Spasticity is common after a stroke and is an independent risk factor for developing pain. BotulinumtoxinA injection is the treatment of choice for focal spasticity. We examined the effect of intramuscular botulinumtoxinA on pain relief in patients in routine clinical practice who were experiencing pain as a primary complaint associated with post-stroke lower limb spasticity.
MethodsProspective, multicentre, post-marketing observational study. The study period was 16 months. The primary effectiveness variable was the mean change from baseline on the pain 0–10 Numerical Rating Scale after four botulinumtoxinA injection cycles. Secondary endpoints included changes from baseline on the pain 0–100 Visual Analogue Scale, Goal Attainment Scale, modified Ashworth Scale, 10-Meter Walk Test, Penn Spasm Frequency Scale, and 36-item Short-Form Health Survey.
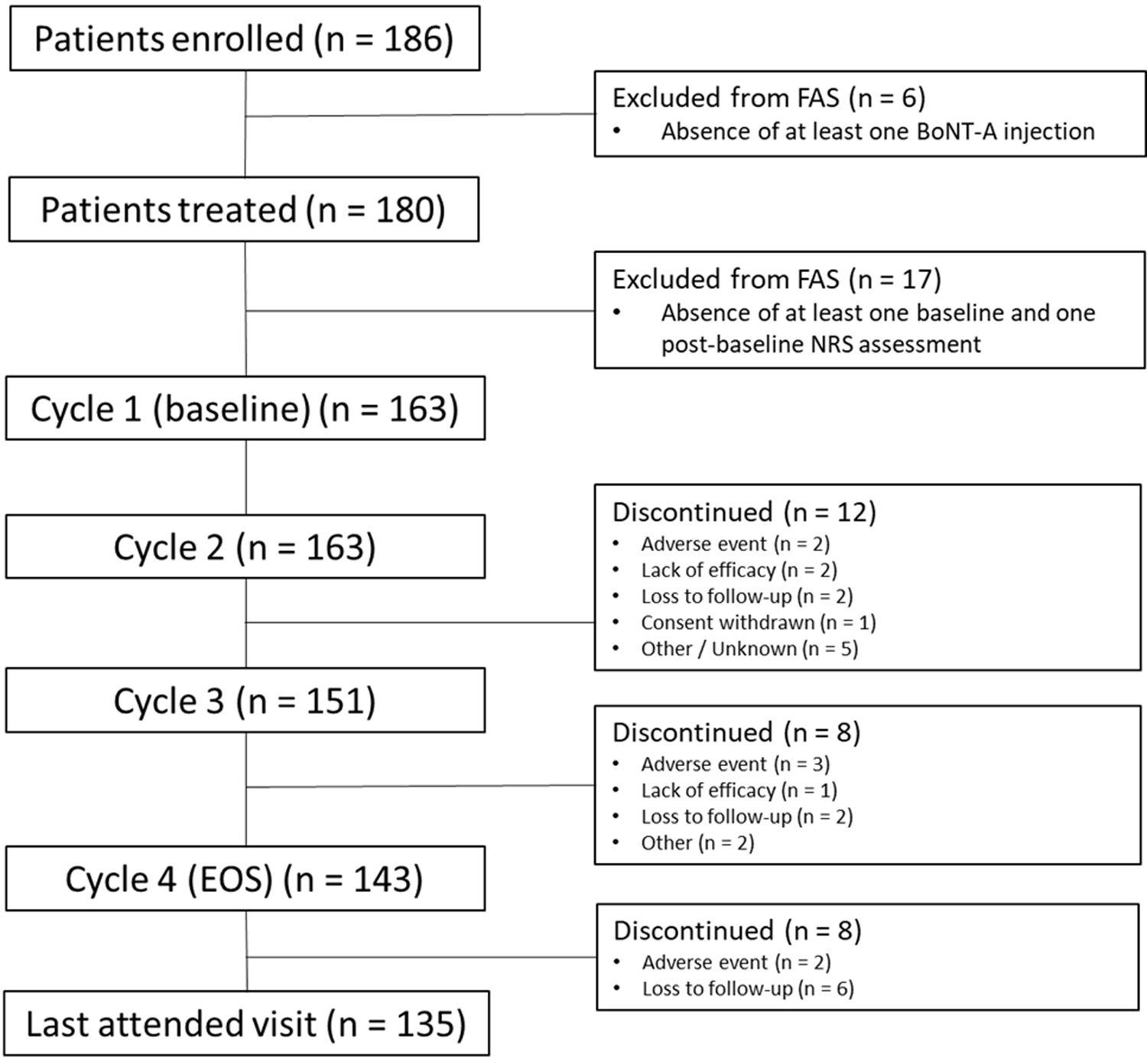
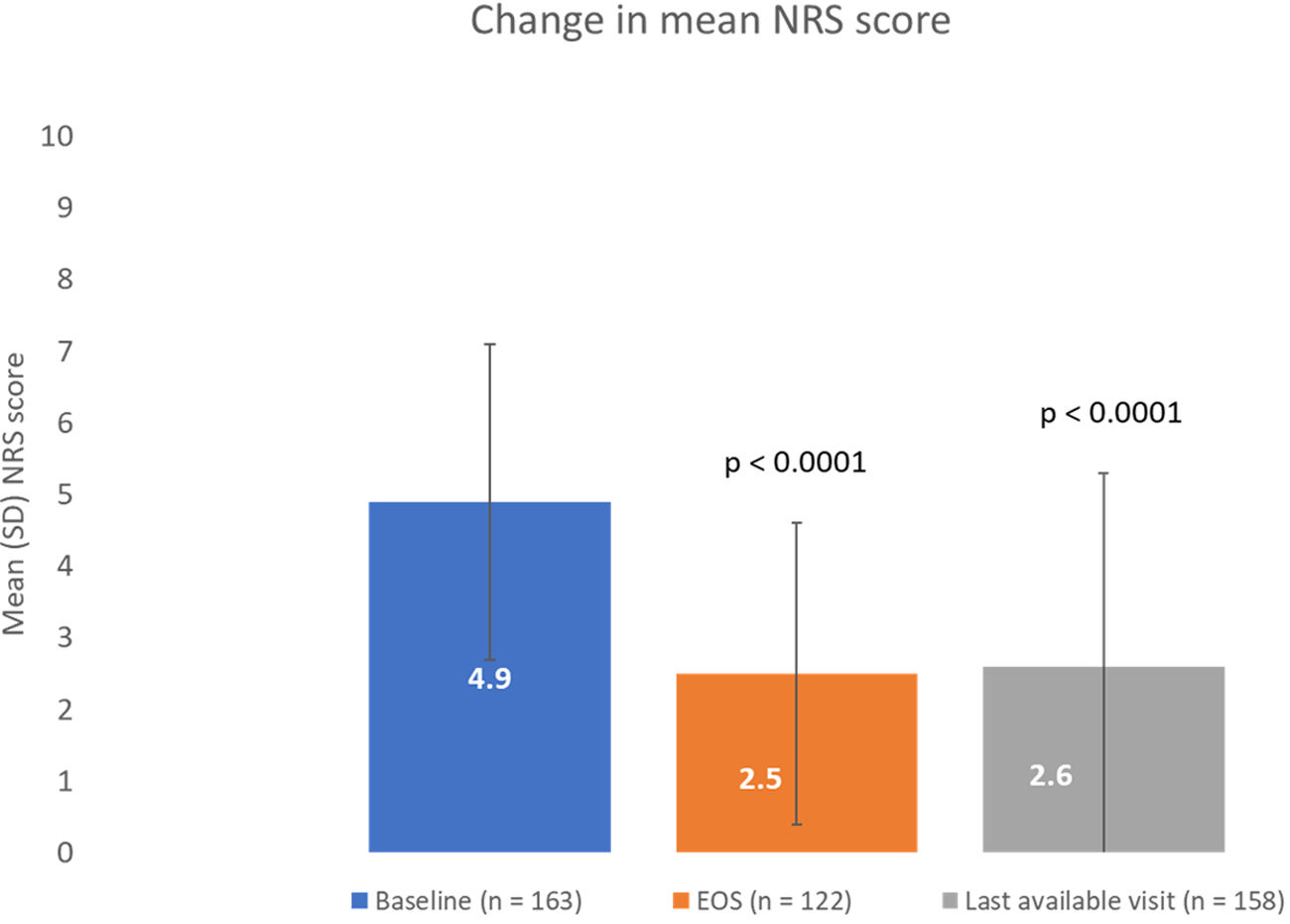
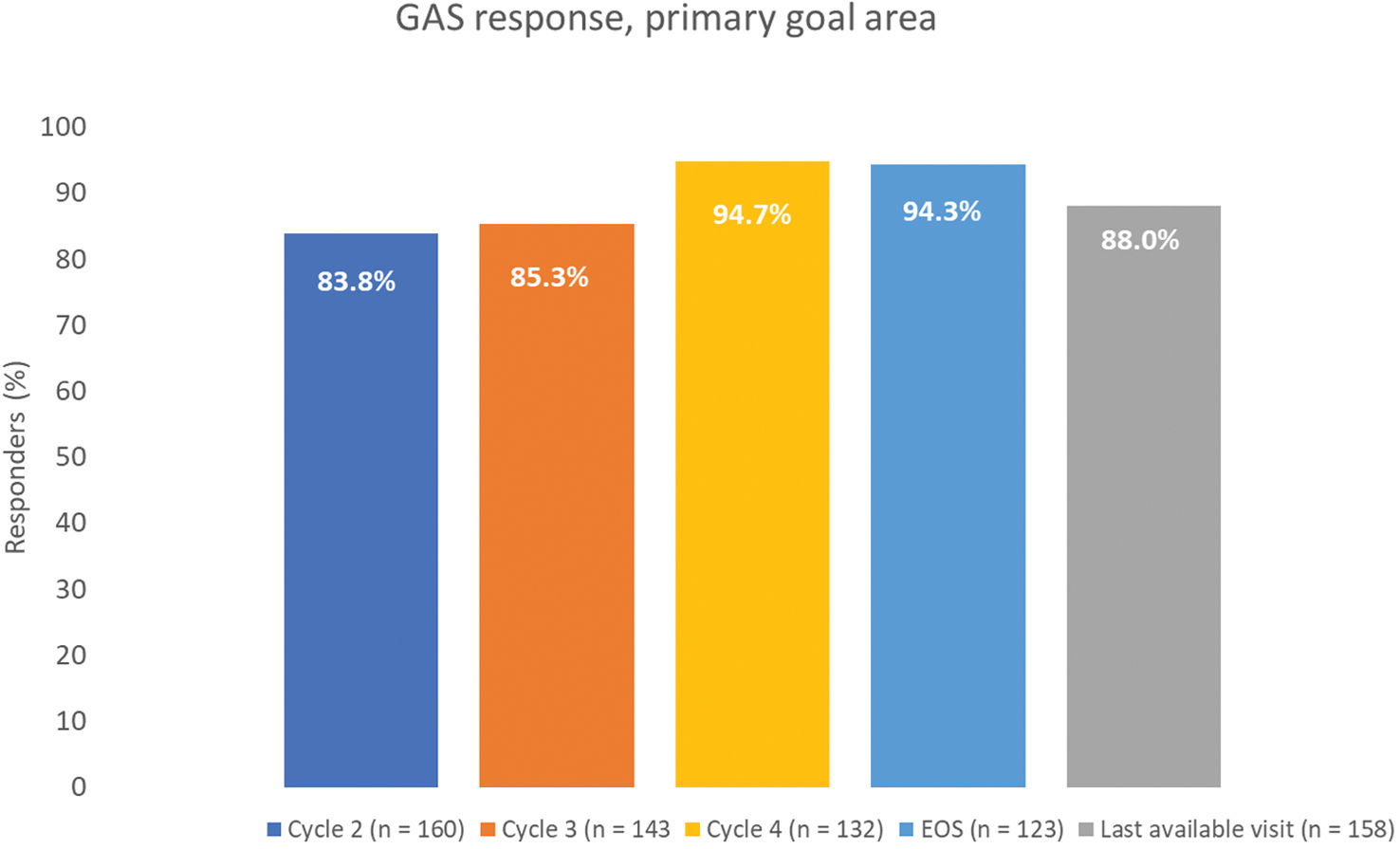
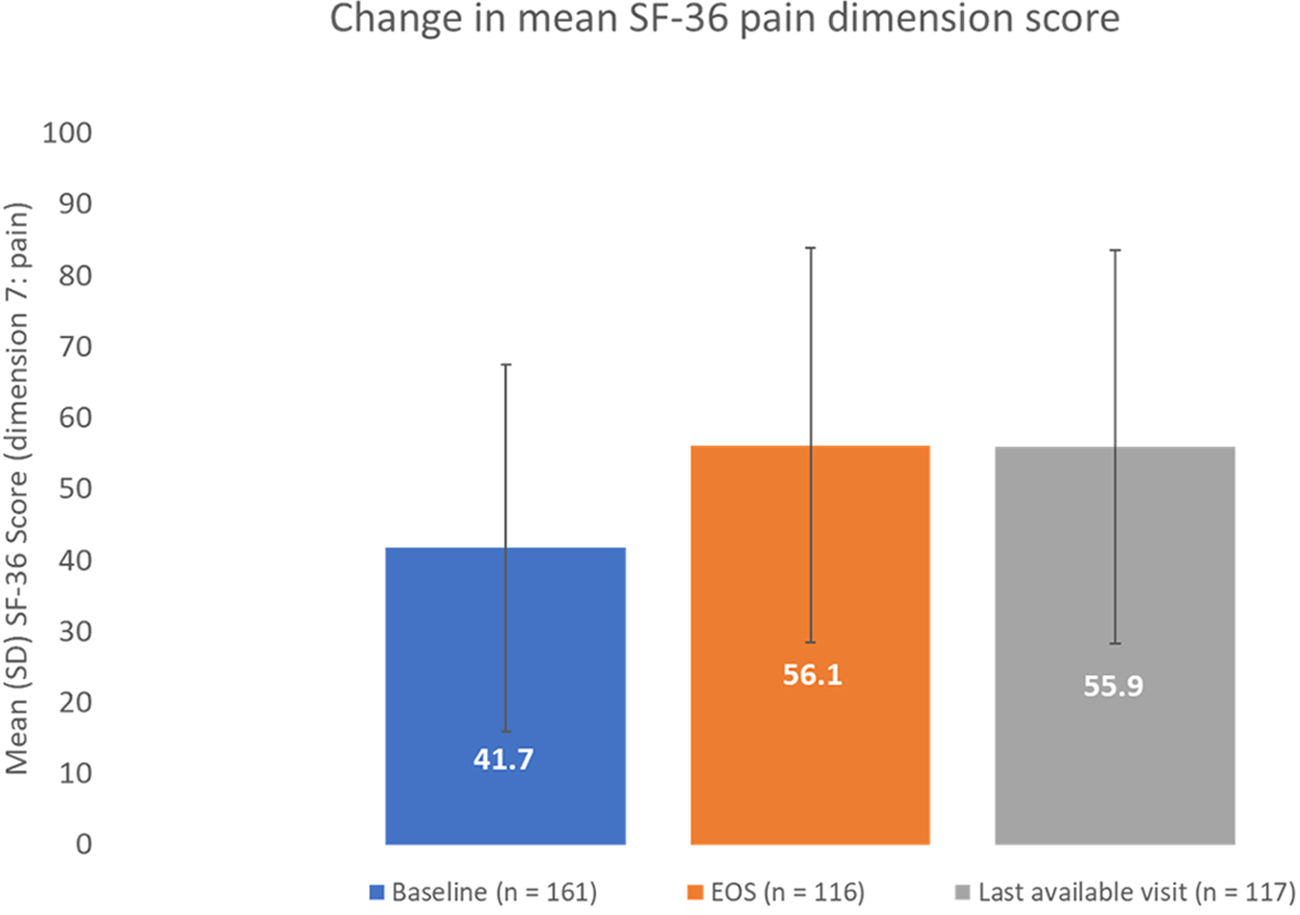
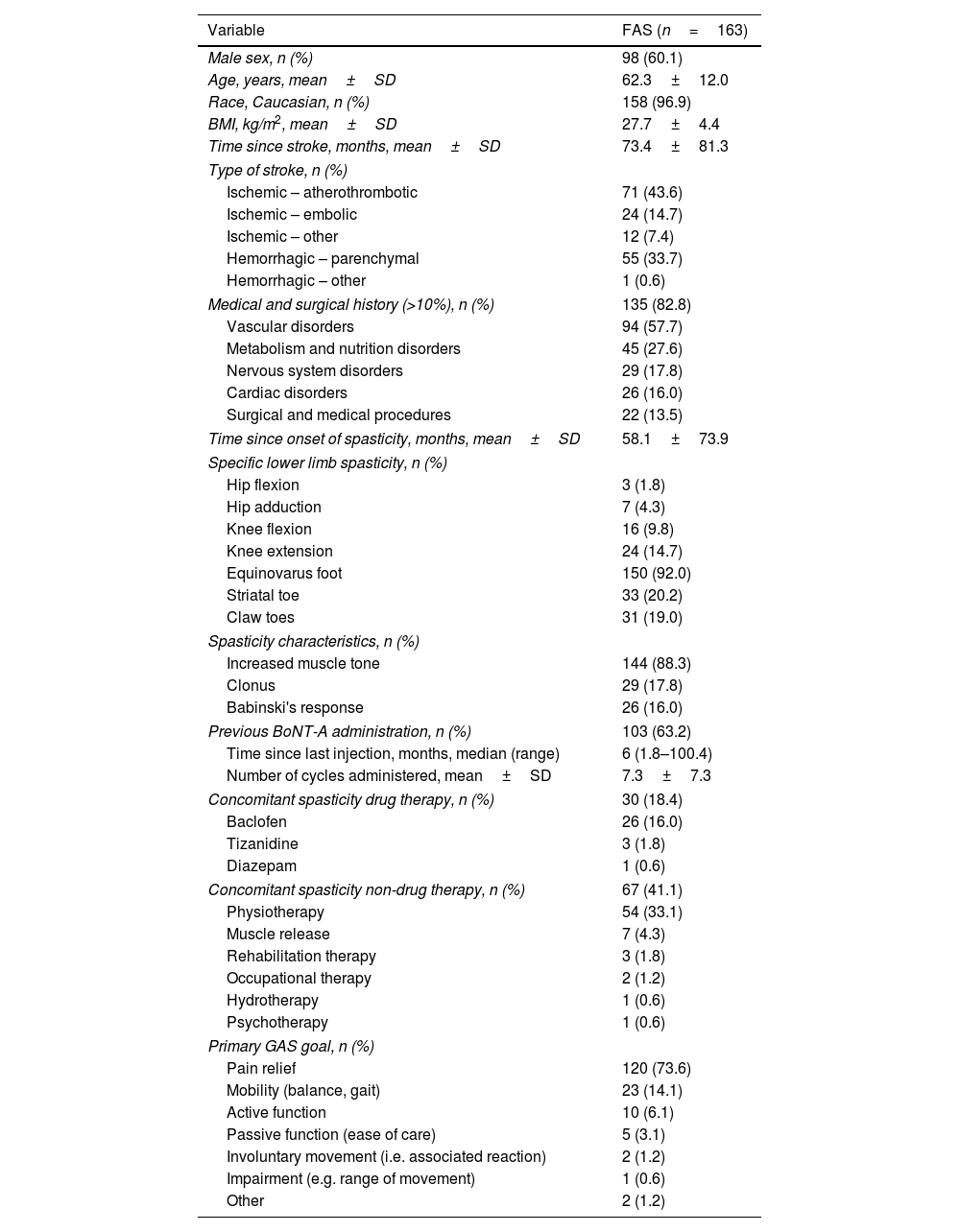
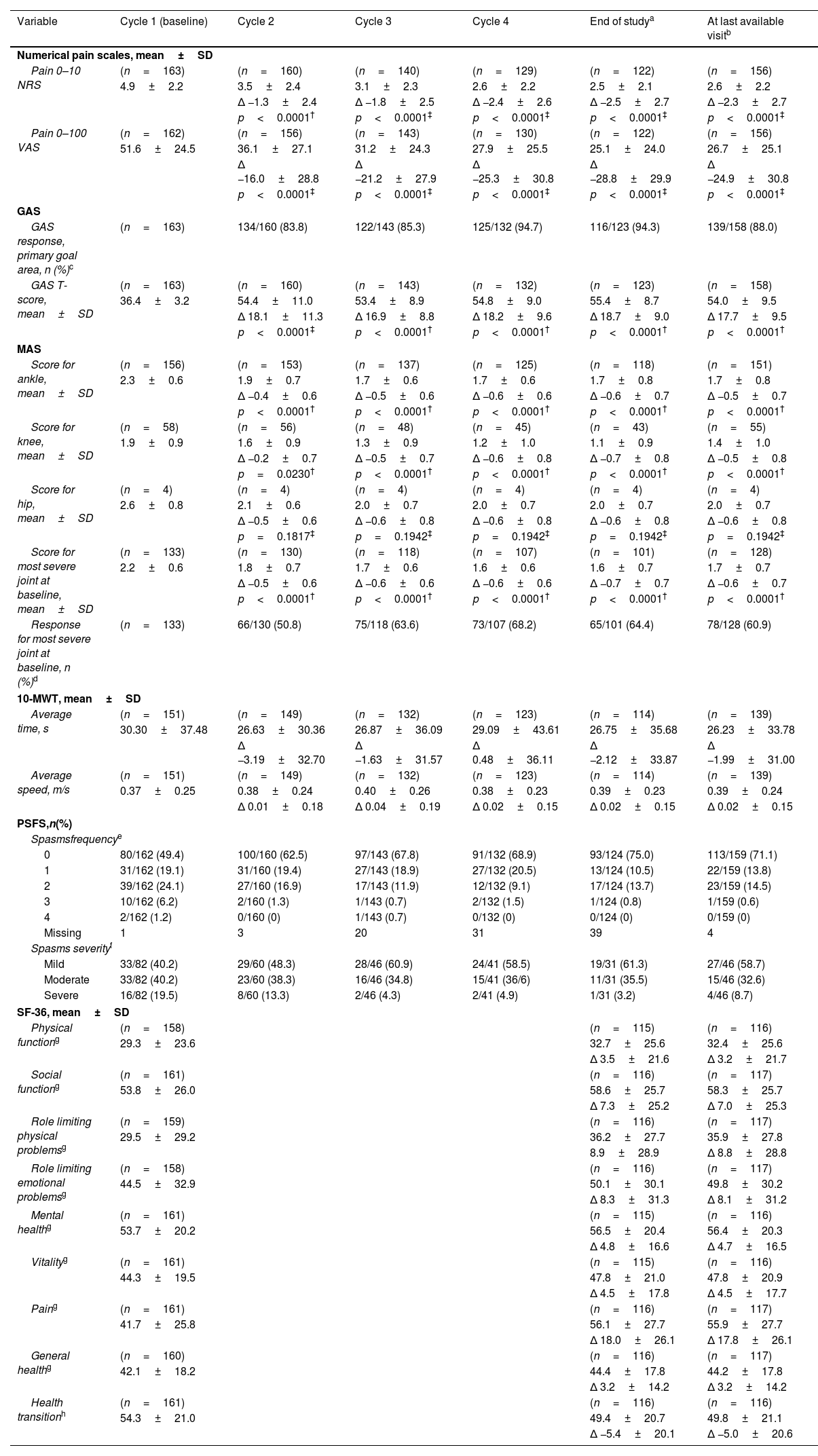
ResultsOf 186 enrolled patients, 180 (96.8%) received botulinumtoxinA at least once. The mean (standard deviation) pain 