Leptolegnia chapmanii is pathogenic to mosquito larvae. The compatibility between L. chapmanii and two insect growth regulators (IGR), diflubenzuron and neem oil, was evaluated. L. chapmanii was grown on culture media containing different concentrations of each IGR. The mycelial growth was significantly reduced with the highest concentrations of IGR (F6,98=268, p<0.05). However, the production of zoospores (F6,56=0.93, p>0.05) and the larval mortality of Aedes aegypti (F6,56=0.95, p>0.05) were not significantly different among treatments. Furthermore, the percentage of adult emergence in the presence of different concentrations of diflubenzuron or a neem formulation was determined, and the pathogenic activity of zoospores was evaluated at the concentrations that inhibit the emergence at 30, 50 and 90%. The pathogenicity of zoospores was not significantly different among treatments (F6,14=0.54, p>0.05), and the larval mortalities were above 90% in all cases.
Leptolegnia chapmanii es un patógeno de larvas de mosquitos. Se evaluó la compatibilidad entre L. chapmanii y dos reguladores del crecimiento de insectos (IGR, por sus siglas en inglés), diflubenzurón y aceite de neem. L. chapmanii creció en medios de cultivo con diferentes concentraciones de estos IGR. El crecimiento micelial fue menor con las concentraciones más altas (F6,98=268; p<0,05). No hubo diferencias significativas en la producción de zoosporas (F6,56=0,93; p>0,05) ni en la mortalidad de las larvas de Aedes aegypti (F6,56=0,95; p>0,05). Se determinó el porcentaje de emergencia de adultos en presencia de diferentes concentraciones de diflubenzurón o de un formulado a base del aceite de neem. También se evaluó la patogenicidad de las zoosporas a las concentraciones de los IGR que inhibieron la emergencia del 30, 50 y 90% de adultos. No hubo diferencias significativas en la actividad de las zoosporas al comparar los tratamientos (F6,14=054; p>0,05). La mortalidad de las larvas fue superior al 90%.
The control of mosquito populations has traditionally been carried out through the use of chemical insecticides, with associated problems such as the increase of resistant populations and the deterioration of health and environment3,6. The use of insecticides with different modes of action, applied in combination or successively at different times, could improve results in the control of mosquitoes, mitigating some of the existing problems.
Leptolegnia chapmanii (Seymour) (Straminipila: Saprolegniales) is a specific pathogen of larval stages of culicids such as Aedes spp., Anopheles spp. and Culex spp., which is harmless to non-target species4. The effectiveness of L. chapmanii as a mosquito pathogen is affected by environmental factors such as temperature, pH, salinity and UV-A radiation, as well as by others related to water quality8,9,11. This microorganism can be maintained under laboratory conditions by in vitro propagation on several nutritive culture media7,12. Diflubenzuron is an Insect Growth Regulator (IGR) that acts by inhibiting chitin synthesis. It persists for a few days in water and soil after application and is non-toxic to humans at concentrations below 0.25ppm15. Azadirachtin is a chemical compound present in neem plants (Azadirachta indica (A. Juss)) that has an antifeedant effect on larval stages of mosquitoes, which impede their development13. Neem-based products have low toxicity to mammals and short residual times in the environment14. The aim of this work was to evaluate the compatibility of L. chapmanii and diflubenzuron and neem oil to provide alternatives for the control of Aedes aegypti (L.) (Diptera: Culicidae).
A. aegypti mosquitoes were reared under laboratory conditions at 27±1°C, 75% relative humidity and 12-h photophase, following standard protocols2. Adults were kept in cages (27l) of aluminum and mesh. Female adults were fed twice a week on chicken blood, and both female and male adults were able to feed continuously on dry grapes and water. The eggs were collected in papers placed inside containers (300ml) with 200ml of water (inside the cages). Larval development took place in plastic containers (3000ml) with 2000ml of water (outside the cages).
An Argentine strain of L. chapmanii was employed in the following assays. The oomycete was isolated from dead larval samples of Aedes albifasciatus (Macquart) found in a temporary water body located near the city of La Plata, Buenos Aires Province5. It was included in the Entomopathogenic Fungi collection of the CEPAVE under accession number CEP 0101, and alternatively in the USDA-ARSEF (ARSEF-5499), in Ithaca (USA). The microorganism was maintained in vitro on PYG-agar medium (Peptone: 1.3g, Yeast Extract: 1.3g, Glucose: 3g, Agar: 10 g, per liter of distilled water) and in vivo on A. aegypti larvae from the laboratory colony. In both cases, the environment was maintained at 25°C and 12-h photophase.
