease | 46 | 28.9 |
| Antiaggregant agents | 138 | 86.79 |
| Type of vascular access | ||
| Internal arteriovenous fistula | 127 | 80.2 |
| Teflon (polytetrafluoroethylene) | 32 | 19.8 |
| Left | 112 | 70.4 |
| Right | 47 | 29.3 |
To be part of our sample patients had to (1) present alterations of at least 3 back-to-back hemodialysis sessions: reduced kinetic values during dialysis (blood flow [Qb]<300ml/min, urea distribution volume [recirculation]>10%, reduction>25% of flow percentage), difficulty in PVA cannulation, prolonged bleeding after hemodialysis, high venous pressures and/or limb edema, and (2) meet all inclusion criteria and no preclusion criteria.
To be included patients needed to undergo a PVA-based hemodialysis, be 18–95 years of age, have undergone ultrasound and angiographic studies for the assessment of PVA and to have signed an informed written consent to be able to participate in the study. Preclusion criteria were the suspicion of iodinated contrast allergy, confirmed or unconfirmed pregnancy, functional PVA but substitute therapy different than hemodialysis, have undergone surgical procedure/s to maintain or reestablish PVA before carrying out the diagnostic radiological studies and hemodialysis performed through a central venous catheter. Withdrawal criteria were the reversal of informed consent at any time of the study, death, or transfer. Fourteen patients were precluded since it was not possible to perform diagnostic proceedings: 6 patients due to the PVA clinical thrombosis needing surgical thrombectomy (n=4) or the placement of a central catheter (n=2), and 8 due to loss in the follow-up (transfer to other institution n=5; death n=2; and unknown n=1). Five patients did not give us their informed consent to be part of the study.