0–10 Numerical Rating Scale score decreased significantly (p<0.0001) from 4.9 (2.2) at baseline to 2.5 (2.1) at study end, representing a 50% decrease in pain severity. Relief of pain due to spasticity was supported by improvement from baseline in all secondary variables except the 10-Meter Walk Test. Two adverse events (erysipelas and phlebitis) in one patient were considered likely to be related to botulinumtoxinA injection.
ConclusionBotulinumtoxinA appears to provide pain relief as an additional benefit of local treatment in patients with post-stroke lower limb spasticity for whom pain relief is a primary therapeutic goal (a Lay Abstract has been provided as Appendix A).
La espasticidad es común tras un accidente cerebrovascular y es un factor de riesgo para desarrollar dolor. La inyección de toxina botulínica A es el tratamiento de elección para la espasticidad focal. Se examinó el efecto de la toxina botulínica A intramuscular sobre el alivio del dolor en pacientes con espasticidad de las extremidades inferiores tras un accidente cerebrovascular.
MétodosEstudio observacional prospectivo, multicéntrico, poscomercialización. Periodo de estudio de 16 meses. La principal variable de eficacia fue el cambio medio con respecto al valor basal en la Escala de Calificación Numérica 0-10 tras cuatro ciclos. Los criterios de valoración secundarios incluyeron los cambios en la Escala visual analógica del dolor 0-100, la escala de consecución de objetivos, la escala modificada de Ashworth, la prueba de marcha de 10-metros, la escala de espasmos de Penn y el cuestionario de salud SF-36.
ResultadosDe los pacientes en estudio, 180 de 186 (96,8%) recibieron toxina botulínica A al menos una vez. La puntuación media de la Escala de Calificación Numérica 0-10 del dolor disminuyó significativamente (p <0,0001) de 4,9 (2,2) a 2,5 (2,1), representando una disminución del 50% en la intensidad del dolor. El alivio del dolor fue respaldado por una mejoría en las variables secundarias, excepto en la prueba de marcha de 10-metros. Dos eventos adversos en un paciente (erisipela y flebitis) se consideraron probablemente relacionados con la toxina botulínica A.
ConclusiónLa toxina botulínica A parece proporcionar alivio del dolor como beneficio adicional del tratamiento local en pacientes con espasticidad de las extremidades inferiores tras un accidente cerebrovascular (Proporcionado Lay abstract en Apéndice-A).