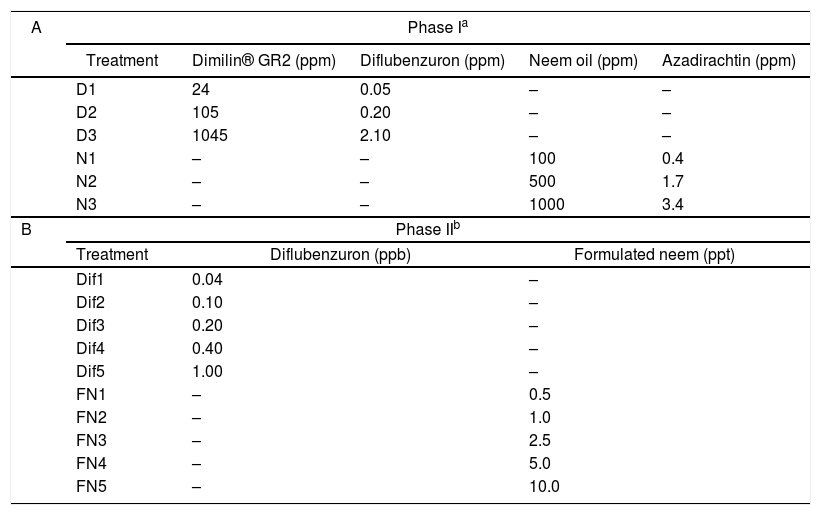
In a first stage, the growth of L. chapmanii was evaluated on culture media with diflubenzuron or neem oil. A granular presentation of diflubenzuron (Dimilin® GR2 with 2g of diflubenzuron per kg, Chemtura, Buenos Aires, Argentina) and neem oil extract with 3400ppm of azadirachtin (Integración Química S.R.L., Quilmes, Buenos Aires) were used. These products were added individually to PYG-agar culture medium at different concentrations (Table 1A) and then distributed in Petri dishes of 90mm in diameter. Five dishes per treatment were inoculated in the center with L. chapmanii and incubated at 25°C and 12-h photophase. The radial growths of L. chapmanii were determined using a millimeter ruler every 24h for four days. The assay was repeated three different times. Subsequently, the pathogenicity of L. chapmanii cultivated for seven days in different media with diflubenzuron or neem oil at the evaluated concentrations was determined. In this way, suspensions of zoospores were prepared by adding five pieces (1cm2 each) of each treatment medium with L. chapmanii into plastic containers (200ml) with 100ml of distilled water. The concentration of zoospores from each container was determined after 48h by placing an aliquot (200μl) of each treatment in a Neubauer chamber and counting under an optical microscope (100×). Ten second or third stage larvae (L2/L3) were added in each container and larval mortality was determined after 48h. The presence of L. chapmanii was confirmed using an optical microscope. Trials involved three replicate containers per treatment and their corresponding control (solid culture medium with diflubenzuron or neem oil but without L. chapmanii). An additional container with water and cubes of PYG-agar without any larvicidal products or L. chapmanii was included as a negative control.
Concentrations of larvicidal products evaluated in combination with L. chapmanii.