Study modalities and data miningThe DDU was performed by a 10+ year experienced radiologist in interventional vascular radiology in DDU always before the angiography. The DDU was performed with a Toshiba SSH-140 Doppler duplex scan kit (Toshiba Medical System Corporation, Shimoishigami, Otawara-shi, Japan) in 35 patients and a Toshiba aplio XV kit (Toshiba Medical System Corporation, Shimoishigami, Otawara-shi, Japan) in 124 patients. In both cases a linear probe with frequencies between 6 and 12MHz was used.
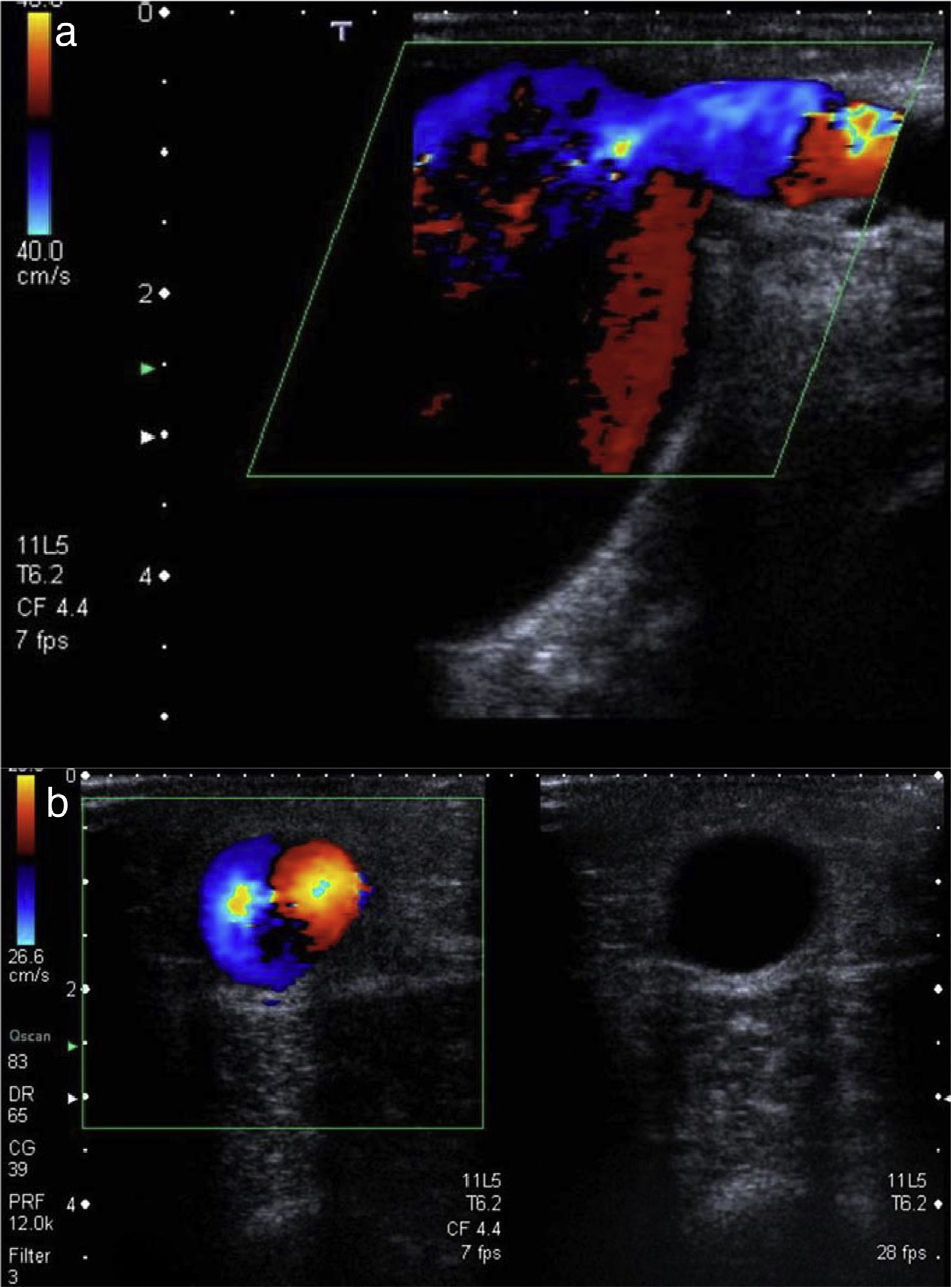
The studies were performed with the patient in the supinus decubitus position, which brings the explorer close to the PVA limb to first assess the afferent arteriole in mode B, the arteriovenous anastomosis and the efferent vein (Fig. 1) and to later perform the DDU used to study the afferent arteriole RI (after obtaining the peak systolic velocity (PSV) and the end diastolic velocity), the flow of the afferent arteriole (ml/min), the diameter of anastomosis (mm), the flow (ml/min) and the PSV of efferent vein (cm/s) (Fig. 2). The estimation of flow both in the afferent arteriole and in the arterialized vein was done using this formula:
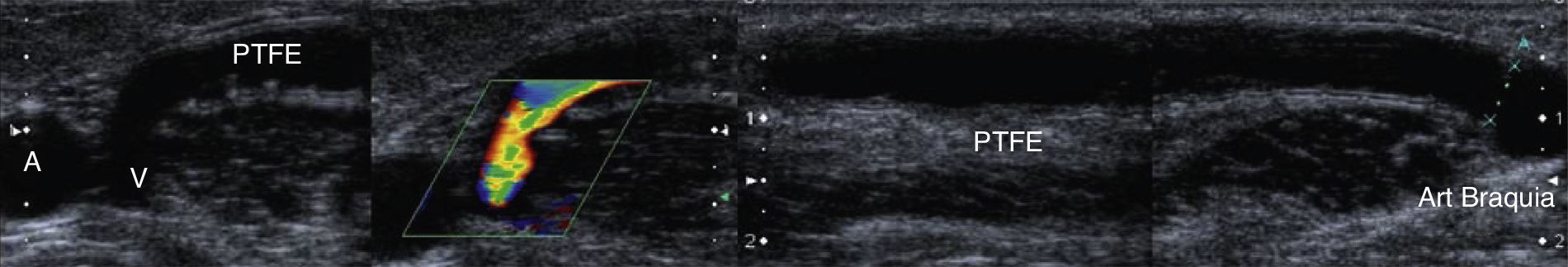
Vascular access with functioning polytetrafluoroethylene (PTFE) prostheses and no ultrasound alterations. Both anastomoses–proximal to the efferent vein (V, efferent vein) and distal to the brachial artery (dotted line). The PTFE prosthesis whose wall can be seen with a triple band with a hypoechoic central line shows some irregularities secondary to repeated punctures.
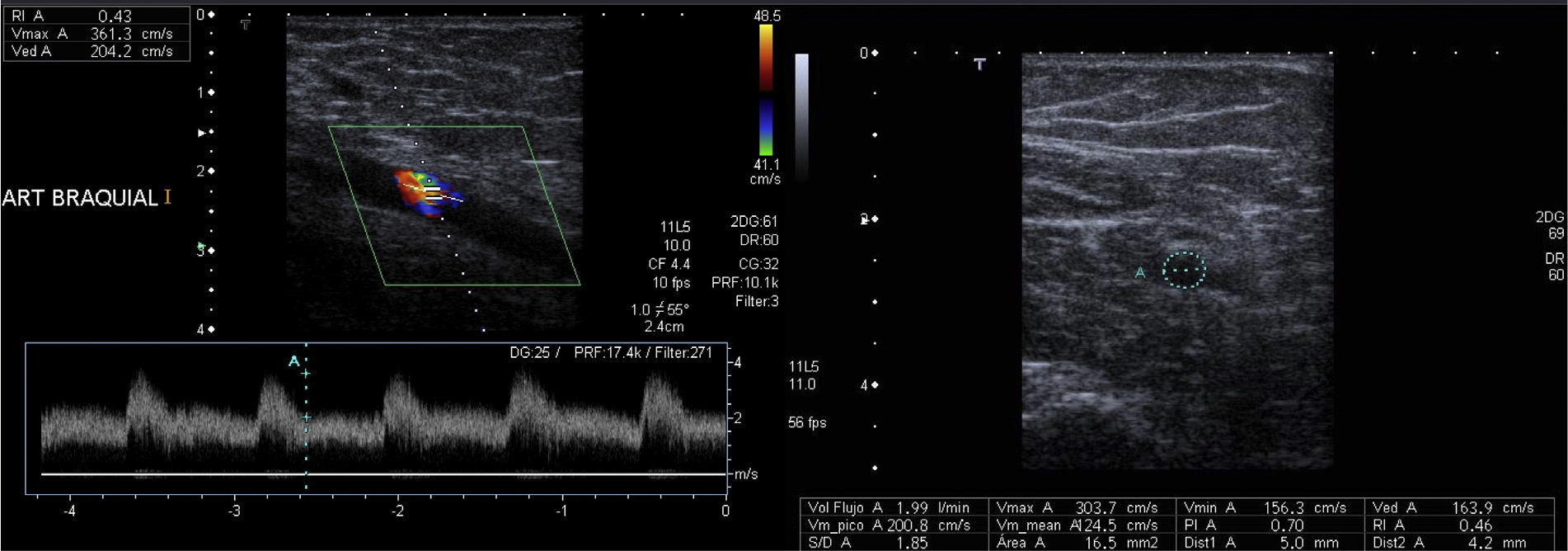
Measurement of flow and RI in the afferent arteriole of a normofuncioning peripheral vascular access. The afferent arteriole is studied with an ultrasound incidence angle <60° to obtain a 4 cycle-range. By knowing the average vessel velocity and area (in the cut that runs perpendicular to its axis) the software of the ultrasound kit gives us the flow (ml/min). RI<0.5 and the shape of the curve indicate that this is a low-resistance artery.
Ultrasound criteria of stenosis include: (1) the vessel lumen stenosis was beyond 50% with respect to the adjacent vascular segment with or without the perivascular color artifact, (2) aliasing and (3) one PSV 2 times the PSV of the non-stenotic adjacent vascular segment (Figs. 3 and 4). Spectra were obtained through an ultrasound angle of incidence ≤60. Blood flow was analyzed in the non-stenotic straight segments of the PVA artery after doing measurements after obtaining the average velocity in ml per minute. PVS, the end diastolic velocity and RI were measured in the afferent arteriole (brachial and radial arterioles) 5cm before anastomosis. The flow from efferent vein was obtained in those accesses with straight segment of uniform diameter at least in 10cm of non-turbulent unidirectional flow and possibility of insonation with an angle <60°.
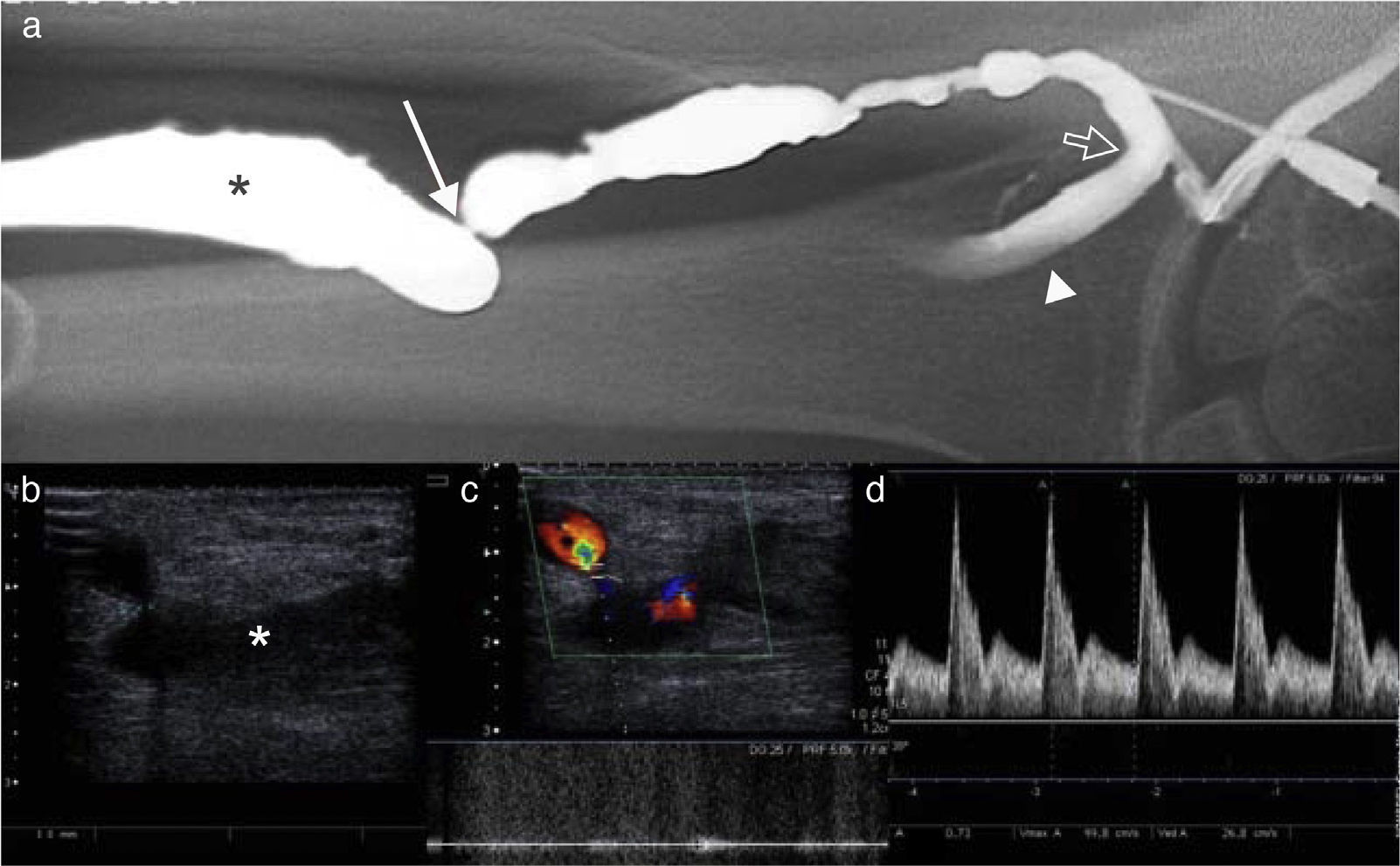
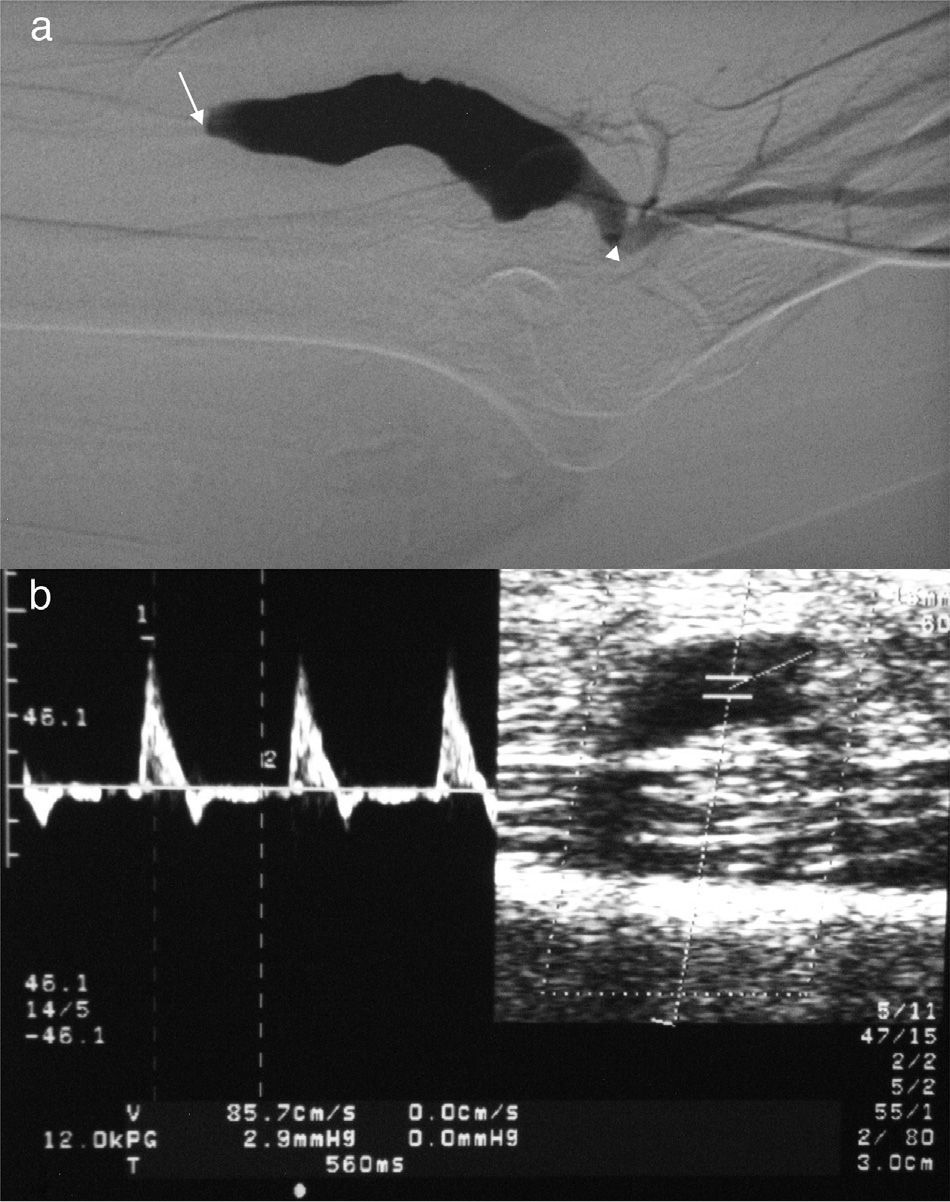
Stenosis in a peripheral vascular access for hemodialysis. (a) Arteriography. Significant stenosis of efferent vein (*). Juxta-anastomotic stenosis of efferent vein followed by filiform stenosis (white arrow) located some inches away from anastomosis (open arrow). Radial artery (arrow head). (b) The same finding can be seen in the mode B-ultrasound. (c) When placing the mouse pointer on the stenosis we can see a high-speed wave with turbulent flow. (d) A high RI and a change in the curve of velocities of the afferent arteriole (radial artery) with elevated systolic peak velocity and low diastole suggest stenosis.