| A | Phase Ia | ||||
|---|---|---|---|---|---|
| Treatment | Dimilin® GR2 (ppm) | Diflubenzuron (ppm) | Neem oil (ppm) | Azadirachtin (ppm) | |
| D1 | 24 | 0.05 | – | – | |
| D2 | 105 | 0.20 | – | – | |
| D3 | 1045 | 2.10 | – | – | |
| N1 | – | – | 100 | 0.4 | |
| N2 | – | – | 500 | 1.7 | |
| N3 | – | – | 1000 | 3.4 | |
| B | Phase IIb | ||||
| Treatment | Diflubenzuron (ppb) | Formulated neem (ppt) | |||
| Dif1 | 0.04 | – | |||
| Dif2 | 0.10 | – | |||
| Dif3 | 0.20 | – | |||
| Dif4 | 0.40 | – | |||
| Dif5 | 1.00 | – | |||
| FN1 | – | 0.5 | |||
| FN2 | – | 1.0 | |||
| FN3 | – | 2.5 | |||
| FN4 | – | 5.0 | |||
| FN5 | – | 10.0 | |||
In a second stage, the pathogenic activity of zoospores was evaluated in the presence of these IGRs in water. On the one hand, a stock solution with 2 parts per million (ppm) of diflubenzuron was prepared in a Falcon tube (15ml) by mixing 10mg of Dimilin® GR2 with 10ml of sterile distilled water in a vortex at 150rpm for 3min. On the other hand, an experimental neem oil emulsion at 10% was prepared by mixing pure neem oil (3ml), Tween 80 at 5% (2ml) and distilled water (25ml) in a Falcon tube (45ml). The mixture was homogenized in an ultrasonic emulsifier chamber (JY96-II, Ningbo Scientz Biotechnology Co., Ltda, China) with a 0.5cm tip at 150W of output power and 20kHz pulses (on-time and off-time durations of 2s respectively) for 5min. Then, a stock solution with 1000ppm of the formulation was prepared by diluting 1ml of emulsion in one liter of tap water. Five concentrations of diflubenzuron and formulated neem (Table 1B) were prepared in plastic containers (200ml) using the stock solutions and chlorine-free tap water to a final volume of 100ml. Later, thirty larvae (L2/L3) of A. aegypti were placed in each container with a pinch of rabbit chow. Containers were maintained at 25°C and 12-h photophase. The mortality of larvae and pupae, and the emergence of adults were recorded every 24h for 20 days. The trial was performed employing one container per treatment and one negative control with tap water. Adult Emergence Inhibition (EI) was analyzed by the probit test using the SPSS v 20.0 (IBM®) software. The pathogenic action of zoospores was evaluated in combination with diflubenzuron or formulated neem at the concentrations established by the probit analysis to inhibit the emergence of adults at 30, 50 and 90% (EI30, EI50, EI90). To this end, a suspension of zoospores was prepared in an Erlenmeyer flask (2000ml) with 1600ml of sterile distilled water and five cubes (1cm2 each) of PYG-agar with seven-day cultured L. chapmanii. The flask was kept at 25°C for 72h allowing the release of zoospores. The concentration was estimated with a Neubauer chamber under an optical microscope, as previously explained, and then adjusted to 1×103 zoospores/ml. Assays were carried out in plastic containers (200ml) with 100ml of water with zoospores in suspension and diflubenzuron or the formulated neem at concentrations to EI30, EI50, EI90. Ten L2/L3A. aegypti larvae were added in each container and their mortality was registered every 24h for four days. The assay was repeated at three different times and involved three containers per treatment and one control for each larvicidal concentration without L. chapmanii.
The statistical analysis included the arcsine-square root transformation of mortality. Significant differences were determined by Analysis of Variance (ANOVA), and the Student–Newman–Keuls (SNK) post hoc test was applied in the cases with significant differences (p<0.05). Statistical analyses were carried out using the SPSS v 20.0 (IBM®) software and graphics were made with SigmaPlot® v. 11.0 (Systat software Inc, UK).
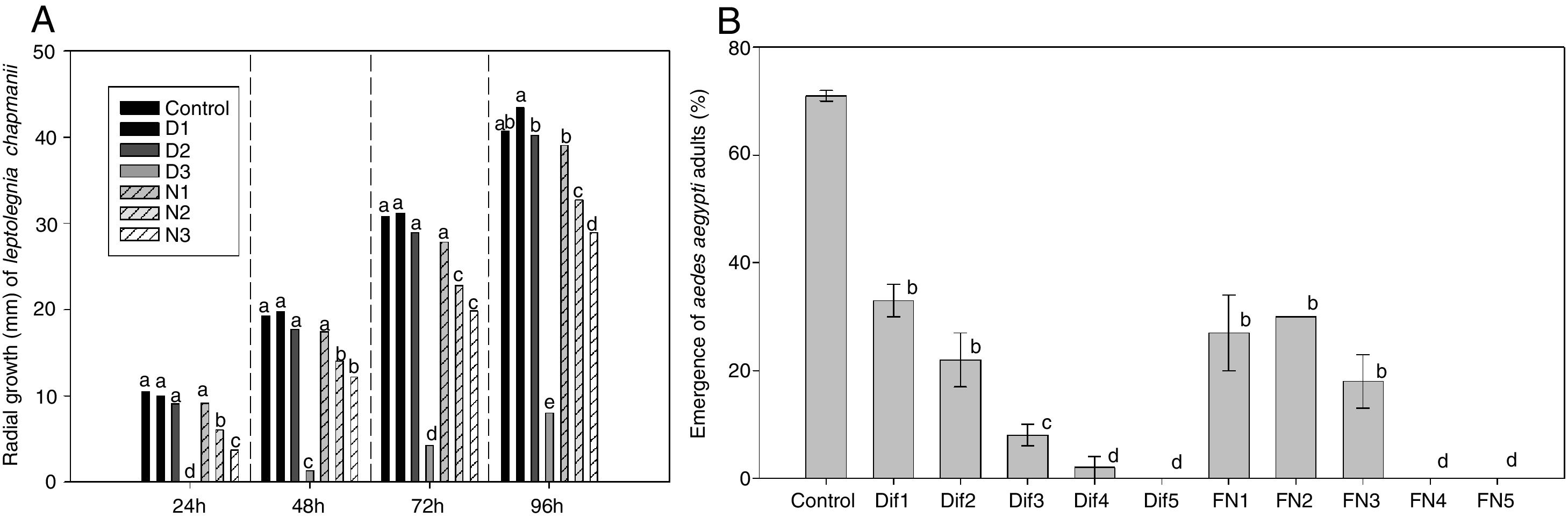