Peripheral vascular access thrombosis. (a) On the angiography the patent segment of the efferent vein is refilled. The occlusion (white arrow) translates into a contrast reflux towards the brachial artery (afferent arteriole) distal to anastomosis (arrow head). (b) Duplex-Doppler ultrasound in brachial artery. Identical wave to the one detected on a triphasic high-resistance normal muscular artery (RI>0.7).
The angiography was performed just a week after the DDU without knowing its result. The images were assessed by a 10+ year experienced radiologist in interventional vascular radiology. The proceeding was carried out with one Artis Zee digital angiography device (SIEMENS Medical Systems, Erlangen, Germany) by introducing a 20-gauge cannula into PVA of the venous branch or into the Teflon (PTFE) together with anastomosis. The contrast agent used was Iohexol 300mg/ml at a 3–4ml/s flow with a 2–5s-injection time and the whole venous outflow tract was initially examined. Then to assess anastomosis and the efferent vein segment proximal to puncture the pressure cuff was compressed while the contrast media agent was administered. Significant stenosis is defined as reductions in the caliber of the efferent vein >50% with respect to the nonaneurysmal venous segment.11,12
PVAs without ultrasound or angiographic alterations were followed both analytically and hemodynamically in hemodialysis sessions during the next 3 months. We tagged normal those PVAs with >300ml/min flow in less than 6 sessions in one month and not showing analytical alterations (recirculation<10%) during this period of time.
Data analysisWe compared the results of DDU and angiography and calculated the sensibility, specificity, positive predictive value, negative predictive value, positive odds ratio, and negative odds ratio of DDU. True positives were PVAs with alterations (stenosis or thrombosis) in DDU and angiography while true negatives were PVAs without lesions in both assays and without clinical or analytical alterations in the hemodialysis sessions 3 months after the angiography. False positives were PVAs with abnormal DDU but without angiographic alterations even in the follow-up during hemodialysis. False negatives were PVAs with normal DDU showing lesions in the angiography.
RI and flow results were expressed as absolute values with respect to the total and as the average standard deviation.
ResultsA hundred and forty of the 159 PVAs studied through angiography showed dysfunction. Nineteen PVAs were normal in the angiography and did not show hemodynamic or analytical alterations in the follow-up during the next 3 months. DDU diagnosed 14 of these PVAs as normal while erroneously diagnosed 5 as not normal after considering there was stenosis in the surgical anastomosis or in the adjacent segment of the efferent vein. DDU categorized 142 PVAs as not normal 5 of which did not show alterations in the subsequent angiography or during the clinical follow-up. Among the 137 PVAs with ultrasound and angiographic dysfunctions 19 were thrombosis (all diagnosed through DDU) and 118 stenoses. Three PVAs were diagnosed as normal in the DDU while the angiography later showed stenosis. In one of the cases post-surgical fibrosis and calcifications produced important perivascular artifacts that did not allow us to measure accurately the diameter of vascular structures and flow velocities. The remaining 2 false negatives were central stenoses (3 stenoses in 2 patients) where the overlapping of bone structures did not allow us to see the veins directly–2 of them affecting the central veins and the other one a proximal segment (proximal third of the arm) of the efferent vein. The DDU sensibility was 0.98 (95% CI 0.88–1), specificity 0.74 (95% CI 0.66–0.81), the positive predictive value 0.96 and the negative predictive value 0.82, the positive odds ratio 3.70 and the negative odds ratio 0.03.