Results from the first stage of this work showed that the growth of L. chapmanii was affected negatively by the presence of the IGRs in PYG-agar (F6,98=268, p<0.05). Thus, the mycelial growth did not vary significantly between control and the treatments at lower concentrations of diflubenzuron and neem oil, but the growth rate was slower as concentrations increased (Fig. 1A). Nevertheless, L. chapmanii developed zoospores at concentrations between 1×103 and 4×103 zoospores/ml not showing statistical differences among treatments (F6,56=0.93, p>0.05), and these structures were always pathogenic for larvae, generating mortalities over 70% (F6,56=0.95, p>0.05). Controls with neem oil added to the media (without L. chapmanii) were neither toxic nor lethal to the larvae. On the other hand, controls with diflubenzuron were toxic, causing larval mortalities of 7±4, 40±4 and 51±2% at 0.05, 0.2 and 2.1ppm, respectively. Mortality of larvae in the negative control (PYG-agar without IGRs or L. chapmanii) was less than 5%.
Effects of diflubenzuron and neem oil. A) On the radial growth of L. chapmanii in PYG-agar culture media with diflubenzuron and neem oil at 24h (F6,98=53, p<0.001), 48h (F6,98=59, p<0.001), 72h (F6,98=76, p<0.001) and 96h (F6,98=268, p<0.001). B) On the emergence of adults of A. aegypti in presence of diflubenzuron or formulated neem, added in water at set concentrations (F10,21=0.29, p<0.01). The same letter groups treatments that did not have significant differences according to the post hoc SNK test (p<0.05).
Regarding the second stage of this work, the emergence of adults was reduced by the presence of diflubenzuron or the neem emulsion in water (Fig. 1B). According to the probit analysis, EI30, EI50 and EI90 were respectively of 0.01, 0.03 and 0.16 part per billion (ppb) of diflubenzuron and 0.1, 0.3 and 2.5 part per thousand (ppt) of emulsion. The pathogenic activity of zoospores was not affected by the presence of diflubenzuron or the neem emulsion at the concentrations evaluated (EI30, EI50, EI90). Mortality of larvae was greater than 90% (after 48h) in all cases, and the presence and development of L. chapmanii in larvae were always confirmed.
It is worth noting that diflubenzuron showed good results reducing the emergence of adults at very low concentrations (concerning the WHO reference value), thus its correct use should not be a risk for human populations. Moreover, the experimental emulsion based on neem oil was easy to prepare and apply. It was diluted properly in water and could be of interest for mosquito control.
These results, both from the first and second stages, suggest that the use of these larvicides (IGRs and L. chapmanii), applied separately, in combination or successively in time, would be viable alternatives to control mosquito populations. In other words, the possible presence of diflubenzuron or neem oil products in the environment should not affect the pathogenic activity of L. chapmanii. On the contrary, the presence of these IGRs would stop and/or delay the development of mosquito larvae, increasing time to interact with pathogens such as L. chapmanii.
Other larvicides successfully tested in combination with L. chapmanii were Bacillus thuringiensis svar israelensis and Temephos, in which case, larvicides presented a synergistic effect, causing higher mortality of the larval population than when applied individually10. Our results reinforce the premise that L. chapmanii has great potential as a biological control agent for mosquito populations.
Conflict of interestThe authors declare that they have no conflicts of interest.
This research work was partially supported by: the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), the Universidad Nacional de La Plata (UNLP) and the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT) [PICT 2012-0622] [PICT 2017-2059].