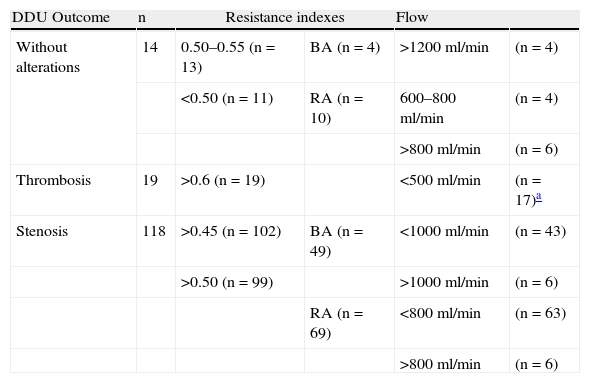
The afferent arteriole RI and flow values are shown in Table 2. We can see a higher flow in the brachial artery with respect to the radial artery both in the accesses with stenosis and in those with no alterations at all. Flow in radial artery is usually underestimated since the supplementary flow from collaterals of the palmar arcade is not measured.13 RI was >0.6 in all cases of access thrombosis and >0.5 in 99 of the 118 accesses with stenosis. Flow of the efferent vein could only be measured reliably in 54 of the 159 accesses due to the presence of inverted flow (n=57), turbulent flow (n=8), lack of pre-bifurcation straight segment (n=12) or insonation angle >60 (n=28). Among 14.9% of native PVAs we found aneurysms (19 patients; 4 PVAs without alterations, 13 PVA with stenosis and 2 cases of thrombosis). The findings were: luminal ectasia (venous diameter>12mm), turbulent local flow and partial or total mural thrombosis (in the case of 2 aneurysms located in the thrombosed PVAs.) Among the 32 PVAs with prosthesis 7 PVAs showed pseudoaneurysms (2 in PVAs without alterations and 5 in PVAs with stenosis) seen as secular formations with flow in the DDU linked to the PTFE. Five of the 26 aneurysms and pseudoaneurysms could not be seen through the fistulography–3 pseudoaneurysms and 2 aneurysms. In 35.8% of the PVAs studied (n=57: 9 PVAs without alterations and 48 PVAs with stenosis) besides the centripetal flow we also observed an inverted retrograde flow depicted on the DDU as areas of different color (Fig. 5).
Resistance indexes and flow velocity reported in peripheral vascular accesses.
| DDU Outcome | n | Resistance indexes | Flow | ||
| Without alterations | 14 | 0.50–0.55 (n=13) | BA (n=4) | >1200ml/min | (n=4) |
| <0.50 (n=11) | RA (n=10) | 600–800ml/min | (n=4) | ||
| >800ml/min | (n=6) | ||||
| Thrombosis | 19 | >0.6 (n=19) | <500ml/min | (n=17)a | |
| Stenosis | 118 | >0.45 (n=102) | BA (n=49) | <1000ml/min | (n=43) |
| >0.50 (n=99) | >1000ml/min | (n=6) | |||
| RA (n=69) | <800ml/min | (n=63) | |||
| >800ml/min | (n=6) | ||||
AB: brachial artery; RA: radial artery; DDU: duplex-Doppler ultrasound.
Most cases of PVA thrombosis are associated with progressive subintimal hyperplasia of venous walls or with the arteriovenous anastomosis causing stenosis in different sites of the PVA.14 Angiography is still considered the standard of reference to find these stenoses and further therapy is through angioplasty or surgery. Yet despite the fact that it is an invasive modality that uses iodinated contrast and ionizing radiations it is still used as the early assay of diagnosis in PVAs with suspicion of dysfunction. However our study showed that the DDU is a useful assay of high sensibility and positive predictive value.
The sensibility of the physical exam is good (85%) for the diagnosis of anastomotic or juxta-anastomotic stenoses (inflow) with moderate specificity (71%) and positive and negative predictive values of 84% and 72% respectively.15,16 As opposed to it our results with DDU are superior showing a 98% sensibility and a 96% positive predictive value while studying vessels, locating stenosis and measuring diameters. Since this is a high-prevalence process within the population with end-stage renal disease in hemodalysis5,17 this positive predictive value almost confirms the condition when ultrasounds are positive. The high-positive odds ratio (3.70) of our study confirms its validity as a diagnostic test to detect stenoses and thromboses.
However specificity has only been moderate (74%) and stands in contrast to the 98% reached in other investigations.18 One reason can be the difficulty in transmitting ultrasound through the edema and post-surgical fibrosis and the other the overestimation of stenosis in the anastomosis due to the artery physiological narrowing after the surgical proceeding10,19 or the difficulty for the ultrasound incidence angle to be adequate given the small amount of subcutaneous cellular tissue in the wrist which prevents us from taking exact measurements of velocities (3 false positives on radiocephalic native PVAs). The information bias was introduced because the radiologist knew that patients who had been referred after showing alterations of PVA might have contributed to the elevated number of false positives.
The sensibility and specificity of DDU in the diagnosis of thrombosis is 100% and its utility is not only diagnostic since it is far more efficient in the location of the segment of the patent proximal efferent vein and the stenosis responsible for thrombosis. When the proximal drainage vein is not patent and size is inadequate thrombectomies, angioplasties or revascularization surgeries cannot be performed and therefore the PVA is not recoverable.20
Elevations of RI have been reported in PVAs with stenosis or thrombosis. These elevations are associated with changes in the wave shape and reductions of flow in the PVA. According to the recommendations by the National Kidney Foundation Disease Outcomes Quality Initiative2 the reduction of flow is considered today as the most reliable measure of PVA dysfunction10,19,21. The normal flow intervals (1053±495ml/min with ultrasound dilution modalities10,19–22 and 1034±527ml/min with catheter thermodilution23) are widely varied.
Most authors perform ultrasound measurements of the efferent vein flow which often leads to imprecise and very variable measurements not associated with the measurements obtained by other modalities.24–26 The tortuosity of venous vein, the wide variations in diameter, how easy the ultrasound probe compresses itself and hemodynamic features (the velocity of blood flow in the efferent vein is not even, it does not show parabolic flow and the curve of velocities as a wide range of frequencies) are the causes of such inaccuracy.13,27 On the contrary the diameter of the afferent arteriole is constant, its course is straight, the spectral curve is clean and flow is parabolic which allows us to perform accurate measurements of flow.13,27 After performing a PVA the brachial artery increases its flow progressively while staying in values of 1000±200ml/min.13 These values are similar to those of our study in normofunctioning PVAs and they are also similar to values measured through saline dilution and thermodilution. In PVAs with stenosis and/or thrombosis flows grow smaller. In our sample 28.5% of normofunctioning PVAs showed flows <800ml/min while 10.1% of dysfunctional PVAs showed normal flow (>800–1000ml/min). From this we deduce the importance of correlating flow values and other hemodynamic data like the wave morphological changes and RI.28 Flow increases in the PVA can be associated with increases of venous pressure non-attributable to stenosis in the outflow.29 Similarly the elevations of flow with constant peripheral diameter and resistances could raise velocities and increase the RI (Poiseuille's law).30 In our opinion it is flow reductions together with RI elevations that are the ones that would more likely be associated with dysfunctions (stenosis and thrombosis). Because there were few normofunctioning PVAs in our sample we did not establish cutoff values for the flow or IR and did not do a ROC curve analysis either–all to be done in future studies.
The PVA morphological alterations and local complications non-associated with flow or dysfunction are (precluding infectious complications) hematomas and aneurysms and pseudoaneurysms adjacent to the native or prosthetic venous branch of the PVA.31
The factors associated with the appearance of aneurysms are repeated punctures, excessive flow distension in the PVA and overpressure in venous segments distal to one stenosis.31 Both the sensibility and specificity of ultrasounds are high to detect these complications that are even higher than the sensibility and specificity of angiographies due to its ability to detect intraluminal repletion defects and hematomas.7,11 In our study we found aneurysms in the ultrasound that went misdiagnosed in the angiography that studies the central residual lumen while overlooking the mural thrombus that takes up a space >50% of the lumen of the affected vessels (19.2% of cases).
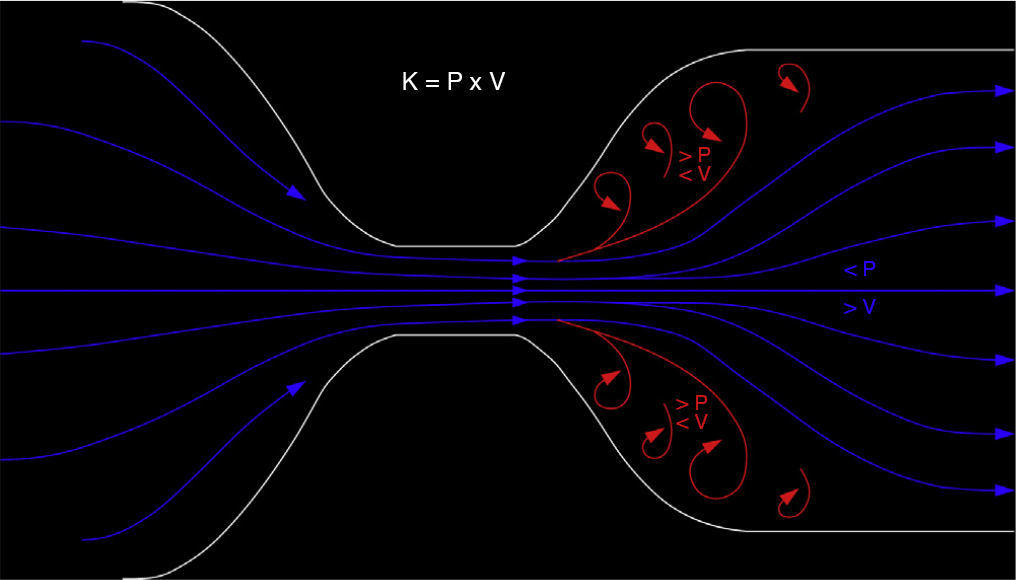
In a high percentage of our PVAs there were segments with inverted flow or double color. Inversion can be explained by the Venturi effect and Bernoulli's equation (Fig. 6). When one vessel widens suddenly–common finding in post-stenotic segments or in one aneurysmal venous segment–the very profile of flow changes, and it lengthens to go back to the parabolic profile.32 During this process the central blood layer (post-stenotic jet) overcomes cohesion among blood layers. Flow then “splits itself” into slow peripheral flow and high pressure and into fast central jet and low pressure (Venturi effect). Different pressure generates inverted flow when heading towards the blood central layer in an effort to reverse directions and even reach the region with the fewest possible pressure. All this produces a double color Doppler image (Bernoulli's equation).30 Even though it is common in our study–both in post-stenotic and outflow segments without alterations–this finding has never been reported before; however we do not believe it is valuable to detect the stenosis of PVA.
The Venturi effect and the Bernoulli's equation. If the flow running through a variable section conduit remains constant when the section diminishes the velocity necessarily increases. According to the Work Energy Theorem the product between velocity (V) and pressure (P) needs to be constant (K). The P difference generated by the high central velocity jet generates a reversed flow (red coils) heading towards the areas with the smallest diameter of all.
An important limitation of our study is that it did not include PVAs with high flows or a sample of similar size of normofunctioning PVAs. However we chose to study a wide sample of patients with PVAs and associated the results not only with the angiographic findings but also with its clinical and analytical evolution. Our study sample size is greater than other existing sizes analyzing not only the ultrasound positive results but also the false positive and negative ones allowing us to secure the ultrasound as the first diagnostic modality in the study of PVAs.
In sum the DDU is a safe efficient modality in the study of PVA stenoses and thromboses while providing us with anatomic and hemodynamic information. However prospective studies will be necessary to correlate flow and RI in the afferent arteriole with the dysfunction of the PVA and other variables like the body mass index, sex, age, type of vascular access or peripheral arteriopathy.
Ethical responsibilitiesProtection of people and animalsAuthors confirm that for this investigation no experiments with human beings or animals have been carried out.
Data confidentialityAuthors confirm that the protocols of their centers have been followed on matters concerning the publishing of data from patients. They also confirm that all patients included in this study have been given enough information and handed over their written informed consent for their participation in this study.
Right to privacy and informed consentAuthors confirm that they have obtained the written informed prior consent from patients and/or subjects appearing in this article. This document is in the possession of the corresponding author.
Authors- 1
Manager of the integrity of the study: TMS.
- 2
Original idea of the study: TMS, CMH, ESM, and FMR.
- 3
Study design: TMS, CMH, ESM.
- 4
Data mining: TMS, ESM.
- 5
Data analysis and interpretation: TMS, ESM, FMR.
- 6
Statistical analysis: TMS, CMH, FMR.
- 7
Reference search: TMS, ESM.
- 8
Writing: TMS, CMH, ESM, FMR.
- 9
Manuscript critical review with intellectually relevant contributions: CMH, FMR.
- 10
Final version approval: TMS, CMH, ESM, FMR.
Authors reported no conflicts of interests.
Please cite this article as: Moreno Sánchez T, Martín Hervás C, Sola Martínez E, Moreno Rodríguez F. Valor de la ecografía doppler en la disfunción de los accesos vasculares periféricos para hemodiálisis. Radiología. 2014;56:420–428.